Abstract
Compatibilization of the partially miscible poly(vinylidene fluoride) (PVDF)/poly(styrene-co-acrylonitrile) (SAN) pair by a third homopolymer, i.e., poly(methyl methacrylate) (PMMA), was investigated in relation to cross section morphology, crystallization behaviors and hydrophilicity of the polyblends. Scanning electron microscopy showed a more regular and homogeneous morphology when more than 15 wt.% PMMA was incorporated. The samples presented only α phase regardless of PMMA content in the blend. As the PMMA content increased in the blends, the interactions between each component were enhanced, and the crystallization of PVDF was limited, leading to a decreasing of the crystallinity and the crystallite thickness. Besides, the hydrophilicity of PVDF was further improved by PMMA addition. The sample containing 15 wt.% PMMA showed a more hydrophilic property due to the more polar part of surface tension induced by PMMA addition. Observed from the cross section of the blends, the miscibility of partially miscible PVDF/SAN blends were efficiently improved by PMMA incorporation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer blends play an important role in the preparing of new high performance polymeric materials for special applications. This method is a versatile, straightforward, and relatively inexpensive one for modifying the properties of polymer materials and has attracted tremendous attention over the past decades. From an exclusively thermodynamic point of view, the blends may be classified as miscible, partially miscible, and immiscible systems [1]. Current theory to identify the miscibility the blend mainly uses the concentration dependences of the glass transition and depression of the melting point [2, 3]. The blends presenting a single T g criterion is possible to be considered as miscible. With regard to the blend containing crystallizable component, the thermodynamic interactions in the blends is described by melting point depression. Recently, there have been numerous reports of miscibility, crystallization, and melting behavior of binary blends of poly (vinylidene) fluoride (PVDF)/amorphous polymer, including PVDF/ poly (methyl methacrylate) (PMMA) [2], PVDF/ poly (vinyl fluoride) (PVF) [4], PVDF/poly(vinyl acetate) (PVAc) [5], PVDF/poly(vinylpyrrolidone) (PVP) [6], PVDF/poly(3-hydroxybutyrate) (PHB) [7] and etc. It has been demonstrated that PVDF could be miscible with oxygen-containing polymers which is related to the interaction between the fluorine atoms and carbonyl groups of the partner polymer [8]. However, as reported in Li’s work [9], not all carbonyl-group-containing polymers are miscible with PVDF. They found that PVDF can be miscible with poly(trimethylene adipate) (PTA) (with a CH2/CO ratio of 3.5) and poly(pentamethylene adipate) (PPA) (with a CH2/CO ratio of 4.5).
PVDF as a semi-crystalline polymer with excellent physical and chemical properties, as well as good thermal stability, has been widely used in many applications. However, because of its hydrophobic nature, PVDF’s wide application in membranes process has been limited. In order to obtain more hydrophilic PVDF blends, PVDF/PMMA [10], and PVDF/polyacrylonitrile (PAN) [11] blends were applied to the membrane preparations. In the result, their hydrophilicity was enhanced and flux rate of the PVDF blend membrane increased by those hydrophilic materials. Among the publications about PVDF blends, few works focused on the ternary system. In Moussaif’s work, the immiscible blend PVDF/PC was compatibilized by PMMA addition [12]. In this ternary blend, the more regular and finer phase dispersion, and better mechanical properties were presented. As far as the thermodynamic properties are concerned, the ternary blend system PVDF/PMMA/PVAc is miscible [13]. Kim et al. use poly(methyl methacrylate-co-methacrylic acid) (P(MMA-co-MAA)) of low methacrylic acid content as the compatibilizer to add into PVDF/nylon 6 blends [14]. It reveals that the compatibility and the energy of rupture in tensile testing for nylon 6 and PVDF is enhanced by adding P(MMA-co-MAA). Therefore, a substantial compatibilization upon the incorporation of a third polymeric component plays an important role in improving the miscibility and physical properties of the immisicible or partially miscible binary blends.
As reported in our previous work [15, 16], the hydrophilicity of PVDF can be improved by random incorporation of hydrophilic polymer (PMMA, SAN) into the PVDF matrices. In the whole range of PMMA composition, PVDF/PMMA blend system has a good miscibility. However, the binary blend of PVDF/SAN is a partially miscible system as the PVDF content is higher than 50 wt.%. As demonstrated in our previous work [15], when PVDF is rich in the binary blend (PVDF/SAN), the one with 70 wt.% PVDF maintains the original properties of PVDF and shows better hydrophilicity, but this system is not miscible. The low miscibility would lead to gross phase separation and poor interfacial adhesion [12]. Therefore, in order to prepare more hydrophilic PVDF/SAN (with 70 wt.% PVDF) blend which maintains good properties of PVDF and SAN component, the miscibility should be improved. As known, PMMA and SAN have the miscibility due to intramolecular repulsive forces in the SAN component [17]. Thus, in this work, in order to improve the miscibility, PMMA, which was considered as a compatibilizer, was incorporated into PVDF/SAN blend. The effect of PMMA composition on the miscibility, morphology, and hydrophilicity of PVDF/SAN blend was investigated.
Experimental
Materials
PVDF (Kynar K-761, \(\overline M w = 441,000\)) was supplied by Elf Atochem of North America Inc. (USA). PMMA (HR1000L) was obtained from Kuraray Co., Ltd (Japan). SAN (D-178, in which the acrylonitrile content is 26 wt.%) was purchased from Zhenjiang Guoheng Chemical Limited Company (China).
Melt blending and specimen preparation
The melt blending of PVDF/PMMA/SAN was carried out with a torque rheogoniometer (Kechuang Machinery XSS-300, China) at 180 °C and 60 rpm for 10 min. A set of the blends with weight fractions of PVDF/PMMA/SAN containing 70/0/30, 70/9/21, 70/15/15, 70/21/9, and 70/30/0 (w/w) were prepared. Then, the blend samples were prepared by compression molding at 180 °C with the platen vulcanizing press (5 MPa) for 10 min, and then cooled at room temperature still under pressure (10 MPa). Thus, the flat sheet samples (about 0.5 mm) possessing smooth surfaces were obtained. Subsequently, the series of measurements were performed.
Scanning electron microscopy (SEM) observation
The samples were fractured in liquid nitrogen, and the surfaces were sputtered with Pt in vacuum. Then, the SEM micrographs of dried and gold-coated cross sections were taken with a JEOL JSM-5900 instrument.
Fourier transform infrared spectroscopy (FTIR) measurement
Fourier transform infrared spectroscopy (FTIR) spectra were obtained by a Bruker IFS 66/s with 4 cm−1 resolution. FIIR-attenuated total reflection (ATR) spectra were applied in this work.
Wide-angle X-ray diffraction measurement
Wide-angle X-ray diffraction (WAXD) was done with a Shimadzu XRD-6000 diffractometer (Cu Kα radiation, 40 kV and 30 mA). The scanning angle ranged from 5°∼50° with the scanning velocity of 4°/min. The interplanar distance (d-value) and the crystallite thickness L was calculated using the Bragg equation and Scherrer equation [18], respectively, as our previous work [16, 19].
Differential scanning calorimetry (DSC)
The calorimetric measurements were made on a TA Instruments Q-200 differential scanning calorimeter in a dry nitrogen atmosphere. In order to erase the thermal history of the samples, the polymer blend samples (ca. 10 mg) were heated from room temperature to 180 °C at a rate of 40 °C/min. Maintained at 180 °C for 5 min, the mixtures were cooled down to 40 °C at a rate of 5 °C/min (the cooling run). After 1 min at 40 °C, a second heating was done upon to 180 °C. The glass transition temperature (Tg) was taken at the inflection point on the thermograms.
The crystallinity of PVDF (X c) was recorded below, as our previous work [20, 21].
Where \(\Delta H_f^ * = {{{\text{104}}{\text{.5J}}} \mathord{\left/ {\vphantom {{{\text{104}}{\text{.5J}}} {\text{g}}}} \right. \kern-\nulldelimiterspace} {\text{g}}}\) [22] is the melting enthalpy for a 100% crystalline PVDF, ΔH f is the melting enthalpy of the mixtures measured in DSC, and ϕ is the weight fraction of PVDF in the blend (70 wt.%).
The crystallization half time (t 1/2), which is defined as the half time of crystallization [23], was used as a characteristic parameter of the crystallization process.
Contact angle test
The measurement of the contact angle of samples was carried out using a Krüss DSA-100 goniometer at 20 °C. Water and diiodomethane were used as the test liquids. The detailed procedure was the same as our previous work [16]. Then, the total solid surface tension γ s, and its dispersive and polar component \(\gamma _s^d \), \(\gamma _s^p \) were calculated by the Owens and Wendt method [24, 25].
Results and discussion
The miscibility of SAN with PVDF
SAN is a hydrophilic polymer material because of its nitrile group (–CN) [11], but just partially compatible with PVDF [15]. This kind of polymer blend will limit its application because of microheterogeneity and small interfacial adhesion. In order to overcome these shortcomings, PVDF/SAN blend should be compatibilized. As is known, PMMA, which has similar physical properties to SAN, is an amorphous and hydrophilic polymer material. As reported, PMMA is miscible with PVDF over the whole range of composition [16, 26–28]. On the other hand, PMMA and SAN have the miscibility due to intramolecular repulsive forces in the SAN component [17]. So PMMA can be used as the compatibilizer of the PVDF/SAN system. As expected, the compatibility of PVDF/SAN blend can be improved by adding with PMMA.
The PMMA component dependence of cross section morphology in PVDF/SAN blends with 70 wt.% PVDF is shown in Fig. 1. The PVDF/SAN (70/30) blend shows a typical morphology of partially compatible polymers, with small dispersed domains of SAN (Fig. 1a). In a ternary system (PVDF/PMMA/SAN; the fraction of PVDF is fixed at 70 wt.%), the increase of PMMA content makes the blend more homogeneous. Therefore, it is obviously observed that an incorporation of PMMA can efficiently improve the compatibility of PVDF/SAN blend. When only PMMA is blended with PVDF, the homogeneous morphology is obtained. This can be confirmed that PVDF/PMMA blend has high compatibility, as illustrated in our previous work [16].
IR spectroscopy
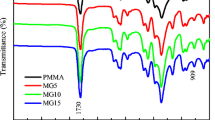
Figure 2 shows the IR spectra (in ATR mode) of the neat PVDF, neat PMMA, and ternary blends (PVDF/PMMA/SAN) with different weight fraction of each component. The samples containing PVDF have the well-defined absorption bands at 1,423, 1,400, 1,383, 1,210, 1,177, 1,068, 975, 870, 855, 794, 761, and 614 cm−1. As reported, these IR absorption bands represent the characteristic spectrum of the α phase of PVDF crystal [29, 30]. It indicates that the only crystallization of α phase PVDF predominates in the crystallization from the melt, which agrees with Gregorio’s results [31]. It can be seen that the intensity of the characteristic α phase bands are not changed. This suggests that the crystalline phase of PVDF is not influenced by the change of the various ratios of PMMA and SAN content, when PVDF content is fixed at 70 wt.%. As for the neat SAN sample, the bands at 759 cm−1 and at 699 cm−1 show the presence of the out-of-plane hydrogen vibration for styrene co-polymer [32]. As PVDF blended with SAN, the band at 759 cm−1 is overlapped by the more intensive α phase PVDF band (761 cm−1).
In addition, when PMMA blended with PVDF or PVDF/SAN blend, the band at 1,722 cm−1, which refers to the carbonyl band in PMMA component, is enhanced and shifts to a higher wavenumber. The enhancing of this band is due to the increased PMMA content in the blend. The small shift of the carbonyl band indicates the occurrence of weak interactions between C=O and –CF2 groups [33]. It imparts a high miscibility to PVDF/PMMA system. So PMMA is selected to improve the miscibility of PVDF/SAN blend.
WAXD analysis
X-ray diffractograms of the ternary blends and neat polymers are shown in Fig. 3. According to the researchers’ work [30, 34, 35], the peaks at 2θ = 18.05, 18.70, 20.27, 26.90, 33.37, 36.17, 37.43, and 39.00° in the curve for the neat PVDF sample (Fig. 3a), represent the diffractions in planes (100), (020), (110), (021), (130), (200), (210), and (002), respectively, which are all characteristic of the α phase of PVDF. It is noted that the values of the diffraction peak obtained in this work are a little higher than those obtained in the reference [30, 34]. This can be attributed to the difference in types of the apparatus which used for WAXD measurement. It is verified that all samples by melt molding, despite the various ratios of PMMA and SAN weight fraction in the blend, presents predominantly the α phase. All these peaks have a decrease in their intensity, as the PMMA composition increased in the blend, especially for the one at 2θ = 18.0°, the decrease is the most remarkable as PMMA content increased. When only PMMA blended with PVDF, the peak at 2θ = 18.0° disappears. This suggests that the existence of PMMA in the blend significantly retards the growth of PVDF lamellae, which is due to the enhanced interactions between PVDF and other two polymer chains. As shown in Fig. 3, the ration of the intensities of the reflections assigned to (100) and (020) change dramatically. This possibly is due to the different limits to the growth of the PVDF lamella. As PMMA is incorporated into PVDF/SAN blend, the lamella L100 is affected remarkably, leading to obvious decrease in the intensities of the reflections (100). It has demonstrated that the crystallinity of a crystalline polymer can be related to the total scattering height of the peak in the WAXD [36]. Therefore, the crystallinity of PVDF in the blends is strongly affected by the increased PMMA content. As the mass ratio of PMMA/SAN is 30/0 in the blend, only two weak peaks at 2θ = 18.7, 20.2° are illustrated, and other peaks disappear (Fig. 3e); i.e., the PVDF crystals are reduced more at this composition. With regard to the neat SAN178, PMMA samples, the amorphous humps instead of diffraction peaks are observed.
Table 1 lists the values of 2θ, interplanar spacing d, according to the three strong peaks observed in the diffractograms of Fig. 3. The crystallite thickness L hkl in the direction perpendicular to the (hkl) crystal plane is calculated by Bragg equation and Scherrer equation [18], as reported in previous work [16, 37]. As shown in Table 1, the value d of all planes has nearly no change with different mass ratios of PMMA and SAN in the blends; i.e., the lattices parameter of the α phase is not affected. The values L (100), L (020) become larger when 9 wt.% PMMA added into PVDF/SAN blend, in comparison with the binary blend of PVDF/SAN (70/30). As demonstrated [16, 37], the miscible binary blend system, such as PVDF/PMMA and PEO/PMMA, the non-crystalline polymer molecules can be sandwiched in interlamellar regions. Therefore, the increased value L could be due to the trapped PMMA chains in the PVDF interlamellae. This confirms that less (9 wt.%) PMMA addition into the binary PVDF/SAN blend can improve their miscibility and favors the growth of PVDF lamellae. However, more presence of PMMA domains will induce some kind of perturbation which hinders the lamellar growth of PVDF in the blends. As detailed in Table 1, when PMMA content increases more (>9 wt.%), the crystallite thickness of PVDF becomes smaller. This result is well in agreement with our previous work [16]. It has been demonstrated that the less PMMA addition (<10 wt.%) into PVDF would favors the lamellae growth in the blend, leading to larger crystallite size. When more PMMA incorporated into PVDF, the growth of PVDF lamellae is reduced. In this work, similar result is obtained. When 9 wt.% PMMA is added into PVDF/SAN blend, the PVDF lamellae growth is favored, while, 15 wt.% or more PMMA incorporation will reduce the lamellae growth of PVDF (especially for L (100) and L (020)). As for the crystallite thickness L 110, the small PMMA addition does not favor this lamella growth. The value L 110 changes a little when the PMMA content is less than 15 wt.%, and it decreases more when more PMMA added. This means that the crystallite thickness L 110 is decreased with PMMA addition.
Thermal properties and crystallinity
Figure 4 shows the DSC crystallization curves of neat polymer and blend samples during cooling from the melt at 5 °C/min after removing the thermal history. It is obviously observed that all the PMMA addition induce a significant decrease in the exothermic peak temperature, comparing with the PVDF/SAN (70/30) blend in which no PMMA added. In polymer/diluent system, the addition of diluent lowers the chemical potential of the mixture, leading to the depression of the crystallization temperature of PVDF diversely [38, 39]. Herein, as PMMA is incorporated into PVDF/SAN (70/30) blend, the dilution effect is enhanced. Therefore, the crystallization of PVDF is influenced more in these samples. This confirms that the interaction between each component of the ternary blend is enhanced by PMMA addition.
The data of the DSC crystallization results from Fig. 4 are summarized in Table 2. The crystallization temperatures and the value ΔH c (enthalpy of crystallization) for PVDF in the blends depend upon various mass ratios of PMMA and SAN. The onset crystallization temperature \(T_c^{on} \), peak crystallization temperature \(T_c^p \), and final crystallization temperature \(T_c^f \) are decreased with an increase of PMMA addition in the ternary blends. The difference between the onset and peak crystallization temperature \(\Delta T_c \) obtained from each sample is listed in Table 2. Beck [40] suggested that the small the values \(\Delta T_c \), the faster the overall crystallization rate, because the free expansion of crystals occurs between the onset and peak crystallization temperature [38]. It is shown that \(\Delta T_c \) is increased with an increase of PMMA content in the ternary blends. This indicates that the PMMA content in the ternary blends has more disturbances on the crystallization of PVDF, and this influence is enhanced as the PMMA content increased. As reported [41, 42], the value t 1/2 is another parameter which characterizes the crystallization rate of polymer. The smaller the value t 1/2, the faster the crystallization rate, and vice versa. In Table 2, the value t 1/2 is increased with PMMA addition in the ternary blend. Therefore, the increased value t 1/2 and \(\Delta T_c \) in the ternary blends suggests that the crystallization rate of PVDF is reduced by PMMA addition. It indicates that the interactions between PVDF and SAN chains can be improved by PMMA addition, leading to better compatibility of the ternary blend. In addition, among these ternary blends, the value \(\Delta T_c \) of each sample has nearly no changes. This is due to the same weight fraction of PVDF in each sample.
Figure 5 shows the melting curves of PVDF, PMMA, SAN, and their blend samples after cooling from the melt. The evidence of a glass transition of the samples, as indicated in the melting curve can be observed in SAN, PMMA, and the blends containing SAN constituent. This glass transition phenomenon in the blends can be attributed to the amorphous domains. In the blend samples, the glass transition is broadened when PMMA content is increased. As only PMMA blended with PVDF (PVDF/PMMA = 70/30), the glass transition is not visible in the melting trace. As detailed in Table 3, when PMMA is incorporated into PVDF/SAN blend, the value T g of the blend is increased with an increase of PMMA addition. The change value of T g indicates the enhanced interactions between the polymer chains in the blends.
The presence of the glass transition can be explained by the distinctly different local segmental mobilities in the blend [43]. As our previous work [15], PVDF/SAN (70/30) is a partially miscible system. So, a microheterogeneity in this blend occurs in the sample (Fig. 1a). As proposed in the work of Kumar’s group [43], even for the miscible blends, the molecular origins of the two different “dynamic microenvironments” (one with mobility approximately equal to the average blend mobility and the other close to the fast component) in systems with interactions and with T g contrast. Herein, the two different microenvironments should be the rich PVDF phase and the additional amorphous polymer phase (SAN or SAN/PMMA), leading to the melting of PVDF crystals and glass transition in the melting traces.
Additionally, in Fig. 5, the melting peak is decreased with an increase of PMMA addition. As reported [2, 26], depression of melting points, due to a decrease in the chemical potential of the crystalline polymer, can provide the information on miscibility and strength of intermolecular interactions in the mixture. The higher depth of melting point depression occurs, the stronger interaction between the components of the mixture exists. So, it is suggested that the interaction between the PVDF, PMMA, and SAN chains can be enhanced by an increase of PMMA addition.
Table 3 lists the data obtained from the melting curves in Fig. 5, and summarizes the influence of the PMMA/SAN mass ratio in the ternary blend on PVDF crystallization during the heating. The onset of melting temperatures \(\left( {T_{\text{m}}^{{\text{on}}} } \right)\), the peak melting temperature \(\left( {T_{\text{m}}^{\text{p}} } \right)\), and the final melting temperatures \(\left( {T_{\text{m}}^{\text{f}} } \right)\) of the PVDF blend samples are decreased with an increase of PMMA addition. This suggests the increased interaction between PVDF, PMMA, and SAN chains when PMMA was added. The difference in value ΔT m \(\left( {\Delta T_{\text{m}} = T_{\text{m}}^{\text{f}} - T_{\text{m}}^{{\text{on}}} } \right)\) indicates the formation of different PVDF crystal size. The higher the value of ΔT m, the less homogeneity of PVDF crystal size. Therefore, the influence on crystallization of PVDF in the ternary blend is enhanced by PMMA addition.
The value ΔH m (proportional to the crystallinity of the blends) for the ternary blend with PMMA addition is smaller than that for PVDF/SAN (70/30) sample. This implies that the crystallinity of PVDF is hindered by PMMA addition. It is well in agreement with the fact that PMMA addition into PVDF/SAN blend can enhance the interactions between PVDF and SAN chains, leading to low crystallinity of PVDF in the ternary blends. In this work, the blend samples have the same composition of PVDF (70 wt.%) with various ratio of PMMA and SAN content. Therefore, the difference in the crystallinity of PVDF in the blends can not be so remarkable.
Contact angle test
The contact angle test was carried out on the smooth surface of the blend samples. The hydrophilicity of PVDF/SAN (70/30) is affected by PMMA addition in blends. The contact angles against two liquids are listed in Table 4, while the surface tension and the components of surface tension are calculated by the device through the application of the Owens–Wendt approach [24], as reported in our previous work [15, 16].
It has been demonstrated that the lower the contact angle against water, the more hydrophilic the sample is [16, 44]. In Table 4, the contact angles against water decrease as the PMMA weight fraction increased. This indicates that the PMMA addition increases the hydrophilicity of the PVDF/SAN (70/30) blend. Hence, a more hydrophilic PVDF/SAN blend can be prepared by incorporating PMMA. As the PMMA content is increased to 15 wt.% in the ternary blend, the contact angle is decreased from 82.7° (PVDF/SAN binary blend) to 78.5°. When more PMMA is added into PVDF/SAN (70/30) blend (>15 wt.%), the water contact angle changes a little. Therefore, in order to obtain the hydrophilic PVDF/PMMA/SAN blend (70 wt.% PVDF), 15 wt.% PMMA is sufficient in the blend.
By proceeding with the contact angle test through two liquids (water and diiodomethane), the total surface tension γ s, which consists of the two components of dispersive part \(\gamma _{\text{s}}^{\text{d}} \) and polar part \(\gamma _{\text{s}}^{\text{p}} \) [24]. The detailed results are given in Table 4. The total surface tension γ s of SAN and PMMA are 47.87, and 49.78 mJ/m2, respectively. The difference in total surface tension between these two polymers can be assigned to the different contributions of functional groups in their chains. Among those blend polymers listed in Table 4, the PMMA sample has the lowest water contact angle and the highest total surface tension. As illustrated in Fig. 6, each sample has a close value to the dispersive part of the surface tension. This implies that the polar component plays the main role in contributions to the surface tension when varying PMMA/SAN composition in the blend (70 wt.% PVDF). It is obvious that when the mass ratio PVDF/PMMA/SAN = 70/15/15, both dispersive and polar parts of the surface tension increase a little. This agrees with the result of water contact angle.
Conclusions
This work confirmed that partially miscible PVDF/SAN polymer blends could be compatibilized by addition of a third polymer, PMMA. As illustrated by SEM, the required amount of PMMA is no more than 15 wt.% in the ternary blends which containing 70 wt.% PVDF, in which a more regular and homogeneous morphology was obtained. The IR and DSC results show that the interactions between polymer chains were enhanced with PMMA content increased in the PVDF/PMMA/SAN blend. The remarkable melting point depression in the ternary blend was indicated that the interactions between each component were enhanced, leading to significant obstruction of PVDF crystallization. As demonstrated by IR and WAXD measurements, only α phase regardless of PMMA content in the blend.
On the other hand, the hydrophilicity of PVDF/SAN (70 wt.% PVDF) can be further improved by adding PMMA. Through contact angle test and calculation of surface tension of the blend surface, the total surface tension increased with PMMA addition because of the increased of the polar component contribution. As the PMMA content reached 15 wt.%, the stable hydrophilicity was obtained. Therefore, this observation is a positive evidence for the blend compatibilization, which indicates that PMMA is an ideal compatibilizer for PVDF/SAN blend to prepare more homogenous and hydrophilic polymer blend.
References
Feldman D (2005) J Macromol Sci A, Pure Appl Chem 42:587
Wang TT, Nishi T (1977) Macromolecules 10:421
Linares A, Acosta JL (1998) J Appl Polym Sci 67:997
Gupta AK, Bajpai R, Keller JM (2006) J Mater Sci 41:5857
Chui HJ (2002) J Polym Res-Taiwan 9:169
Chen NP, Hong L (2002) Polymer 43:1429
Liu JP, Jungnickel BJ (2003) J Polym Sci Part B, Polym Phys 41:873
Alfonso GC, Turturro A, Pizzoli M, Scandola M, Ceccorulli G (1989) J Polym Sc B, Polym Phys 27:1195
Li SH, Woo EM, Weak (2008) J Appl Polym Sci 107:766
Nunes SP, Peinemann KV (1992) J Membr Sci 73:25
Yang MC, Liu TY (2003) J Membr Sci 226:119
Moussaif N, Jerome R (1999) Polymer 40:3919
Classen S, Vogt M, Wendorff JH (1995) Polym Adv Technol 6:616
Kim KJ, Cho HW, Yoon KJ (2003) Eur Polym J 39:1249
Ma W, Zhang J, Chen S, Wang X (2008) Colloid Polym Sci 286:1193
Ma W, Zhang J, Wang X, Wang S (2007) Appl Surf Sci 253:8377
Kumaraswamy GN, Ranganathaiah C, Deepa Urs MV, Ravikumar HB (2006) Eur Polym J 42:2655
Suryanarayana C, Grant NM (1998) X-ray diffraction: a practical approach. Plenum, New York
Ma W, Zhang J, Wang X (2007) Appl Surf Sci 254:2947
Gu M, Zhang J, Wang X, Ma W (2006) J Appl Polym Sci 102:3714
Tao H, Zhang J, Wang X, Gao J (2007) J Polym Sci B, Polym Phys 45:153
Nakawa K, Ishida Y (1973) J Polym Sci, Polym Phys Ed 11:2153
Long Y, Shanks RA, Stachurski ZH (1995) Polymer 20:651
Owens DK, Wendt RC (1969) J Appl Polym Sci 13:1741
Spelt JK (1990) Colloids Surf 43:389
Nishi T, Wang TT (1975) Macromolecules 8:909
Sasaki H, Bala PK, Yoshida H, Ito E (1995) Polymer 36:4805
Roerdink E, Challa G (1978) Polymer 19:173
Kobayashi M, Tashiro K, Tadokoro H (1975) Macromolecules 8:158
Gregorio Jr R (2006) J Appl Polym Sci 100:3272
Gregorio Jr R, Nociti NCPS (1995) J Phys D, Appl Phys 28:432
Sun YP, Lawson GE, Bunker CE, Johnson RA, Ma B, Farmer C, Riggs JE, Kitaygorodskiy A (1996) Macromolecules 29:8441
Benedetti E, Catanorchi S, D’Alessio A, Vergamini P, Ciardelli F, Pracella M (1998) Polym Int 45:373
Davis GT, McKinney JE, Broadhurst MG, Roth SC (1978) J Appl Phys 49:4998
Gregorio Jr R, Cestari M (1994) J Polym Sci B, Polym Phys 32:859
Hodge RM, Edward GH, Simon GP (1996) Polymer 3:1371
Martuscelli E, Canetti M, Vicini L, Seves A (1982) Polymer 23:331
Lim GBA, Lloyd DR (1993) Polym Eng Sci 33:529
Kim SS, Lloyd DR (1992) Polymer 33:1036
Beck HN, Ledbetter HD (1965) J Appl Polym Sci 9:2131
Pillin I, Pimbert S, Levesque G (2002) Polym Eng Sci 42:2193
Wang J, Dou Q, Wu S, Chen X (2007) Polym Eng Sci 47:889
Kumar SK, Colby RH, Anastasiadis SH, Fytas G (1996) J Chem Phys 105:3777
Huang C, Zhang L (2004) J Appl Polym Sci 92:1
Acknowledgment
Financial support from the Key Project of BMSTC (D0406003040191) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, W., Chen, S., Zhang, J. et al. Crystallization behavior and hydrophilicity of poly(vinylidene fluoride) (PVDF)/poly(methylmethacrylate) (PMMA)/poly(styrene-co-acrylonitrile) (SAN) ternary blends. Colloid Polym Sci 287, 147–155 (2009). https://doi.org/10.1007/s00396-008-1958-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-008-1958-z