Abstract
The surface tension of aqueous mixtures of dodecyltrimethylammonium tetrafluoroborate (DTABF4) and sodium tetrafluoroborate (NaBF4) was measured as a function of total molality and composition of DTABF4 at 298.15 K. The results were analyzed by originally developed thermodynamic equations and compared with those of dodecyltrimethylammonium bromide (DTAB)–sodium bromide (NaBr) mixed system. It was indicated that BF −4 ions reduce the repulsion between DTA+ ions more effectively than Br− ions in the adsorbed film. To investigate this difference more closely, the surface tension of DTAB–NaBF4 and DTABF4–NaBr mixed system was also measured. The data analysis revealed that BF −4 ions are adsorbed positively even for the pure NaBF4 system and preferentially to Br− ions in these mixtures. Furthermore, it was concluded that the side-by-side arrangement suggested in the adsorbed film of 1-hexyl-3-methylimidazolium tetrafluoroborate (HMIMBF4) is due to not only the positive adsorption of BF −4 ions but also the capability of hydrogen bond formation between imidazolium ion and BF −4 ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For past decades, ionic liquids have been received increasing attention as a substitute for existing reaction solvents because of their low vapor pressure, non-flammability, and good solvent ability for a wide range of materials [1–3] and as a novel media for batteries, fuel cells, and solar cells because of their high conductivities and wide electrochemical windows [4–6]. The ionic liquids based of 1-alkyl-3-methylimidazolium ion have been investigated recently as short-chain cationic surfactant in the field of colloid and interface science because the imidazolium cations have an amphiphilic structure and their assembled states are, at least partly, similar to structure of neat ionic liquids [7–11]. We have previously studied the surface property of the aqueous solutions of 1-hexyl-3-methylimidazolium tetrafluoroborate (HMIMBF4) and a usual cationic surfactant with BF −4 as its counter anion, dodecyltrimethylammonium tetrafluoroborate (DTABF4), by employing the surface thermodynamics and X-ray absorption fine structure under total reflection condition (TRXAFS) [12, 13]. It was demonstrated that for example, the surface pressure π vs. the average area per molecule A curve of the HMIMBF4 system is almost vertical, which suggests an incompressible adsorbed film, at the saturation adsorption whereas those of DTABF4 and dodecyltrimethylammonium bromide (DTAB) systems show a higher compressibility even at their saturation adsorption (Fig. 1). Furthermore, in the adsorbed film of the HMIMBF4–1-hexyl-3-methylimidazolium bromide (HMIMBr) mixture, BF −4 ions are rather near cations, but Br− ions are distributed in the electric double layer, and thus, a segregation of BF −4 and Br− ions takes place in the adsorbed film [13]. Such counterion distribution is probably examined by employing the theory of Ivanov et al. [14].
For the neat ionic liquid surface, it is indicated that the BF −4 ion occupies the surface at the same level of the imidazolium ring because the BF −4 ion forms two hydrogen bonds with C(2)–H and –CH on the side chain, and as a result, it positions on top of the imidazolium ring [15–17]. From the fact that the compressibility of the film is very low irrespective of that the observed minimum occupied area of HMIMBF4 is still larger than the expected area for the fully packed monolayer of HMIM+ ions (0.36 nm2), we have concluded that the adsorbed film has similar structure to the neat ionic liquid surface. Probably, the adsorption of imidazolium cations at the air/water interface plays an important role for providing the environment where BF −4 ions are highly concentrated as the neat ionic liquid surface.
In the present study, in order to examine this unique adsorption behavior, we will focus on the effect of the counterions and the role of imidazolium cation on the adsorption behavior. For this purpose, we adopted the dodecyltrimethylammonium ion (DTA+ ion) as a surfactant cation which is less capable of forming hydrogen bonds than imidazolium ion and measured the surface tension of the DTABF4–NaBF4, DTAB–NaBF4, and DTABF4–NaBr systems. It was found that BF −4 ion adsorbs at the air/water interface preferentially to Br− ion, and the capability of hydrogen bond formation between imidazolium ion and counterion works effectively for the side-by-side arrangement in the adsorbed film of HMIMBF4.
Experimental
Material
Sodium tetrafluoroborate (NaBF4) was purchased from Kanto Kagaku Co., Ltd. (98%) and recrystallized from water and then baked at 170 °C for 7 h under reduced pressure. Sodium bromide (NaBr) was purchased from Wako Pure Chemical Industries (99.9%) and used as received. DTAB purchased from Wako Pure Chemical Industries (99%) was purified by recrystallizing it five times from the mixture of acetone and ethanol (1:5 volume ratio). DTABF4 was synthesized from DTAB with equimolar amount of NaBF4 in the aqueous solution and then recrystallized twice from water. These purities were checked by elemental analysis. Water used for all experiments was distilled three times; the second and third stages were done from alkaline permanganate solution.
Surface tension measurement
The surface tension γ of the aqueous solutions was measured by the drop volume method at 298.15 K under atmospheric pressure. The total molality \( \hat{m} \) and mole fraction of surfactant \( {\hat{X}_2} \) were adopted as the experimental concentration variables, where they are defined as
and
Here, \( {m_{{1} + }} \) and \( {m_{{1 - }}} \) represent the molalities of cationic and anionic ions dissociated from inorganic salts and \( {m_{{2} + }} \) and \( {m_{{2 - }}} \) those from ionic surfactants, respectively.
The γ value was calculated by the following equation [18, 19]
where V is the volume of a drop, g the local acceleration of gravity, r the capillary radius, and F the correction factor calculated from the following equation,
and is Δρ the density difference between air and aqueous solution. The densities of aqueous solutions of NaBr and NaBF4 ρ solution were measured by the oscillating tube method (Anton Par, D60/602) and shown in Fig. 2. It was found that ρ solution increases linearly with molality as
where \( {\rho_{\rm{water}}} \) was 0.997047 g cm−3, and \( a = 7.65052 \times {10^{ - 5}} \) (NaBr) \( 6.69881 \times {10^{ - 5}} \) (NaBF4), respectively.
The experimental error of the surface tension measurement was within 0.05 mN m−1.
Result and discussion
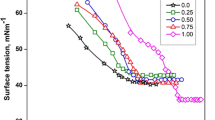
The results of surface tension measurements of the aqueous solutions of the DTABF4–NaBF4 mixtures were plotted against \( \hat{m} \) at fixed mole fractions of DTABF4 \( {\hat{X}_2} \) in Fig. 3. The γ value decreases with increasing \( \hat{m} \) and the shape of γ vs. \( \hat{m} \) changes regularly with \( {\hat{X}_2} \). We have previously proved that the break points on these curves are corresponding to the phase transition of the adsorbed film from the gaseous to the expanded states [20]. It should be noted that the γ values of the aqueous solution of NaBF4 slightly decreases with \( \hat{m} \), in contrast to that of NaBr, as shown in the wide concentration ranges in Fig. 4.
The total differential of surface tension at constant temperature and pressure is given by [21]
where the total surface density of ions and the surface composition of the second surfactant, DTABF4, are defined as
where the electroneutrality condition \( \Gamma_{{\rm{DT}}{{\rm{A}}^{+} }}^{\rm{H}} + \Gamma_{{\rm{N}}{{\rm{a}}^{+} }}^{\rm{H}} = \Gamma_{{\rm{B}}{{\rm{F}}_4}^{-} }^{\rm{H}} \) is taken into account, and
respectively. From Eq. (6), the surface density of surfactant was calculated by using
and shown in Fig. 5. From the linear decrease of γ with \( \hat{m} \) for the pure NaBF4 solution, it was noticed that NaBF4 adsorbs positively at the air/water interface, and its amount increases with increasing \( \hat{m} \). It is well known that Na+ ion shows negative adsorption due to the image force from the interface; hence, it was revealed that BF −4 ion adsorbs positively in the pure NaBF4 system. On the other hand, \( {\hat{\Gamma }^{\rm{H}}} \) of DTABF4 and the mixtures increases with increasing \( \hat{m} \) and shows the discontinuous change at the phase transition concentrations.
Next, let us evaluate the compositions of DTABF4 in the adsorbed film \( \hat{X}_2^{\rm{H}} \) by plotting \( \hat{m} \) values against \( {\hat{X}_2} \) at fixed γ and then applying
to the \( \hat{m} \) vs. \( {\hat{X}_2} \) curves. The results were drawn in the form of the phase diagram of adsorption (PDA) in which the composition in the bulk solution and that in the adsorbed film were represented together (Fig. 6).
It is worth noting that the value of \( \hat{X}_2^{\rm{H}} \) is larger than unity in the whole bulk composition range. Considering that \( \hat{X}_2^{\rm{H}} \) is rewritten from (Eqs. 7 and 8) as
it can be understood that one of the reason for this is the negative value of \( \Gamma_{{\rm{N}}{{\rm{a}}^{+} }}^{\rm{H}} \), that is, exclusion of Na+ ions from the surface region by the electrostatic repulsion toward DTA+ ions adsorbed at the interface. Figure 7 represents the surface densities of DTA+ ion evaluated by combining the \( {\hat{\Gamma }^{\rm{H}}} \) values in Fig. 5 and \( \hat{X}_2^{\rm{H}} \) values in Fig. 6, where the results of the DTAB–NaBr system [22] is also drawn. The \( \Gamma_{{\rm{DT}}{{\rm{A}}^{+} }}^{\rm{H}} \) value increases with decreasing \( {\hat{X}_2} \), but its dependence on \( {\hat{X}_2} \) is much weaker than that observed in the DTAB–NaBr mixtures. These results clearly demonstrate that the shielding effect of BF −4 ion on the electrostatic repulsion between DTA+ ions is quite effective compared with that of Br− ion, and the electric charge of DTA+ ions is almost completely shielded even in the pure DTABF4 system. Therefore, the further adsorption of BF −4 ion does not take place when NaBF4 is added.
Furthermore, the investigation of adsorption behavior of the DTAB–NaBF4 system revealed that BF −4 ion adsorbs at the air/water interface preferentially to Br− ion as follows. Figures 8 and 9 show the γ vs. \( \hat{m} \) curves and the PDA for the DTAB–NaBF4 mixtures. It should be noted in Fig. 8 that the γ vs. \( \hat{m} \) curve at a given \( {\hat{X}_2} \) moves to the left from \( {\hat{X}_2} = 1 \) (DTAB) to \( {\hat{X}_2} = 0.5 \) and then turns to the right with decreasing \( {\hat{X}_2} \). The \( \hat{m} \) vs. \( {\hat{X}_2} \) curves at given surface tensions (solid curves in Fig. 9) demonstrates this situation more clearly; the curves have a minimum at around \( {\hat{X}_2} = 0.5 \). The \( \hat{X}_2^{\rm{H}} \) values calculated from the solid curves are shown by the broken lines, which form the PDA together with the \( \hat{m} \) vs. \( {\hat{X}_2} \) curves. There are two important points. First, the PDA exhibits a negative azeotropic point, and thus, two components are mixed favorably in the adsorbed film in the whole range of \( {\hat{X}_2} \). Secondary the \( \hat{X}_2^{\rm{H}} \) values are around \( \hat{X}_2^{\rm{H}} = 0.5 \) irrespective of \( {\hat{X}_2} \) when \( {\hat{X}_2} < 0.5 \). Taking account of the relation \( \hat{X}_2^{\rm{H}} = X_{{\rm{DT}}{{\rm{A}}^{+} }}^{\rm{H}} + X_{{\rm{B}}{{\rm{r}}^{ - }}}^{\rm{H}} \) from (Eq. 8) and
and supposing that \( X_{{\rm{N}}{{\rm{a}}^{+} }}^{\rm{H}} \approx 0 \), we have \( X_{{\rm{DT}}{{\rm{A}}^{+} }}^{\rm{H}} + X_{{\rm{B}}{{\rm{r}}^{ - }}}^{\rm{H}} = 0.5 \) and \( X_{{\rm{B}}{{\rm{F}}_4}^{-} }^{\rm{H}} = 0.5 \) when \( \hat{X}_2^{\rm{H}} = 0.5 \). Therefore, the electroneutrality condition \( X_{{\rm{DT}}{{\rm{A}}^{+} }}^{\rm{H}} = X_{{\rm{B}}{{\rm{F}}_4}^{-} }^{\rm{H}} + X_{{\rm{B}}{{\rm{r}}^{ - }}}^{\rm{H}} \) yields \( X_{{\rm{B}}{{\rm{r}}^{ - }}}^{\rm{H}} = 0 \); it is suggested that the adsorbed film is almost composed of DTA+ and BF −4 ions when \( {\hat{X}_2} < 0.5 \). This is demonstrated more precisely by combining the results of the DTAB–NaBF4 system with those of the DTABF4–NaBr system as shown later.
We have previously reported that the Br− ions in the adsorbed film of DTAB aqueous solution have two hydration states by the TRXAFS measurement [12]: one corresponding to the Br− ions with smaller hydration number of 3 to 4 distributed near the surfactant head groups (bound ions) and the other the Br− ions with hydration number of 6 similar to those in the bulk solution (free ions). The fraction of the free ions decreases with increasing \( \Gamma_{{\rm{DT}}{{\rm{A}}^{+} }}^{\rm{H}} \), and the fraction of the bound ions increases instead. However, in the mixed adsorbed film of HMIMBF4–HMIMBr system, the TRXAFS revealed that the Br− ions are all in the free states at almost all the bulk compositions [13]. Taking these results into consideration, it is indicated that the fraction of the bound BF −4 is considerably high even in the pure DTABF4 system, and Br− ions distribute mainly in the electrical double layer as the free ion.
Finally, let us calculate the ratio of BF −4 ion and Br− ion in the mixed adsorbed film. Here, it should be remembered that the surface densities of the respective ions cannot be calculated in principle for the binary ionic surfactant mixture without common ions like the DTAB–NaBF4 system [21]. However, they can be evaluated at \( {\hat{X}_2} = 0.5 \) by combining the results of the DTABF4–NaBr system as follows. Figures 10 and 11 show the \( \gamma \) vs. \( \hat{m} \) curves and the PDA for the DTABF4–NaBr mixtures. The \( \gamma \) vs. \( \hat{m} \) curve gradually changes its position from \( {\hat{X}_2} = 1 \) (DTABF4) by adding NaBr, which gives the similar feature to that of the DTABF4–NaBF4 mixture in Fig. 3, but the different one from that of the DTAB–NaBF4 mixture in Fig. 8. Since the numbers of constituent ions are the same for the DTABF4–NaBr and DTAB–NaBF4 systems at a given \( \hat{m} \) and at \( {\hat{X}_2} = 0.5 \), we have four equations: the one is Eq. (12) from the mass balance relation, and other three are
and
from the electroneutrality condition in adsorbed film. The \( X_{\rm{\alpha }}^{\rm{H}}\,\left( {\alpha {;}\,{\hbox{DT}}{{\hbox{A}}^{+} },\,{\hbox{N}}{{\hbox{a}}^{+} },\,{\hbox{B}}{{\hbox{r}}^{-} },\,{\hbox{BF}}_4^{-} } \right) \) values thus estimated are listed at γ = 65 and 46 mN m−1 in Table 1. It is evident that the ratio \( {{X_{{\rm{B}}{{\rm{r}}^{ - }}}^{\rm{H}}} \mathord{\left/{\vphantom {{X_{{\rm{B}}{{\rm{r}}^{ - }}}^{\rm{H}}} {X_{{\rm{B}}{{\rm{F}}_4}^{-} }^{\rm{H}}}}} \right.} {X_{{\rm{B}}{{\rm{F}}_4}^{-} }^{\rm{H}}}} = 0.009 \) and 0.053, and thus, the adsorbed film is almost composed of DTA+ and BF −4 ions when \( {\hat{X}_2} = 0.5 \). This is in accord with the suggestion obtained from the PDA of the DTAB–NaBF4 system and the examination on it by supposing \( X_{{\rm{N}}{{\rm{a}}^{+} }}^{\rm{H}} \approx 0. \)
In the present study, for the purpose of examining the side-by-side arrangement of HMIMBF4 in the adsorbed film, we investigated the counterion effect on surface adsorption of the several cationic surfactants. The important conclusions are as follows: BF −4 ions show strong shield effect on the electric repulsion between surfactant ions, and they are preferentially adsorbed at the air/water interface even when the bulk solutions are enriched with Br− ion. Taking into account that the BF −4 ion adsorbs positively while Br− ion negatively at the air/water interface in the respective pure inorganic electrolyte solutions, the differences in adsorption behavior observed in the binary mixtures of DTABF4–NaBF4, DTAB–NaBF4, and DTABF4–NaBr are partially attributable to the difference in surface activity of the counterions. Although the adsorption ability of BF −4 ion is undoubtedly important factor in governing the adsorption behavior of cationic surfactant having BF −4 as counter anion, the interaction between the surfactant cation and BF −4 is also essential in the adsorption behavior. For example, the side-by-side arrangement is suggested due to the ion paring between HMIM+ and BF −4 ions in the adsorbed film, whereas DTABF4 itself does not show such arrangement as examined from the π vs. A curves (Fig. 1). Therefore, it is indicated that the capability of hydrogen bond formation between imidazolium ion and counterion works effectively for the side-by-side arrangement.
References
Wasserscheid P, Welton T (2003) Ionic liquids in synthesis. Wiley, New York
Welton T (1999) Chem Rev 99:2071
Wasserscheid P, Keim W (2000) Angew Chem Int Edi 39:3773
Bonhote P, Dias A, Papageorgiou N, Kalyanasundaram K, Gratzel M (1996) Inorg Chem 35:1168
Fuller J, Breda AC, Carlin RT (1998) J Electroanal Chem 459:29
Quinn BM, Ding ZF, Moulton R, Bard AJ (2002) Langmuir 18:1734
Firestone MA, Dzielawa JA, Zapol P, Curtiss LA, Seifert S, Dietz ML (2002) Langmuir 18:7258
Dong B, Zhao XY, Zhang J (2008) Li N. Inoue T 317:666
Bowers J, Butts CP, Martin PJ, Vergara-Gutierrez MC, Heenan RK (2004) Langmuir 20:2191
Jiang W, Wang YT, Voth GA (2007) J Phy Chem B 111:4812
Vanyur R, Biczok L, Miskolczy Z (2007) Colloids Surfaces A—Physico Eng Asp 299:256
Aratono M, Shimamoto K, Onohara A, Murakami D, Tanida H, Watanabe I, Ozeki T, Matsubara H, Takiue T (2008) Anal Sci 24:1279
Shimamoto K, Onohara A, Takumi T, Watanabe I, Tanida H, Matsubara H, Takiue T, Aratono M (2009) Langmuir 25:9954
Ivanov IB, Marinova KG, Danov KD, Dirnitrova D, Ananthapadmanabhan KP, Lips A (2007) Adv Colloid Interface Sci 134:105
Jeon Y, Sung J, Seo C, Lim H, Cheong H, Kang M, Moon B, Ouchi Y, Kim D (2008) J Phys Chem B 112:4735
Jeon Y, Sung J, Bu W, Vaknin D, Ouchi Y, Kim D (2008) J Phys Chem C 112:19649
Kolbeck C, Cremer T, Lovelock KRJ, Paape N, Schulz PS, Wasserscheid P, Maier F, Steinruck HP (2009) J Phys Chem B 113:8682
Harkins WD, Brown FE (1919) J Am Chem Soc 41:499
Lando JL, Oakley HT (1967) J Colloid Interface Sci 25:526
Motomura K, Iwanaga S, Hayami Y, Uryu S, Matsuura R (1981) J Colloid Interface Sci 80:32
Aratono M, Villeneuve M, Takiue T, Ikeda N, Iyota H (1998) J Colloid Interface Sci 200:161
Kashimoto K, Takata Y, Matsuda T, Ikeda N, Matsubara H, Takiue T, Aratono M, Tanida H, Watanabe I (2006) Langmuir 22:8403
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research of Priority Area (No. 20031020) from the Ministry of Education, Science, Sports and Culture.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Imai, Y., Shimamoto, K., Takiue, T. et al. Study on surface adsorption from cationic surfactant–electrolyte mixed aqueous solution including BF −4 ion. Colloid Polym Sci 288, 1005–1011 (2010). https://doi.org/10.1007/s00396-010-2221-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-010-2221-y