Abstract
Multi-arm star amphiphilic hyperbranched copolymers with poly(2-(dimethylamino) ethyl methacrylate) (PDMAEMA) shell and hyperbranched poly(3-ethyl-3-(hydroxymethyl)oxetane) (HBPO) core were synthesized by reversible addition–fragmentation chain transfer method. The hyperbranched copolymers were further modified by succinic anhydride (SUC) to obtain the novel pH- and thermosensitive hyperbranched copolymer HBPO-star-PDMAEMAs-SUC. The composition and morphology of synthesized copolymers were investigated by 1H NMR, dynamic light scattering, and transmission electron microscopy. These copolymers exhibited phase transitions in response to pH and temperature. The pH-dependent release properties of the drug-loaded micelles were also investigated using indomethacin (IND) as a model drug. The IND-loaded micelles displayed a rapid drug release at an alkaline pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to numerous potential applications in the controlled drug delivery and release, hyperbranched polymers have attracted great attention recently [1–3]. Hyperbranched polymers are three-dimensional macromolecules with good solubility and high reactivity. They are usually prepared by one-pot synthesis from specific monomers with branched potential [4, 5]. To enhance the application of hyperbranched polymers, it is crucial to impart desired functionality to target hyperbranched polymers with appropriate structures and properties [6–8].
Stimuli-sensitive properties are among the most useful functions of polymers for various fields of application. In particular, temperature and pH responsiveness are of great importance for biomedical applications. It is known that certain cancers as well as inflamed or wound tissue exhibit a pH different from 7.4, as it is in circulation. For example, chronic wounds have been reported to have pH values between 7.4 and 5.4, and cancer tissue is also reported to be acidic extracellularly. Moreover, the temperature of the tumor tissues in the body can be changed safely by hyperthermia [4, 9]. For these reasons, many efforts have been made to developing pH- and temperature-sensitive hyperbranched polymers for biomedical use [4–7].
A general strategy to prepare pH- and temperature-sensitive hyperbranched polymers is to introduce function groups or chains into the outer surface of hyperbranched polymers. As is known to all, poly(N-isopropylacrylamide) (PNIPAM) and poly(2-(dimethylamino) ethyl methacrylate) (PDMAEMA) homopolymers are temperature-responsive [10–13], and there are a great deal of research reports on the synthesis of temperature-sensitive hyperbranched polymers by introducing PNIPAM and/or PDMAEMA homopolymer into the outer surface of hyperbranched polymers [14–16]. For the pH-sensitive group, there are two types of pH-sensitive block copolymers: (1) those with basic core monomeric units (e.g., amines) which are uncharged and thus hydrophobic at high pH and become hydrophilic upon protonation at low pH [17–21] and (2) those with acidic core units (e.g., carboxylic acids) which are uncharged when protonated at low pH and become negatively charged at a relatively high pH [22]. Some previous studies have prepared the pH-sensitive hyperbranched polymers successfully, but most of the polymers display pH sensitivity only at acidic or alkaline conditions [23, 24].
In this study, we have succeeded in fabricating hyperbranched copolymers HBPO-star-PDMAEMAs-SUC by introducing both two types of pH-sensitive groups (PDMAEMA and succinyl groups) and temperature-sensitive homopolymer (PDMAEMA) onto the outer surface of HBPO. The resultant HBPO-star-PDMAEMAs-SUC copolymers have pH and temperature sensitivity both at acidic and alkaline conditions. To explore the potential application of the novel pH- and temperature-sensitive star copolymer, drug loading and in vitro release properties were also investigated. Indomethacin, a nonsteroidal anti-inflammatory antipyretic and analgesic lipophilic drug, was used as the model drug. We also investigated the influence of the lipophilic drug on the pH- and thermosensitivity of the HBPO-star-PDMAEMAs-SUC. The results showed that the indomethacin (IND)-loaded HBPO-star-PDMAEMAs-SUC exhibited pH-dependent release behaviors.
Experimental section
Materials
HBPO and S-1-dodecyl-S′-(α,α′-dimethyl-α″-acetic acid) trithiocarbonate (DMP) were synthesized according to a previously published procedure [25, 26]. Succinic anhydride (SUC), pyridine, tetrahydrofuran, and n-hexane were purchased from East China Chemical Co. (Shanghai, China) and used as received. Azobisisobutyronitrile (AIBN, 98%) was recrystallized twice from ethanol and dried in vacuum prior to use. AIBN was purchased from East China Chemical Co. 4-Dimethylaminopyridine (DMAP), N,N′-dicyclohexycarbodiimide (DCC), and 2-(dimethylamino) ethyl methacrylate (DMAEMA, 99%) were purchased from Aladdin Reagent Co. (Shanghai, China) and used as received.
Synthesis of macroinitiator HBPO-DMP
The hydroxyl terminal groups of HBPO were reacted with DMP by esterification to produce the HBPO-DMP reversible addition–fragmentation chain transfer (RAFT) macroinitiator. Typically, in a three-necked flask, 2.00 g (1.38 mmol) HBPO and 1.00 g (2.75 mmol) DMP were dissolved in 25 mL tetrahydrofuran (THF). About 1.00 g (4.84 mmol) DCC and 0.09 g (0.74 mmol) DMAP were added and the solution was stirred at 45 °C for 24 h under N2 atmosphere. The resultant solution was filtered and the products were precipitated in cold n-hexane. Then, the crude product was redissolved in THF and precipitated in cold n-hexane three times. The resultant product was dried in vacuum at 45 °C for 12 h to obtain a yellow solid. The Mn of HBPO and HBPO-DMP were 1,645 and 3,125 g/mol, determined by gel permeation chromatography (GPC), and about four initiation sites were introduced to HBPO-DMP. δ H (HBPO-DMP, DMSO-d6): 0.72–0.85(–CH 3), 1.21–1.33 (–CH 2CH3), 3.11–3.18 (–OCH 2–), 3.25–3.33 (–CH 2OH), 1.00–1.20 (–CH 2–), 1.40–1.80 (–CH 3).
Synthesis of HBPO-star-PDMAEMAs
HBPO-star-PDMAEMAs were synthesized through a RAFT path using HBPO-DMP as macroinitiator and AIBN as catalyst. The general procedure was as follows. The macroinitiator and AIBN were dissolved in THF and the solution was transferred to the flask. After the DMAEMA monomer was added by syringe, the flask was immersed in liquid nitrogen followed by three cycles of freeze–pump–thaw procedures. Finally, the flask was flame-sealed under vacuum and placed in a preheated oil-bath at 80 °C for 24 h. The resulting sample was further diluted with THF and then precipitated in cold n-hexane. The product was dried in vacuum at 45 °C for over 24 h. The feed ratio of DMAEMA to the initiation site of macroinitiator is controlled at 10, 20, 30, and 40 for P-1 to P-4. The yields of the P-1 to P-4 were 75.4%, 82.9%, 85.7%, and 88.1%. δ H (P-1, DMSO-d6): 0.8–0.9 (–CH 3), 1.8–1.9 (–CH 2–), 2.20–2.30 (–N(CH 3)2), 2.50–2.60 (–CH 2–N), 3.90–4.10 (–O–CH 2–CH2–).
Synthesis of succinylated HBPO-star-PDMAEMAs (HBPO-star-PDMAEMAs-SUC)
HBPO-star-PDMAEMA-SUC copolymers (PS-1 to PS-4) were synthesized by modifying the HBPO-star-PDMAEMAs with SUC. HBPO-star-PDMAEMAs (P-1 to P-4) were dissolved in pyridine. After excess SUC was added, the solution was stirred at 45 °C for 24 h under N2 in the dark. The reaction mixtures were filtered to remove the solid deposit and concentrated by evaporation. The crude products were redissolved in diethyl ether and the solution were filtered and concentrated by evaporation to remove the unreacted SUC for three times. The products were dried in vacuum at 45 °C for 24 h. The yields of polymerizations were determined gravimetrically (listed in Table 1). δ H (PS-1, DMSO-d6): 0.8–0.9 (–CH 3), 2.20–2.30 (–N(CH 3)2), 2.4 (–CH 2COOH) 2.50–2.60 (–CH 2–N), 3.90–4.10 (–O–CH 2–CH2–).
Drug loading and release
Indomethacin was loaded in HBPO-star-PDMAEMAs-SUC copolymer according to the published procedure [27]. The release experiment was conducted at pH 6.0, 7.0, and 8.0, respectively, and the temperature was maintained at 37 °C. IND-loaded copolymer (0.5 mg) was dissolved in 5 mL PBS, then the solution was put into a dialysis bag (MWCO = 3.5 kDa) and immersed in a beaker containing 200 mL PBS under stirring. At predetermined time intervals, 5 mL of liquid was sampled from the outer solution and then replaced with the same volume of release medium. The drug concentration was detected by measuring UV–vis absorbance at 320 nm.
1H NMR characterization
The 1H NMR spectra were recorded on an AVANCE AV 400-MHz digital FT-NMR spectrometer operating at 400 MHz using DMSO-d6 as solvent and tetramethylsilane as internal standard.
GPC measurements
Molecular weight and polydispersity of the copolymers were determined by GPC at 40 °C. An Agilent 1100 series GPC system equipped with a LC pump, PL gel MIXED-C column, and RI detector was used. The column was calibrated with polystyrene standards of narrow molecular weight distribution. The HPLC grade THF was used as a mobile phase and the flow rate was 1.0 mL/min.
Dynamic light scattering
Dynamic light scattering (DLS) measurements were performed in aqueous solution using a HORIBA Zetasizer LB-550V apparatus at 25 °C. PS-4 copolymers (1 mg/mL) were dissolved in PBS 6.0, 7.0, and 8.0.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed using a JEM-2100 TEM operated at an accelerating voltage of 200 kV, whereby a small drop of solution was deposited onto a copper EM grid and dried at 40 °C under atmospheric pressure. PS-4 copolymers (1 mg/mL) were dissolved in PBS 6.0, 7.0, and 8.0.
Thermogravimetric analysis
Thermogravimetric analysis (TGA) was performed on a Pyris Diamond 1 instrument (America) at a heating rate of 20 °C/min from 25 °C to 600 °C in a flow of nitrogen.
Optical transmittance measurements
Transition temperatures of the IND-loaded HBPO-star-PDMAEMAs-SUC copolymers (1 mg/mL) in various pH PBS (0.1 M) at 500 nm was monitored using a Hitachi U-3010 spectrophotometer with a water-jacketed cell holder coupled with a circulating bath. Temperature was raised at 0.5 °C/min. Values for the lower critical solution temperatures (LCST) of copolymer solutions were determined as the temperature corresponding to 50% of the optical transmittance.
Results and discussion
Synthesis of HBPO-star-PDMAEMAs-SUC
The synthesis procedure of HBPO-star-PDMAEMAs-SUC was shown in Scheme 1. HBPO with hydroxyl terminal groups was reacted with DMP by esterification to produce the HBPO-DMP as the RAFT macroinitiator. Then, the star copolymers of HBPO-star-PDMAEMAs (P-1 to P-4) were synthesized through a RAFT path using HBPO-DMP as macroinitiator, DMAEMA as monomer, and AIBN as catalyst. The synthesized HBPO-star-PDMAEMAs were further modified by SUC to obtain HBPO-star-PDMAEMAs-SUC (PS-1 to PS-4). The molecular weight (M n) and molecular weight distribution (M w/M n) of these copolymers were obtained by GPC measurement, and the data were collected in Table 1.
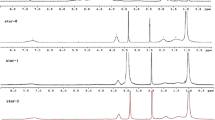
The structure and composition of synthesized copolymers were investigated by 1H NMR. As shown in Fig. 1, the proton signal of –CH 2–OH for HBPO at 3.30 ppm can be observed. After the hydroxy groups of HBPO are partly esterified by DMP, the new signals at 1.00–1.20 and 1.40–1.80 ppm from methylene and methyl of DMP can be found. Comparing the 1H NMR data of P-1 and HBPO-DMP, a new peak at 2.20–2.30 ppm from methyl of (N–CH 3) groups can be observed, which indicates that P-1 copolymer is successfully synthesized. Comparing the 1H NMR data of P-1 and PS-1, a new signal at 2.40 ppm of –CH 2– from succinyl group appears in the spectrum of PS-1, which confirms that PS-1 is succinylated successfully.
The curves of the loss weight rate of the HBPO, P-2, P-4, PS-2, and PS-4 in a nitrogen stream from room temperature to 600 °C were gained by way of TGA. Figure 2 shows the results of their TGA curves. It can be found that the thermal stability of these polymers is different. For HBPO, it begins to decompose when the temperature increases to 300 °C and decomposes almost completely at 470 °C. In the case of HBPO-star-PDMAMEAs (P-2, P-4), two decomposition temperatures are observed. The first decomposition temperature is at 178 °C, and loss weight reaches about 50% up to 350 °C, which is attributed to the decomposition of the PDMAEMAs arms. The second decomposition temperature is at 350 °C, which is attributed to the decomposition of the copolymer HBPO-star-PDMAEMAs. The decomposition of multi-arms star copolymers is almost completed at 470 °C. After the HBPO-star-PDMAEMAs were modified by SUC, three decomposition temperatures appeared in the curves of PS-2 and PS-4. The first stage of thermal decomposition is attributed to the decomposition of succinyl groups and PDMAEMAs arms. The second and the third stages are attributed to the decomposition of PDMAEMAs arms and HBPO cores, respectively. The results of TGA further confirmed that the HBPO-star-PDMAEMA-SUC copolymers have been successfully synthesized.
Morphology and pH-dependant size of micellar nanoparticles
It is well known that the star amphiphilic copolymers with PDMAEMA arms will self-assemble into large compound micelles and the spherical nanoparticles may have advantages as a tumor drug delivery system [10, 11, 19]. Figure 3 shows the TEM images of the PS-4 micelles at pH 6.0, 7.0, and 8.0 which indicate that the self-assembled micelles are well dispersed with spherical shape at different pH values. For DLS, as shown in Fig. 4, the size of the micelles is about 41 nm at pH 6.0, 33 nm at pH 7.0, and 24 nm at pH 8.0, respectively. The slight decrease in size of the PS-4 micelles can be attributed to the deprotonation of the tertiary amine groups of DMAEMA at various pH values. At low pH, the tertiary amino groups of the star copolymer are completely charged. Hence, the PDMAEMA segments are stretched fully due to the electrostatic repulsion among PDMAEMA segments, and the star copolymer shows a larger hydrodynamic diameter. When pH increases, the micelles experience the deprotonation of DMAEMA, which shrink the chains of micellar shell and result in a smaller size [20, 21]. Comparing the TEM and DLS measurement, we find that the average micelle diameter determined by TEM is larger than that measured by DLS. This discrepancy is widely considered to be induced by the process of sample preparation and the difference of investigation method between DLS and TEM. In the process of preparing samples for TEM measurements, the solvent evaporation at 40 °C may lead to the collapse and shrinkage of the micelles, which increases the hydrophobicity of the micelles. So the micelles can join together to form the larger aggregates driven by the increasing intermicellar hydrophobic interaction. In addition, DLS is sensitive to the hydrodynamic diameter that includes the highly solvated PDMAEMA chains [28].
Phase transitions of HBPO-star-PDMAEMAs-SUC
PDMAEMA is a water-soluble smart polymer with temperature, pH, and ionic strength responsibilities, and the HBPO-star-PDMAEMAs synthesized here also displayed a thermosensitive behavior. With the increase of temperature from room temperature to above 70 °C, the solution of P-2 at pH 7.5 gradually turned to a white opaque suspension, and the phase transition is reversible (Fig. 5b). After P-2 was further modified by SUC, the phase transition phenomena disappeared at the range of pH 7.5–6.0 (Figs. 5a and 6a, b). The hydrophobic drug, IND, was selected as a model drug and loaded in HBPO-star-PDMAEMAs-SUC micelles to prepare the polymer–drug complex IPS-1 to IPS-4. The solution of IPS-2 exhibited the phase transition behavior at pH 6.0 and became turbid at pH 5.0, as shown in Fig. 6d, e.
The illustration for the phase transition behavior of thermosensitive HBPO-star-PDMAEMAs and HBPO-star-PDMAEMAs-SUC micelles is shown in Scheme 2. When the solution of HBPO-star-PDMAEMAs is heated, the PDMAEMA arms dehydrated and collapsed onto the surface of the micelles and the micelles change from a hydrophilic state into a hydrophobic state. Then, the hydrophobic micelles join together to form the even larger aggregates driven by the increasing intermicellar hydrophobic interaction. The dehydration and aggregation of the micelles will certainly increase the turbidity of the solution [28]. After the HBPO-star-PDMAEMAs are further modified by SUC, the carboxyl groups which are hydrophilic and negatively charged at the alkaline conditions are introduced onto the surface of the HBPO [29]. Thus, when the pH of the solution is above 7, the steric hindrance of stretching succinyl chains and the electric repulsion between succinyl chains will prevent HBPO-star-PDMAEMAs-SUC molecules from intermolecular aggregations to large micelles. When pH is decreased to 6.0, both of the PDMAEMA groups and succinyl groups are partially protonated. Thus, PDMAEMA groups become positively charged and more strongly hydrophilic, while succinyl groups become uncharged and more weakly hydrophilic [30–32]. At the lower pH (pH < 7.0), the steric hindrance of stretching PDMAEMA arms and the electric repulsion between PDMAEMA chains will also prevent the HBPO-star-PDMAEMAs-SUC aggregations to form larger micelles. Indomethacin, a kind of lipophilic drug, containing carboxyl group in its structure was loaded in HBPO-star-PDAMEMAs-SUC. The hydrophobicity of the copolymer is enhanced and the micelles can join together to form the larger aggregates driven by the increasing intermicellar hydrophobic interaction. Thus, the solution of drug-loaded HBPO-star-PDMAEMAs-SUC is transparent at alkaline condition, has the phase transition behavior at pH around 6.0, and becomes turbid when pH is below 5.7.
The influence of the proportion of PDMAEMA blocks on the thermosensitivity of the copolymers was investigated. PDMAEMA was a water-soluble smart polymer with temperature responsibility. The hydrophilicity and temperature sensitivity of the IND-loaded copolymers would be enhanced after more proportion of PDMAEMA was introduced [10, 11, 28]. As shown in Fig. 7, the LCST of the four kinds of IND-loaded copolymers are 31, 59, 51, and 55 °C, respectively, indicating that the proportion of PDMAEMA groups will affect the phase transitions of HBPO-star-PDMAEMAs-SUC solution distinctly.
The influence of pH on the thermosensitivity of the IND-loaded copolymers was also investigated by UV–vis spectrometry. As shown in Fig. 8, the solution of IPS-4 at pH 6.0 and 5.9 exhibits phase transition at 39 and 43 °C, respectively. The solution at pH 5.7 is turbid at room temperature. As we all know, the carboxyl groups of the IPS and indomethacin can act as a pH sensor. The protonated carboxyl groups at lower pH may increase the hydrophobicity and induce the formation of hydrogen bond, leading to the lower cloud point. When the pH is below 5.7, most of the carboxyl groups are protonated and the solution turned into an opaque suspension at room temperature.
In vitro drug release
IPS-4, which has the best hydrophilicity in the four kinds of IND-loaded copolymers, is selected to investigate the pH-dependent release properties of the IND-loaded micelles. The drug content in the IPS-4 micelles is about 30% (w/w) as determined by UV–vis spectrometry (see Electronic supplementary material). As shown in Fig. 9, every curve shows an initial burst release stage which is thought to be the release of drugs that packed in the corona of micelles. However, the subsequent release depends greatly on the pH of the solution. The accumulative time of release of 95% of the loaded drugs from IPS micelles is 300 min at pH 8.0, 450 min at pH 7.0, and 1,200 min at pH 6.0, respectively. As illustrated in Scheme 2, the PDMAEMA groups of the PS-4 was protonated at lower pH and the copolymer had a larger hydrodynamic for the fully stretched PDMAEMA segments, which was confirmed by DLS. So the release of the IND from the IPS-4 micelles was relatively slower at lower pH for the longer route for the diffusion.
Conclusions
pH- and thermosensitive multi-arms star amphiphilic hyperbranched copolymers were synthesized successfully. The copolymer contained the carboxyl groups as pH-sensitive units and PDMAEMA groups as pH- and thermosensitive units. The pH- and thermosensitivity of the copolymer can be adjusted by controlling the proportion of the PDMAEMAs units. The HBPO-star-PDMAEMAs-SUC micelles showed phase transitions after loading indomethacin as a model drug. The drug release rate depends greatly on the pH of the solution, which indicated that the copolymer is a candidate as a functional material for biomedical applications. The investigation of targeted delivery of indomethacin to folate receptor-positive cancer cells is in progress.
References
Pang Y, Zhu Q, Liu JY (2010) Biomacromolecules 11:575
Chen S, Zhang XZ, Cheng SX (2008) Biomacromolecules 9:2578
Calderón M, Quadir MA, Sharma SK (2009) Adv Mater 21:1
Jiang GH, Wang Y, Sun XK (2010) Polym Chem 1:618
Yan DY, Zhou YF, Hou J (2004) Science 303:65
Burakowska E, Zimmerman SC, Haag R (2009) Small 5:199
Shen Y, Kuang M, Shen Z et al (2008) Angew Chem Int Ed 47:2227
Gao M, Jia XR, Li Y et al (2009) Macromolecules 42:4273
Schmaljohann D (2006) Adv Drug Deliv Rev 58:1655
Liu YH, Cao XH, Luo MB et al (2009) J Colloid Interface Sci 329:244
Zhou JH, Wang L, Ma JZ et al (2010) Eur Polym J 46:1288
Bao HQ, Hu JH, Gan LH et al (2009) J Polym Sci A, Polym Chem 47:6682
Chen WX, Fan XD, Huang Y et al (2009) React Funct Polym 69:97
Kojima C, Kohei Y, Harada A et al (2009) Bioconjug Chem 20:1054
You YZ, Hong CY, Pan CY (2009) Macromolecules 42:573
Zhang BY, He WD, Li WT et al (2010) Polymer 51:3039
Yuan WZ, Yuan JY, Zheng SX et al (2007) Polymer 48:2585
Georgiou TK, Vamvakaki M, Patrickios CS (2004) Biomacromolecules 5:2221
Xu FJ, Zhang ZX, Ping Y et al (2009) Biomacromolecules 10:285
Georgiou TK, Vamvakaki M, Phylactou LA et al (2005) Biomacromolecules 6:2990
Georgiou TK, Phylactou LA, Patrickios CS et al (2006) Biomacromolecules 7:3505
Cheng HX, Xie SA, Zhou YF (2010) J Phys Chem B 114:6291
Rijcken CJF, Soga O, Hennink WE et al (2007) J Control Release 120:131
Liu HJ, Chen Y, Shen Z (2007) J Polym Sci A, Polym Chem 45:1177
Lai JT, Filla D, Shea R (2002) Macromolecules 35:6754
Jiang GH, Wang L, Chen WX (2006) Eur Polym J 42:3333
Kim SY, Shin ILG, Lee YM (1998) J Control Release 51:13
Hong HY, Mai YY, Zhou YF et al (2008) J Polym Sci A, Polym Chem 46:668
Xu YY, Bolisetty S, Drechsler M (2008) Polymer 49:3957
Sakaguchi NK, Kojima C, Harada A (2008) Bioconjug Chem 19:1040
Plamper FA, Ruppel M, Schmalz A (2007) Macromolecules 40:8361
Zhang BY, He WD, Li WT (2010) Polymer 51:3039
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (20604024), Natural Science Foundation of Zhejiang Province (Y4100045), Qianjiang Talents Project of Zhejiang Province (2010R10023), Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (1001603-C), Scientific Research Foundation of Zhejiang Sci-Tech University (0901808-Y), Key Bidding Project of Zhejiang Provincal Key Lab of Fiber Materials and Manufacturing Technology, Zhejiang Sci-Tech University (S2010002), and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT: 0654).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 547 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Jiang, G., Wang, Y. et al. Synthesis and drug release properties of novel pH- and temperature-sensitive copolymers based on a hyperbranched polyether core. Colloid Polym Sci 289, 677–684 (2011). https://doi.org/10.1007/s00396-010-2314-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-010-2314-7