Abstract
This paper is focused on the synthesis and characterization of hydrophobically modified polyelectrolytes and their use as reducing as well as stabilizing agents for the formation of gold nanoparticles. Commercially available poly(acrylic acid) has been hydrophobically modified with various degrees of grafting of butylamine introduced randomly along the chain. Different analytical methods are performed, i.e., IR and 1H-NMR spectroscopy in combination with elemental analysis to determine the degree of grafting. The modified polymers can successfully be used for the controlled single-step synthesis and stabilization of gold nanoparticles. The process of nanoparticle formation is investigated by means of UV-vis spectroscopy. The size and shape of the particles obtained in the presence of unmodified or modified polyelectrolytes are characterized by dynamic light scattering, zeta potential measurements and transmission electron microscopy. The polyelectrolytes were involved in the crystallization process of the nanoparticles, and in the presence of hydrophobic microdomains at the particle surface, a better stabilization at higher temperature can be observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colloidal noble metals, e.g., gold and silver, have been well known for long. Alchimists have already tried to use the colloidal gold (aurum potabile) as an “elixier of life.” Later, colloidal gold was used for coloring glass, as seen in old cathedrals. In the last decades, the preparation of ultrafine metal particles attracted significant attention again because of their special optical, electronic, magnetic, and catalytic properties when the particle size is fixed between 5 nm and 50 nm [1–5]. For example, 5 nm-sized gold nanoparticles exhibit intense photoluminescence [6]. However, the unusual size-dependent chemical and physical properties make them attractive for quite different new fields of application, e.g., electron-dense labelling agents in histochemistry and cytochemistry [7, 8], in catalysis, nanotechnologies, electrooptical devices and pharmaceutics [9, 10].
Several methods can be employed for the synthesis of metal colloids [11]. Concerning the formation of gold nanoparticles, different in situ methods can be applied for reducing a diluted tetrachloroaureate solution. The reduction can occur in diluted aqueous or alcoholic solutions by adding organic reducing agents (e.g., sodium salt of citric acid already used by Faraday [12]) to the refluxed aqueous solution of the HAuCl4 precursor, or by using the reducing agent borohydride BH −4 . Another way to prepare gold nanoparticles is to use photoreduction by UV irradiation, or by refluxing alcoholic solutions of the precursor (alcohol reduction) [13]. Often the borohydride reduction is a fast-reduction method performed at room temperature and leads to small spherical particles in contrast to the slow reduction by UV irradiation resulting in significant larger particles [14]. However, the reduction speed can be adjusted via the pH also [15].
The reduction process can be realized in different types of template phases, e.g., in polymer gel templates [16, 17], in polymer dendrimers [18, 19] or in polymeric nanoenvironments [20, 21]. Another possibility is to use well defined microemulsion droplets as templates. A detailed overview of the preparation of metal nanoparticles in water-in-oil microemulsions was recently published [22].
However, the stability of the nanoparticles formed mainly depends on their surface charge and it can be influenced by some further added protective components. Quite different types of polymers, including polyelectrolytes and amphiphilic block copolymers, can be used as protecting agents [23–25]. The most commonly used polymers are poly(1-vinylpyrrolidone), poly(ethylene glycol) or their copolymers [23–26]. Moreover the choice of polyelectrolytes as stabilizers has also been of interest for the stabilization of nanoparticles due to the several advantages that these macromolecules can offer. Pugh and Heller [27] have confirmed the stabilization of colloidal particles by the use of polyelectrolytes, which can combine both steric and electrostatic effects. Most of polyelectrolytes employed to stabilize gold particles are cationic ones [28]. Recently, it was shown that polyelectrolytes can act as both reducing and stabilizing agents for the gold nanoparticles. Protected gold particles have successfully been obtained with linear polyethylenimine, which serves as reducing and protective agent [29]. However, linear polyethylenimine has some special features, e.g., solubility in water. The polymer becomes soluble in water only at pH<7, or by heating up to higher temperatures (>70 °C). Recently, it was demonstrated that polyanions, i.e., polyacrylates, can act as reducing agents, too, when the precursor solution was refluxed [30].
Another example for a single-step synthesis and stabilization of metal nanoparticles was shown by adding pluronic copolymers with PEO-PPO-PEO blocks [31]. However, “double-hydrophilic” block copolymers, e.g., polyethyleneoxide-polyethyleneimine block copolymers, used as nanoreactors can additionally serve as templates. Moreover, variations of different parameters, such as concentration, pH, type of reducing agent can affect the nanoparticle morphology as well [15].
In the present work the reduction and stabilization of gold colloids by adding hydrophobically modified anionic polyelectrolytes is investigated in much more detail. The polymers used are derivatives of poly(acrylic acid). The aim of the study was to show the influence of the degree of substitution on reduction and stabilization behavior. However, this opens a way to achieve effectively stabilized gold nanoparticles in apolar solvents. First of all the polymers were added at room temperature and the reduction process was investigated over a longer period (up to 8 days). In comparison, the reduction process is realized at a higher temperature, i.e., 100 °C. The particle formation was checked by means of UV-vis spectroscopy and the particle size and size distribution were investigated by dynamic light scattering in combination with transmission electron microscopy.
Experimental
Materials
Polyacrylic acid sodium salt (PAA) was purchased from Fluka: the average molecular weight given by the producer was 5.100 g/mol. In order to obtain a pure PAA in the acidic form, the commercial sample was purified by ultrafiltration and turned into its acid form with a cation exchanger obtained from Merck. Methanol (>99%), Butylamine (purum>99%), 1-methyl-2-pyrrolidone (NMP), Dicyclohexylcarbodiimine (CDI), were supplied by Fluka and used without further purification. The metal precursor hydrogen tetrachloroaureate HAuCl4 and the sodium borohydride NaBH4 were purchased from Aldrich. Water was purified with the Modulab PureOne water purification system (Continental).
Synthesis of modified poly(acrylic acid)
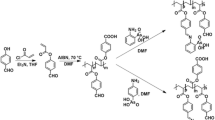
Commercially available poly(acrylic acid) has been hydrophobically modified with various degrees of grafting x (x=3; x=10; x=20 mol%) of butylamine introduced randomly along the chain as shown on the reaction Scheme 1 [32, 33]:
The PAA is dissolved (for 3 h) in 1-methyl-2-pyrrolidone (NMP) at 60 °C, in a three-neck flask fitted with a condenser, a thermometer, and a magnetic stirrer. Butylamine and N-N′-dicyclohexylcarbodiimide (CDI) (dissolved in NMP) are successively introduced into the PAA solution under vigorous stirring. The temperature is maintained at 60 °C and the reaction mixture is stirred for approximately 24 h in the dark. The system is then cooled down to room temperature, the dicyclohexylurea crystals formed are eliminated by filtration and the polymer is precipitated in its sodium salt form by dropwise addition of concentrated (40% by volume) NaOH. The precipitate is washed by hot (60 °C) NMP three times, and three times with cold methanol by vacuum filtration. The crude product is dissolved in water, precipitated in methanol (2 times) and finally freeze-dried.
Note, that the polymers formed are referred as follows: the polymers named PA3C4, PA10C4 and PA20C4 are a PAA respectively modified by 3%, 10%, or 20% of butylamine along the polymer backbone.
Colloidal gold preparation
Aqueous solutions of polyelectrolyte (1% by weight) were prepared, and stirred 24 h before use. An aqueous tetrachloroaureate precursor solution (2×10−3 M) was prepared.
Each polymer solution was mixed with the metal precursor solution in a molar ratio of polymer:metal=3:1 at room temperature. In addition, measurements at a polymer:metal ratio 10:1 were carried out. It has to be noted that at a molar ratio of 3:1 there is already a large excess of polymer chains per particle.
UV-vis spectra were taken immediately after mixing, and from time to time up to 8 days.
In addition, the experiments were carried out under heating conditions. The HAuCl4 solution (2×10−3 M) was refluxed for 10 min, and a warm (55–60 °C) aqueous solution of polyelectrolyte (molar ratio of polymer:metal=3:1) was quickly added. Reflux was continued 15–20 min and the color effect was noted.
Methods
To verify the degree of amide groups along the polymers backbone, different analytical methods, i.e., 1H NMR, IR spectroscopy, and elemental analysis were performed.
1H NMR measurements were carried out using a Bruker AMX-300 spectrometer operating at a proton resonance frequency of 300 MHz. Infrared spectra were recorded with a FTS 66/5 Infrared Fourrier Transform Spectrometer (Bruker). Elemental analysis was realized by using an EA1110 (CHNS-O) elemental analyzer from CE instruments.
To determine the particle size and particle size distribution particle, dynamic light scattering measurements were carried out at 25 °C at a fixed angle of 173° by using a Nano Zetasizer 3600 (Malvern) equipped with a He–Ne laser (4 mW) and a digital autocorrelator. The solutions were measured as prepared without any further dilution.
Electrophoretic light scattering was used for detecting the surface charge of the nanoparticles. This technique allows therefore the determination of the zeta potential of the nanoparticles at the effective shear plane between the moveable and non-moveable parts of the double layer. Measurements were carried out with a Zetasizer 4 (Malvern) at a fixed angle of 90°.
The nanoparticle formation was directly investigated by measuring the absorption band at about 530 nm with a Cary 5000 UV-vis NIR spectrometer (Varian).
In addition, the morphology and size of the particles were determined by transmission electron microscopy (EM 902, Zeiss). Samples were prepared by dropping a small amount of the aqueous dispersion on copper grids, dried and examined in the transmission electron microscope at an acceleration voltage of 90 kV.
Results and discussion
Characterization of the polymers
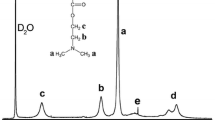
1H NMR spectroscopy is a useful method to confirm the modification of the polyelectrolytes. The spectra of the modified polymers in D2O were compared to that of the unmodified PAA (Fig. 1) [34]. First of all the results show characteristic peaks due to the presence of an amide chain. At around 0.8 ppm (peak ε) the typical chemical shift of the methyl end group of the grafted chain can be observed. Moreover the chemical shift at 3.05 ppm (peak χ) is characteristic for the first ethyl group of the grafted chain: the peak deshielding is due to the presence of the amide group. Moreover, as the percentage of grafting increases, the intensities of the peaks χ and ε increase, as expected.
The IR spectroscopy can successfully be used as a method to observe the substitution of polyelectrolytes, too. The infrared spectrum of the PAA sample, in the form of KBr pellets [35], is shown in Fig. 2a. The spectrum shows the characteristic stretching absorption band of the carbonyl group C=O at 1,720 cm−1 . Bending vibrations of the –CH2– ad CH–CO groups are respectively located at 1,457 and 1,417 cm−1 . Additional bands at 1,241 and 1,164 cm−1 can be related to the coupling between inplane OH bending and C–O stretching vibrations of neighboring carboxyl groups. The infrared spectra of the modified polymers (Fig. 2b–d) show significant changes in comparison to the PAA. The most characteristic absorption band of the amide group located at 1,569 cm−1 can be correlated to the C=O band.
Elemental analysis is a useful method to calculate the degree of grafting of polyacrylamides via the carbon/nitrogen (C/N) ratio. Table 1 summarizes the results of the analysis. It is shown that the yield of incorporation is above 80% of the theoretically expected value, indicating a successful chemical modification.
Gold nanoparticle formation
After adding the various polymer solutions to the metal precursor, the aqueous solutions were characterized by means of UV-vis spectroscopy to study the direct formation of the gold nanoparticles, which absorb, depending on their particle size, between 500 nm and 600 nm. Figure 3 shows the time-dependent absorption spectra for the tetrachloroaureate solution in presence of PA3C4. The first absorption peak can be detected after a few hours demonstrating that the reduction process is quite slow. Tables 2 and 3 show the results for the gold nanoparticle formation depending on the different polyelectrolytes added and the procedure employed for the synthesis. Notable features given here are the color of the solutions, the UV-vis absorption maxima, and the particle size determined by dynamic light scattering. Depending on the particle size and the degree of agglomeration, gold nanoparticles can present a wide range of color from red to blue. For example, monodisperse small gold nanoparticles with a particle size of 18 nm exhibit a red color. A change in color to purple or blue indicates the formation of larger particles and/or aggregation phenomena.
In addition the particles were studied by means of electrophoretic light scattering to measure the electrokinetic potential (zeta potential) at the particle surface. The particles reveal a negative zeta potential of about −30 to −50 mV, according to the polymer used for the reduction. Indeed, the zeta potential depends on the degree of hydrophobicity of the polyanion added. It has to be noted that the lowest value of −50 mV was observed for the non modified PAA, as to be expected. One can conclude that the surface charge of the particles is dominated by the adsorption layer of the polymer. This means that the nanoparticles produced are stabilized due to the adsorption of the polyelectrolyte layer.
Reduction process realized at room temperature
In the case of the unmodified PAA a red solution was obtained, which is stable over several months. Dynamic light scattering measurements (Table 2, Fig. 4a) and TEM micrographs (Fig. 5a, b) confirm that gold nanoparticles with an average particle size of 19 nm were formed. In this case, the polyelectrolytes adsorbed at the particle surface stabilize the nanoparticles against flocculation due to electrostatic repulsion forces. However, in addition to spherical nanoparticles, normally observed by using a fast sodium borohydride reduction, triangular and cylindrical structures were observed (Fig. 5b). Because of the adsorption of the anionic polyelectrolytes onto the nanoparticles, the morphology of the particles seems to be influenced in a characteristic way. These results suggest a polymer-controlled crystallization during the primary cristallization as well as the superstructure formation process [36, 37].
At low degrees of substitution by hydrophobic side chains (PA3C4) the individual nanoparticles start to aggregate, and the color of the sample changes to pink. Electron micrographs show particle aggregates in the order of about 120 nm, consisting of individuals much smaller, primary nanoparticles (ca. 20 nm in size), which correlates very well with the mean particle size of 24.5 nm determined by DLS (Table 2). This behavior can be explained predominantly by hydrophob–hydrophob interactions, which leads to a partial aggregation of these individual particles. When the degree of hydrophobicity of the polymer is increased to 10%, a purple-blue dispersion was obtained. The tendency to form aggregates is enhanced and much larger aggregates of about 130 nm are detected by means of dynamic light scattering and transmission electron microscopy.
A further increase of the number of hydrophobic side chains induces the formation of smaller nanoparticles. However, the mean particle size (25.5 nm) is somewhat larger in comparison to the unmodified PAA, and the size distribution too, as to be seen by dynamic light scattering (Table 2, Fig. 4b). This increase of the mean particle size correlates with the fusion to some larger tri- or multi-angular gold nanoparticles (compare EM micrograph in Fig. 5c, d). The color of the dispersion is pink again. The particles seem to be once again better stabilized against collision and this behavior can be explained by an additional sterical stabilization effect. Nevertheless, this sterical stabilization does not prevent an increase of the polydispersity in comparison to the unmodified PAA.
It has to be mentioned here that a later heating of the polyelectrolyte-gold dispersions up to 100 °C does not change the particle size of the gold nanoparticles, and consequently no color change can be observed.
Reduction process realized at 100 °C
By using the unmodified PAA, a purple-pink color was obtained after just 20 min at reflux. The mean particle diameter measured by DLS was 37.5 nm (Table 3) with a somewhat larger particle distribution (Fig. 6a). In good agreement, the TEM micrograph (Fig. 7) shows particle aggregate structures between 30 nm and 50 nm. When the reaction mixture was heated for a longer time, coagulation occurred (blue color and precipitation).
By using the hydrophobically modified polymers (degree of substitution ≥10%), red-pink colored solutions containing nanoparticles with an average particle size of 18 nm (Table 3) can be obtained, and visualised by TEM (compare Fig. 8). Moreover, the nanoparticles formed are stable at 100 °C for a longer time and the polydispersity of the nanoparticles formed decreases (Fig. 6b).
It seems to be of interest to note that all the particles obtained are spherical, whatever the polymer used, in contrast to the synthesis at room temperature.
Additional measurements carried out at a higher polymer concentration (polymer:metal=10:1) show bigger particle dimensions. This means a tendency to aggregation has to be mentioned here, when higher polymer concentrations were used.
Conclusions
We have confirmed in this paper that hydrophobically modified polyelectrolytes can act as both reducing and stabilizing agents for the formation of gold nanoparticles.
One advantage of the method used here is that the reduction can be realized in water at room temperature very slowly or much faster by heating up the system to 100 °C. In both cases gold nanoparticles of colloidal dimensions can be produced. However, the size and shape of the individual nanoparticles mainly depends on the polyanion added and the temperature procedure used.
Regarding synthesis at room temperature in the presence of polyacrylates, a tendency to form triangular or rod-like nanoparticles has to be mentioned. When hydrophobic side chains are incorporated into the polymer chain, the stability of the primary gold nanoparticles can be strongly influenced. In general, the colloidal stability is decreased and aggregation phenomena were observed. However, the most unstable situation is observed at a degree of substitution of 10%. This means that at higher degrees of substitution (≥20%) the hydrophobic stabilization effect becomes dominant. This behavior can be understood with regard to the different types of stabilization that have to be taken into account here. When the degree of substitution is low, at about 3%, the hydrophob–hydrophob interactions can lead to a weak aggregation, in comparison to effects observed by using associating polymers. When the degree of hydrophobic side chains is increased, up to 10%, the flocculation tendency is increased furthermore. However, at about 20% of substitution, the particles are stabilized again due to an additional steric repulsion in combination with the electrostatic repulsion effect. Taking into account that hydrophobic polyelectrolytes, with both ionic and hydrophobic groups, have been well known to form local micelles in water [38–40], the additional steric repulsion can be understood on the basis of the formation of microdomains at the particle surface, as schematized in Fig. 9.
When the reduction process is realized much more quickly at 100 °C, a tendency to form spherical particles is observed whatever the polymers used. Moreover, the degree of hydrophobic modification of the polymer is of real interest for the stability of the particles. It was observed that with higher degree of hydrophobicity (≥10%) more stable nanoparticles were formed, due to the combination of an electrostatic with a steric stabilization effect. The quite different temperature-dependent flocculation behavior can be explained by two different types of steric stabilization. In the case of the hydrophobic side chain modified PAA, an entropic repulsion, characterized by a lower flocculation temperature, leads to aggregation phenomena at room temperature, but not yet at 100 °C. When PAA is used, an enthalpic repulsion, characterized by an upper flocculation temperature, leads to aggregation phenomena at 100 °C, but not yet at room temperature.
Based on this knowledge it becomes possible to tune first of all the size and shape of the primary nanoparticles by varying the degree of hydrophobic side chains of the polyacrylates as well as the temperature.
References
Demaille C, Brust M, Tsionsky M, Bard AJ (1997) Anal Chem 69(13):2323
Hayward RC, Saville DA, Aksay IA (2000) Nature 404:56
Braun E, Eichen Y, Sivan U, Ben-Yoseph G (1998) Nature 391:775
Chen M, Yamaruro S, Farrell D, Majetich SA (2003) J Appl Phys 93:7551
Ciebien JF, Cohen RE, Duran A (1998) Supramol Sci 5:31
Wilcoxon JP, Martin JE, Parsapour F, Wiedenman B, Kelley DF (1998) J Chem Phys 108(21):9137
Hayat MA (ed) (1989) Colloidal gold: principles, methods and applications. Academic, San Diego
Hyatt AD, Eaton BT (1993) Immuno-gold electron microscopy in virus diagnosis and research. CRC, Boca Raton
Quinn M, Mills GJ (1994) Phys Chem 98:9840
Wei Y, Cao C, Jin R, Mirkin CA (2002) Science 297:1536
Roucoux A, Schulz J, Patin H (2002) Chem Rev 102:3757
Faraday M (1857) Philos Trans R Soc Lond 147:145
Esumi K, Suzuki A, Aihara N, Usui K, Torigoe K (1998) Langmuir 14:3157
Mayer ABR, Mark JE (1998) Eur Polym J 34(1):103
Sidorov SN, Bronstein LM, Valetsky PM, Hartmann J, Cölfen H, Schnablegger H, Antonietti M (1998) J Colloid Interface Sci 212:197
Caruso RA, Giersig M, Willig F, Antonietti M (1998) Langmuir 14:6333
Caruso RA, Antonietti M, Giersig M, Hentze HP, Jia J (2001) Chem Mater 13:1114
Faul CFJ, Antonietti M, Hentze HP, Smarsly B (2003) Colloids Surf 212(2–3):115
Grohn F, Kim G, Bauer AJ, Amis EJ (2001) Macromolecules 34(7):2179
Saito H, Okamura S, Ishizu K (1992) Polymer 33:1099
Chan YNC, Schrock RR, Cohen RE (1992) Chem Mater 4:24
Capek I (2004) Adv Coll Int Sci 110:49
Mayer ABR, Mark JE (2000) Polymer 41:1627
Mayer ABR, Mark JE (1997) Colloid Polym Sci 275:333
Corbierre MK, Cameron NS, Lennox RB (2004) Langmuir 20:2867
Kim F, Connor S, Song H, Kuykendall T, Yang P (2004) Angew Chem Int Ed 43:3673
Pugh TL, Heller WJ (1960) Polym Sci 47(149):219
Mayer ABR, Mark JE (1997) J Macromol Sci A 34(11):2151
Sun X, Dong S, Wang E (2004) Polymer 45:2181
Hussain I, Brust M, Papworth AJ, Cooper AI (2003) Langmuir 19:4831
Sakai T, Alexandridis P (2004) Langmuir 208:426
Wang TK, Iliopoulos I, Audebert R (1988) Polym Bull 20:577
Anghel DF, Alderson V, Winnik FM, Mizusaki M, Morishima Y (1998) Polymer 39(14):3035
Garces FO, Sivadasan K, Somasundaran P, Turro NJ (1994) Macromolecules 27:272
Moharram MA, Rabie SM, El-Gendy HM (2002) J Appl Polym Sci 85:1619
Limin Q, Cölfen H, Antonietti M (2000) Angew Chem Int Ed 39(3):604
Shu-Hong Y, Cölfen H, Antonietti M (2003) Adv Mater 15(2):133
Dubin PL (1985) Microdomains in polymer solutions. Plenum, New York
Laschewski A (1995) Adv Polym Sci 124:1
Koetz J, Kosmella S, Beitz T (2001) Prog Polym Sci 26:1199
Acknowledgements
The authors thank A. Laschewsky, University of Potsdam & Fraunhofer Institut für Angewandte Polymerforschung Golm, for useful discussions on polymer analysis, for the use of elemental analysis facilities and for providing access to the Infrared and the UV-vis spectroscopy instruments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Note, C., Koetz, J., Kosmella, S. et al. Hydrophobically modified polyelectrolytes used as reducing and stabilizing agent for the formation of gold nanoparticles. Colloid Polym Sci 283, 1334–1342 (2005). https://doi.org/10.1007/s00396-005-1349-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-005-1349-7