Abstract
The self-aggregation of sodium perfluorooctanoate (SPFO) has been studied by pH, ion-selective electrodes, changes in Rhodamine 6G colour and fluorescence, conductivity, surface tension and viscosity measurements. It has been determined that the aggregation of SPFO is gradual. It starts to aggregate at 0.01 mol dm−3 with the formation of pre-micelles of perfluorooctanoate ions, which capture counterions at C=0.02 mol dm−3 and form micelles at a critical micelle concentration (CMC) of 0.03 mol dm−3. Micelles at the CMC are highly ionised and strongly hydrated. At C*=0.06 mol dm−3, a low ionisation degree was found, indicating the formation of a more compact micellar structure. Micelles at the CMC show energetic advantage in comparison with the adsorbed state at the air/solution interface, and this advantage increases at C* originating desorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Substituting the hydrogen atoms with fluorine produces dramatic changes on surfactant properties [1, 2]. Among them, very low surface tensions, high fluidity, low dielectric constant, high vapour pressure, high compressibility and high gas solubility can be mentioned [1, 3, 4]. Fluorinated surfactants are much more surface active than their hydrocarbon counterpart. As a consequence, they have lower critical micelle concentrations than the hydrogenated analogous of the same chain length. The hydrophobic part of the fluorinated surfactant not only repels water but also repels hydrocarbon oil and fat. Because of their unique properties, fluorinated surfactants are irreplaceable in many applications, and their study appears to be of high scientific interest.
Sodium perfluorooctanoate (SPFO) is a good model to study the properties of fluorinated surfactants. It has a fluorocarbon tail, which can be compared with that of sodium undecanoate (it has been estimated that the effect of each CF2 group on micelle formation is roughly equivalent to approximately 1.5 CH2 group) [5]. Some studies on the SPFO micellisation process exist in the literature [5–12]. Despite the significance of these works, a complete understanding of the mechanism of self aggregation of this compound requires more studies. In this line is our more recent investigation on the dependence of the SPFO partial molar volume and compressibility on temperature [13] since hydration effects in solutions are known to be strongly sensitive to temperature.
In this work, pH, ion-selective electrodes, conductivity, surface tension and viscosity measurements have been used to study the aggregation of SPFO. Results enabled us to postulate a gradual self-aggregation mechanism for SPFO in aqueous solutions.
2 Experimental section
2.1 Materials
Sodium perfluorooctanoate (SPFO, CAS 1984-06-1) was obtained from Lancaster Synthesis Ltd. (No. 16988). A stock solution of concentration 0.1 mol dm−3 was prepared in distilled water and then different solutions in the range 0.001–0.1 mol dm−3 were obtained by dilution. The confidence intervals were calculated with a level of 0.9 using the Student’s t distribution.
2.2 Apparatuses and procedures
The pH determinations were made at 25.0±0.1 °C with a millivoltmeter and pH-meter CRIBABB and a Broade and James glass electrode.
Conductivity measurements were made with an immersion cell and an automatic conductimeter Antares II of Instrumentalia. Measurements were made at 20.0±0.1 °C.
The ion-selective measurements were made with a CRIBABB millivoltmeter with an Orion 8411 Ross Na+-ion-selective electrode and a perfluorooctanoate (PFO−) ion-selective electrode. Both electrodes were used against a saturated calomel electrode. The PFO− -ion selective electrode was prepared by gluing a PFO−-ion exchange membrane at one end of a PVC tube. This membrane was prepared with 300 mg of PVC dissolved in 50 ml of tetrahydrofuran (THF)+0.2 ml dibutyl phtalate (plasticiser) and 0.100 g of Ba(PFO)2. Ba(PFO)2 was prepared by mixing the appropriate amounts of BaCl2 and NaPFO aqueous solutions. The insoluble Ba(PFO)2 salt was centrifuged and washed several times with double-distilled water and then left to dry. The Ba(PFO)2 crystals were powdered and suspended in the above solution. Then, the PVC mixture was left in a Petri dish to let the THF to evaporate, and the resulting membrane was cut and glued to the tube. The tube was filled with an aqueous solution of 0.01 mol dm−3 in NaPFO+0.1 mol dm−3 in KCl having a small amount of solid AgCl. An Ag/AgCl electrode made with a silver wire was placed into the tube, which was sealed with a rubber plug.
Surface tension was measured by the Wilhelmy plate method using a Krüss K12 surface tension apparatus, equipped with a processor to acquire data automatically, once adsorption equilibrium was reached. The reproducibility of surface tension measurements was ±0.01 mN m−1. The temperature was maintained at 25.00±0.01 °C by re-circulation of water from a bath. Surfactant solutions were prepared by dilution, determining the concentration by mass with a precision of ±0.00001 g.
To carry out Rhodamine 6G colour and fluorescence experiments, a series of test tubes containing the same volume of solutions with different concentration was prepared. A drop of aqueous solution of Rhodamine 6G was added to each tube, and the colour and fluorescence of the dye was observed by eye.
Viscosity measurements were made with a capillary viscometer through titration of a concentrated solution of sodium perfluorooctanoate with water. Experiments were carried out at 25.0, 30.0 and 35.0 (±0.1) °C by water re-circulation. A Chainomatic density balance was used to determine the density of solutions.
3 Results and discussion
3.1 pH measurements
From pH measurements the hydrolysis constant can be calculated. Since sodium perfluorooctanoate is a salt of a weak fatty acid and a strong base, it undergoes hydrolysis. So there is some fatty acid AH in solution, in equilibrium with anions A− and OH− ions. If only these components are present and the HA formed is soluble in water, the pH versus log C, (C=total surfactant concentration) must be a straight line with a slope of +0.5 [14]:
where K a and K w are the acidity (ionisation) constant of the fatty acid and the self-ionisation constant of water. Thus, from the intercept of this line the ionisation constant of the acid may be obtained.
A theory on surfactant hydrolysis on micellisation was developed by Stainsby and Alexander [15]. They explain the characteristic shape of the hydrolysis degree, β=[OH−]/C dependence on C of soap solutions by the solubilisation of the strongly hydrophobic fatty acid molecules by micelles. If no micelles are present, β decreases with increasing concentration, as in common (non-self-aggregating) hydrolysable salt solutions. The solubilisation of the fatty acid by micelles shifts the hydrolysis equilibrium to the formation of more HA and OH−, giving a sudden increase in β. Since micelles have a limited capacity of solubilisation, when that capacity is saturated, the hydrolysis becomes proportional to the concentration of micelles and a maximum of β is obtained, followed by a diminution at a higher concentration. The involved equilibriums are:
in which HA M is the fatty acid dissolved in micelles and HA S is that dissolved in monomeric solution. Thus, a distribution constant of HA between micelles and aqueous solution K F =[HA M ]/[HA S ] is involved. Here, [HA M ] is the concentration of acid in micelles per unit volume of micelles and [HA S ] that of acid per unit volume of aqueous solution. Stainsby and Alexander computed curves β versus C equal to the experimental curves and found that the concentration Cmax where β has its maximum is related to the CMC value by
The distribution constant of HA between micelles and water, K F , is computed with the density of micelles, ρ, the hydrolysis constant, K h =K w /K a , and the value of the hydrolysis degree at the maximum, β max, by
The micelle density ρ can be computed as
where M is the molar surfactant weight, vMmolecule the micellised molecule volume and NA the Avogadro’s number. Finally, they found that the hydrolysis degree at the minimum, β min, is related to the concentration at which the first aggregates form, C F , by
These first aggregates may be oligomers (dimers, trimers, etc.), solid HA or acid soap HNaA2.
The pH versus log C graph (not shown) was similar to those of other common soaps [14], showing a straight line with a slope of about +0.5 below the CMC, whose least-squares fitting equation was
having a regression coefficient of 0.9928. From Eq. 7 we can obtain K a =0.049±0.008, (pK a =1.31±0.07) and K h =(2.02±0.32)×10−13. Moroi et al. [16] reported K a values of several perfluorinated acids: perfluoroundecanoic (K a =2.48× 10− 3 at 25 °C), perfluorododecanoic (K a =7.48× 10− 3 at 25 °C), perfluoropropionic (K a =0.36531±0.00007 at 25 °C), perfluorobutanoic (K a =0.4263±0.0002 at 25 °C), perfluoropentanoic (K a =0.2873±0.0002 at 25 °C) and perfluorohexanoic (K a =0.13152±0.00005 at 25 °C). From the almost linear relationship between log K a and the number of carbon atoms in the hydrophobic chain, the value obtained for perfluorooctanoic is K a =0.050. The agreement is then excellent.
The pH suddenly increases at the CMC. This agrees with the limited tendency to hydrolysis, due to the high value of K a . The abrupt change indicates that micelles solubilise the acid shifting the hydrolysis equilibrium to more acid formation. However, at a concentration of 0.06 mol dm−3, the capacity to solubilise micelles decreases, which can be related with a change in the micellar structure. Cylindrical and discoid micelles have been reported for perfluorinated surfactants of six to eight carbon atoms [17]. The most probable shape of micelles at CMC is spherical, so the change at 0.06 M should be related with a modification from spherical to anisometric micelles. Ouriques et al. [18] found cylindrical micelles of NH4PFO in D2O below 0.537 mol dm−3 and discoid above 0.578 mol dm−3. The reported aggregation number of sodium perfluorooctanoate micelles, 20 [19] or 15 [6, 20], is coherent with the presence of spherical micelles, whose maximum aggregation number, obtained by geometric considerations for this ion is 18 [18].
Figure 1 shows the degree of hydrolysis as a function of concentration. From this curve, the following values were obtained: Cmin=0.03 mol dm−3, β min=0.0215, Cmax=0.06 mol dm−3 and β max=0.0473. From the partial molar volume of micellised sodium perfluorooctanoate, Vmp,M=213±0.02 cm3 mol−1 (25 °C) [21], the micelle density was ρ =M/Vmp,M=2.2635±0.0002 g cm−3. From Eq. 3, CMC=0.03 mol dm−3. From Eq. 6, C F =(4.6±1.4)×10−12 mol dm−3; for commercial soap, C F =3.44× 10−12 mol dm−3 [22]. Frequently, the solubility of the acid, SHA, is equal to C F in pure soaps systems. The distribution constant of HA between micelles and intermicellar solution, computed with equation (4) is K F =(1.00±0.16)×1011. For comparison, the K F value for pure sodium dodecanoate at 40 °C was 4.5× 105, and 1× 106 for sodium tatradecanoate [15]. This indicates that practically all molecules of the acid formed by hydrolysis are captured by micelles, producing an increased hydrolysis, which is detectable in spite of the low value of the hydrolysis constant. This is caused by the high hydrophobicity of the acid.
3.2 Conductivity measurements
The specific conductivity as a function of molar concentration plot (not shown) shows two straight lines. The pre-CMC straight line least-squares fitting equation was
and that of the post-CMC line
The intersection between both lines gives CMC=0.0304±0.0051 mol dm−3.
The micelle ionisation degree α=1−m/n, in which m is the number of counterions in the micelle Stern layer and n the aggregation number, may be computed from specific conductivity measurements by the equation of Evans [23]
where (dκ/dC)2 and (dκ/dC)1 are the slopes of κ versus C straight lines prior and after the CMC, and λ X is the equivalent conductivity of the counterion. This equation is a function of n. However, this dependence is very weak, and any reasonable n value may be used without any significant error [23].
Assuming the aggregation number n=17.5, which is the average reported in the literature [6, 19, 20], a value of α=0.409±0.011 is obtained, in good agreement with α=0.455 reported in the literature from conductivity measurements [6] and α=0.464 obtained from dependence of the CMC on the concentration of electrolyte added [6].
The equivalent conductivity at infinite dilution obtained from the equivalent conductivity plot (not shown) was Λ°=68.2±0.6 S cm2 eq−1. There is a maximum at the CMC zone that suggests that at the beginning in the aggregation, highly charged aggregates are produced.
From Λ°, the average equivalent conductivity of the fluorinated soap anion can be estimated, obtaining λanion=Λ°−λ°Na+=68.2±0.6−50.9=18.3±0.6 S cm2 eq−1. For comparison, in the literature λ anion=18.1 for perfluorodecanoate [24], λ anion=16.2 for perfluorohexanoate [25] and λ anion=16.9 for perfluoropentanoate [25]. Interpolation from these data gives λ anion=17 S cm2 eq−1 for perfluorooctanoate, approximately the value we found.
The differential conductivity, Λd=1,000 (dκ/dC)=1,000(κ1-κ2)/(C1−C2), plotted as a function of the average concentration, Caverage=(C1 +C2)/2, [26–28], or the square root of the average concentration, \(\sqrt {C_{{\text{average}}} } \) [29, 30], can be used to obtain information about the CMC, micelles and the pre-CMC behaviour of the system. Here, κ i is the specific conductivity associated with the concentration C i of the surfactant, and the indices 1 and 2, two successive points in the specific conductivity plot. The classical differential conductivity plots show two straight, almost horizontal lines, one of them at C<CMC and the other at C>CMC, connected by a sigmoid curve. The inflection point is taken as the CMC. If the pre-CMC line is not horizontal, this may be an indication of pre-micellar association. The larger the CMC interval, the larger the concentration interval containing the sigmoid curve is. A square, abrupt step must replace the sigmoid curve if the aggregation number of micelles is infinite; thus, small micelles must give an elongated sigmoid [27]. Some differential conductivity curves have a maximum at the CMC. This indicates the formation of highly charged micelles [28]. The Λd value at the minimum of the post-CMC curve is taken as ΛM, the molar conductivity of micelles
where umic is the micelle electrical mobility, F the Faraday constant and λ X the equivalent conductivity of the counterion.
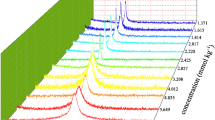
Figure 2 shows the differential conductivity as a function of average concentration. The slope below the CMC indicates that there is pre-micellar association; the maximum occurring immediately below the CMC indicates the formation of highly charged aggregates. The inflexion point, approximately at 0.03 mol dm−3 is commonly taken as the CMC. However, the amplitude of the sigmoid region indicates that the aggregation is not abrupt but gradual, and micelles are small. Above 0.055 mol dm−3, the differential conductivity is practically constant with concentration. The micellar conductivity, Λ M °, for these stabilized micelles can be estimated by extrapolation at zero concentration, obtaining Λ M °=35 S cm2 eq−1.
The micellar conductivity at the CMC is conventionally assumed as the differential conductivity at the minimum, ΛM,CMC°=31 S cm2 eq−1. From Eq. 10, the micelle electrical mobility may be computed, obtaining umic C*=3.6×10−4 cm2 V−1 s−1 at C* and umicCMC=2.6×10−4 cm2 V−1 s−1 at the CMC. These values may be compared with that reported in the literature for sodium perfluorooctanoate [19], u micCMC =3.9× 10− 4 (cm2 V−1 s−1), and are close to the values found for other surfactants; u micCMC ≈ 3.7× 10−4 (cm2 V−1 s−1) for sodium dodecyl sulphate micelles [31] and u micCMC =3.94× 10−4 (cm2 V−1 s−1) for dodecyltrimethylammonium hydroxide micelles [32]. However, because of the limited points used to compute our values, they must be considered only as estimations.
3.3 Ion-selective electrodes measurements
The responses of the Na+ and PFO− ion-selective electrodes (not shown) were similar to those reported in the literature for other ions. The aggregation of PFO− starts at C≈ 0.01 mol dm−3, and Na+ is incorporated by aggregates at C≈ 0.02 mol dm−3, i. e. both ions aggregate at concentrations below the CMC value found by other methods. The concentrations of the non-micellised Na+ and PFO−, [Na+]free and [PFO−]free, the concentration of the aggregated perfluorooctanoate, [M]=Ctotal−[PFO−]free, and the ionisation degree, α=1−(Ctotal−[Na+]free)/Ctotal−[PFO−]free, were calculated following the usual methods [33]. All concentrations are shown in Fig. 3 and α in Fig. 4. As stated above, from C≈ 0.01 mol dm−3 pre-micellar aggregates are formed and at C≈0.02 mol dm−3, these aggregates begin to capture counterions. At this last concentration, the aggregates have an average ionisation degree of 0.88 that decreases to 0.30 at the CMC and to 0.05–0.07 at higher concentrations. This is reflected in the maximum just below the CMC in Fig. 2. The α value at the CMC agrees reasonably well with that obtained from conductivity measurements of 0.409±0.011. Assuming that CMC=0.028 mol dm−3, which is probably inside the CMC uncertainty, a value of α=0.4 is obtained. The presence of highly charged pre-micellar aggregates and the increased hydrolysis at the CMC producing highly conductive OH− ions can increase the α value obtained by conductivity measurements. Moreover, this latter value is a unique value that averages the effects of structural changes before and after CMC.
With the α value obtained from Fig. 4, the micellar electrophoretic mobility at CMC can be recalculated, obtaining umic CMC=5.4×10−4 cm2 V−1 s−1, a value that seems reasonable because of the small size of micelles and their high charge.
3.4 Surface tension measurements
Figure 5 shows the surface tension as a function of the concentration. The CMC obtained is 0.03 mol dm−3. A slight maximum can be seen at C*=0.059 mol dm−3. By a combination of ion-selective electrode and surface tension results, the Gibbs equation could be applied in all the range of concentrations, including those above CMC,
where Γ i is the surface concentration excess of the solute molecules, σ is the interfacial tension, R the ideal gas constant, T the absolute temperature and afree salt the activity of the unmicellised salt, since micelles are not surface active. This activity was calculated as afree salt=[Na+]free[PFO−]free. The area per adsorbed molecule at the air–solution interface was computed by amolecule=(Γ i NA)− 1, where NA is the Avogadro’s number. Figure 6 plots the surface tension versus log afree salt, including the best fitting curve to obtain \(\partial \sigma /\partial \ln \,a_{{\text{free salt}}} \) at different free salt activities. The resulting area per molecule is shown in Fig. 7 as a function of total concentration. A decrease of amolecule with increasing concentration below the CMC up to approximately 0.01 mol dm−3 is observed. Above this concentration, a competition between aggregates and the surface monolayer exists. Since desorption is produced, pre-micellar aggregates and micelles are energetically more favourable alternatives for the surfactant molecules than the adsorbed state at the air–water interface. At 0.06 mol dm−3, there is a structural change that apparently increases the energetic advantage of the micelles giving rise to a new desorption. The surface was never saturated, and the adsorbed layer acts as a strongly non-ideal gaseous monolayer. This can be seen in Fig. 8 where π amolecule versus π has been plotted, π=σ 0−σ being the surface pressure, σ0 the surface tension of water and σ that of the solution. In all cases, the value of π amolecule is above the ideal value of k B T=4 mN nm2 m−1 at 25 °C, k B being the Boltzmann constant.
In soluble monolayers, there is some immersion of the hydrophobic chains into the water phase [34–39] producing high fluorocarbon-water contact of the adsorbed surfactant molecules. The increasing compactness of the structure of aggregates produces a decrease of the fluorocarbon-water contact by each surfactant molecule, while that in the air–solution interface is constant. Then, this must produce an increased energetic advantage of aggregates compared with the adsorbed monolayer.
By inspection of Fig. 7, it can be concluded that at 0.01 mol dm−3, where pre-micellar aggregates appear, the free energy change to take the chain from the bulk solutions to the air–water interface is approximately equal to that produced in forming the pre-micellar aggregates and then stabilising the adsorption at the interface. When counterions are captured by aggregates, at 0.02 mol dm−3, the aggregates probably become more compact, perhaps by increasing the aggregation number and producing an additional reduction of the water/perfluorocarbon interface area which generates the desorption. This desorption increases at the CMC. Micelles must be relatively compact, and the exposition of perfluorinated chains to water must be lesser than at the air/solution interface. Results of ion-selective electrodes show a quick fall of micellar ionisation degree above the CMC. This strong capture of counterions indicates a strong increase of surface potential of “naked” micelles that suggests the presence of micelles of small radius with a surface covered by a layer of polar groups close to each other. This is coherent with a strong reduction of the contact between perfluorinated chains and water in micelles, which means a free-energy advantage of the micelles when compared with the adsorbed layer and explains the initial desorption at the CMC. If, moreover, a structural change is produced at 0.06 mol dm−3, possibly with the formation of discoid micelles [17, 18], the additional reduction of the micellar perfluorocarbon/water interface explains the strong subsequent desorption.
3.5 Rhodamine 6G experiments
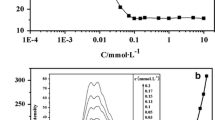
The experiment with Rhodamine 6G colour and fluorescence was employed to confirm that the CMC is 0.03 mol dm−3. Rhodamine 6G was studied by Lewschin [40]. Their spectrum varies with concentration in water with the appearance of a new band when concentration increases. Colour change comes with fluoresce variations. With anionic surfactants and constant concentration of Rhodamine 6G, the solution above the CMC is orange and highly fluorescent, whereas below the CMC it is red and without fluorescence. This is not a very precise method to determine the CMC, but it is a good tool to choose among various alternatives for the CMC, because it detects the presence of micelles.
The colour of the solutions below 0.03 mol dm−3 was the typical light red of Rhodamine 6G solutions when no micelles are present, whereas at concentration ≥0.03 mol dm−3, it was orange with changes in the fluorescence, indicating the existence of micelles. This result confirms that between 0.01 and 0.03 the aggregates are not true micelles. There were neither changes in colour nor fluorescence at C*=0.06 mol dm−3, as expected, because this method is not sensitive to structural micelle changes.
3.6 Viscosity measurements
The reduced specific viscosity of micelles is defined as [41]:
where η and η0 are the respective viscosities of the micellar solution and solvent, and C M the concentration of the micellised surfactant in g cm−3.
By extrapolation to C M =0, the intrinsic viscosity may be obtained [42]
where v is the shape factor, v1 and v2 are the specific volumes of the solvent (water) and the dry surfactant and w is the grams of water per gram of surfactant ratio in micelles. E is the electroviscous effect correction, which may be computed by Booth theory [43]:
where c i and z i are the concentration and the charge (in e units) of the ion i, whose electrophoretic mobility is u i ; ɛ, κ sp , and η0 are the dielectric constants of the solvent, the specific conductivity and the viscosity of the solution at the CMC; ζ is the zeta potential of micelles, whose radius is a; and κ−1 is the Debye distance. Z is a function of κ a, which may be obtained from the literature [44].
To compute the electroviscous effect correction, the micelle radius a must be known. The zeta potential of micelles was estimated by the equation [45]
where ɛ0 is the vacuum permittivity, Q=neα, n being the aggregation number, e the elementary charge and α the ionisation degree of micelles.
Figure 9 shows the relative viscosity at 30 °C, η r , as a function of concentration, those at 25 and 35 °C being similar. Below the CMC, η r <1, i. e. the viscosity of the solution is lower than that of pure water. Consequently, the effect of the unmicellised monomer is to break the structure of water. A minimum exists below the CMC at approximately 0.015–0.02 mol dm−3 and then the viscosity increases the CMC is close to the concentration at which η r ≈ 1. At C≈ 0.06 mol dm−3, there is a slope change.
The rupture of the 3-dimensional structure of water by several substances is named the “structure braking” effect [46] or negative hydration [47]. It was suggested that water molecules in structure breaking aqueous solutions are more mobile than in pure water [48, 49]. These terms are widely used for describing the effect of large ions on the water structure. In particular, it is evidenced by a decrease in viscosity. From neutron diffraction studies [49], it is inferred that the water structure is weakened around some ions having low surface potential, causing a serious disruption of the H-bond structure.
There is evidence of the fact that the hydrogen bonds of the water molecules become weaker around the halogenated groups of organic compounds [50, 51]. Since the CF2 groups have a dipole moment of 1.8 D, it is polar enough to retain some hydration water [52]. Then, it may act as a large, low-charged ion and produce disruption of water, which may explain the low viscosity below the CMC.
When aggregates are formed, the contact between water and perfluorinated chains decreases and a perturbation of flow lines is produced due to the presence of aggregates. This originates an increase of viscosity. The slope change in C≈ 0.06 mol dm−3 corresponds to a change in micelle shape.
Plots of reduced viscosity, η sp /C M , versus concentration of the micellised surfactant, (C M =C−CMC), were made and one of them is shown in Fig. 10. In all cases, two straight lines (I and II) are observed. In order to analyse these lines, the Na+ ion conductivity at infinite dilution, (λ 0Na+ =50.9 S cm2 eq−1), and the Na+ ion electrophoretic mobility, (uNa+=5.275 × 10−4 cm2 s−1 V−1), were taken from the literature [53]. Using the value of λ°PFO-=18.3±0.6 S cm2 eq−1, uPFO-=1.89×10−4 cm2 s−1 V−1 was computed. The ionic strength at the CMC was computed as ICMC=0.03 mol dm−3 giving the inverse of the Debye length κ=1.802 nm−1.
The micellar volume used to compute the micellar radius was Vmicelle=nVmp,M/NA=6.215 nm3 with n=17.5 and Vmp,M=213.87±0.02 cm3 mol−1 (25 °C) [21]. Assuming a spherical micelle, a=1.14 nm and κ a=2.054, giving values of Z=0.0003576 and ζ=52.9 mV. If the relative viscosity at the CMC and 25 °C is 1.01816 (the water viscosity is 0.8904 cp), then the viscosity of the solution at the CMC and 25 °C is ηCMC=0.9066 cp and consequently E=1.11. The calculations at 30 and 35 °C did not give significant changes in E.
From the Vmp,M value of NaPFO partial molar volume, its specific volume was computed as v2=0.4904713±0.000046 cm3 g−1. The specific volume of water was v1=0.81659 cm3 g−1 computed from data in the literature [54]. We took the Einstein shape factor v=2.5 for spherical micelles.
The analysis of the viscosity data at the employed temperatures gave the values of w, the weight of water per gram of surfactant and the number of water molecules per micellised surfactant molecule n W /n S , at the CMC and at the intersection between lines I and II, which occurs at C*≈0.06 mol dm−3. These results are shown in Table 1.
In comparison with these findings, dodecyltrimetylammonim hydroxide micelles have n W /n S =39.2±0.7 [55], alkyltrimethylammonium bromides have 60–70 [56], sodium soaps have about 10 [57, 58], the non-ionic surfactant triton X-100 has 43 [59] and sodium dodecyl sulphate has 6 [60]. All these data were obtained at the CMC and at room temperature.
Micelles at the CMC are strongly hydrated. This can be due to strong repulsion among the polar head groups originated by the high degree of micellar ionisation at the CMC. This produces a scarcely compact micellar surface, with pockets of water among polar groups and probably between the CF2 groups adjacent to the polar groups. Some authors, as Clifford [61, 62] and others [63–66], suggest the existence of water in the closeness of the first methylene group. Muller et al. [63, 65], by NMR measurements of fluorinated amphyphilics, concluded that there is water inside the lipophilic body. The number of wet perfluoromethylene groups varies between four [61, 62] and two or three [56, 61, 67–71].
The inspection of Table 1 leads to the assertion that when the concentration increases and the repulsion among the polar head groups decreases, a reduction in hydration is produced because of the increased compactness of micelles. A temperature increment also produces a partial dehydration.
4 Conclusions
This work throws some light on many important details of SPFO aggregation. Results show that SPFO aggregates gradually. Monomers exhibit a character of water structure breakers. The aggregation starts at C≈ 0.01 mol dm−3 with the formation of pre-micelles of PFO− ions. This concentration was detected with ion-PFO− selective electrode and pH. At C≈ 0.02 mol dm−3, the pre-micellar aggregates capture counterions Na+, with a very high ionisation degree (α≈0.88) that quickly decreases when the concentration increases. This concentration was confirmed by Na+ ion-selective electrode, surface tension and viscosity measurements.
The CMC value, determined by pH, conductivity, Rhodamine 6G colour and fluorescence changes and surface tension experiments, was C≈ 0.03 mol dm−3. Micelles at the CMC are small and highly ionised (α=0.409±0.011 by conductivity, 0.30 by ion-selective electrodes). This suggests that the polar groups in the Stern layer are away enough from one another causing the formation of pockets of water trapped inside the spaces. Consequently, the micelles at the CMC are strongly hydrated. This hydration decreases when the temperature increases. Solubilisation capacity of perfluorooctanoic acid by micelles is extremely high, K F =(1.00±0.16)× 1011, which explains that the increased hydrolysis on micellisation can be detected despite the high value of K a (pK a =1.306±0.074).
At the concentration of C*≈ 0.06 mol dm−3 (detected by measurements of pH, surface tension and viscosity measurements), where the capacity of acid solubility decreases, the ionisation degree is low (0.04–0.07), and the hydration is reduced indicating a more compact micellar structure, probably discoid micelles, as has been reported in the literature.
Micelles at the CMC show energetic advantage compared with the adsorbed state at the air/solution interface, and this advantage increases at C*, originating desorption. This fact is an additional argument favourable to a larger compactness of micelles at C* compared with that of spherical micelles at the CMC.
References
Krafft MP, Riess JG (1998) Biochimie 80:489
Kissa E (2001) Fluorinated surfactants and repellents, surfactant science series. Marcel Dekker, New York
Riess JG (2000) Fascinated by fluorine. Elsevier, Amsterdam
Riess JG, Krafft MP(1999) Mat Res Soc Bull 24:42
Shinoda K, Hato M, Hayshi T (1972) J Phys Chem 76:909
Mukerjee P, Korematsu M, Okawauchi M, Sugihara G (1985) J Phys Chem 89:5308
Tamaki K, Ohara Y, Watanabe S (1989) Bull Chem Soc Jpn 62:2497
Kato S, Harada H, Nakashima H, Nomura H (1992) J Colloid Interface Sci 150:305
de Lisi R, Inglese A, Milioto S, Pellerito A (1997) Langmuir 13:192
de Lisi R, Milioto A, de Giacomo A, Inglese A (1999) Langmuir 15:5014
Fukada K, Kobayashi Y, Ota Y, Fujii M, Kato T, Seimiya T (2000) Thermochimica Acta 352:189
Oelschlaeger C, Waton G, Buhler E, Candau SJ, Cates ME (2002) Langmuir 18:3076
González-Pérez A, Ruso JM, Prieto G, Sarmiento F (2004) J Surf Deterg (in press)
Lucassen J (1966) J Phys Chem 70:1824
Stainsby G, Alexander AE (1949) Trans Faraday Soc 54:585
Moroi Y, Yano H, Shibata O, Yonemitsu T (2001) Bull Chem Soc Jpn 74:667
Hoffman HJ (1984) Ber Bunsenges Phys Chem 88:1078
Ouriques GR, Sander RB, Dmitriev V (2000) Langmuir 16:7900
Sugihara G, Mukerjee P (1981) J Phys Chem 85:1612
Shinoda K, Katsura K (1964) J Phys Chem 68:1568
Milioto S, Crisantino R, de Lisi R, Inglese A (1995) Langmuir 111:718
Schulz PC, Clausse D (2003) J Chem Ed 80:1053
Evans HC (1956) J Chem Soc Pt 1:579
Moroi Y, Takeuchi M, Yoshida N, Yamauchi A (1998) J Colloid Interface Sci 197:221
Moroi Y, Yano H, Shibata O, Yonemitsu T (2001) Bull Chem Soc Jpn 74:667
Hartley GS (1936) Aqueous solutions of paraffin chain salts. Herman et Cie Paris
Stigter D (1954) Rec Trav Chim Pays Bas 73:611
McDowell MJ, Kraus A (1951) J Am Chem Soc 73:2173
Sugihara G, Era Y, Funatsu M, Kunitake T, Lee S, Sasaki Y (1987) J Colloid Interface Sci 187:435
Mukerjee P, Yang AYS (1976) J Phys Chem 80:1388
Stigter D, Mysels KJ (1955) J Phys Chem 59:45
Morini MA, Schulz PC (1997) Colloid Polym Sci 275:802
Schulz PC (1988–1989) Colloid Surf 3:69
Lu JR, Lee EM, Thomas RK, Penfold J, Flitsch SL (1993) Langmuir 9:1352
Lu JR, Simister EA, Lee EM, Thomas RK, Rennie AR, Penfold J (1992) Langmuir 8:1837
Lu JR, Marrocco A, Su TJ, Thomas RK, Penfold J (1993) J Colloid Interface Sci 158:303
Lu JR, Simister EA, Thomasand RK, Penfold J (1993) J Phys Chem 97:6024
Lu JR, Li ZX, Smalwood J, Thomas RK, Penfold J (1995) J Phys Chem 99:8233
Lu JR, Hromadova M, Thomas RK (1993) Langmuir 9:2417
Lewschin WL (1934) Acta Physicochim URSS 1:685
Schulz PC (1984) Anal Assoc Quim Argentina 72:529
Oncley LJ (1949) Ann N Y Acad Sci 41:121
Parkerand RA, Wasik SP (1958) J Phys Chem 62:967
Booth F (1959) Proc R Soc Lond A 203:33
Alexander AE, Johnson P (1950) Colloid science. University Press, Oxford, p 128
Frank HS, Evans MW (1943) J Phys Chem 13:507
Engel G, Hertz HG (1968) Ber Bunsenges Phys Chem 72:808
Samoilov OY (1955) Zhur Fiz Khim 29:1582
Krestov JA (1962) J Struct Chem 3:125
Mizuno K, Oda K, Shindo Y (1996) J Phys Chem 100:10310
Mizuno K, Oda K, Maeda S, Sindo Y (1995) J Phys Chem 99:3225
Drakenberg T, Lindman B (1973) J Colloid Interface Sci 44:184
Handbook of chemistry and physics (1970) CRC Press, Boca Raton
Spernol A (1956) J Phys Chem 60:703
Schulz PC, Rodriguez JL, Morini MA, Puig JE (1997) Colloid Polym Sci 276:396
Ekwall P, Mandell L, Solymon P (1971) J Colloid Interface Sci 35:519
Solyom P, Ekwall P (1968) Chimie Physique Appliqué. Treatements Agents de Surface. CR Congrès Internationale des Detergents 2:1041
McBain JW, Kawakami J, Lucz HP (1933) J Am Chem Soc 55:2762
Kushner LM, Hubbard WD (1954) J Phys Chem 58:1163
Mukerjee P (1964) J Colloid Sci 19:722
Clifford J, Pethica BA (1965) Trans Faraday Soc 61:182
Clifford J (1965) Trans Faraday Soc 61:1276
Muller N, Birkhahn RH (1967) J Phys Chem 71:957
Muller N, Simsohn H (1971) J Phys Chem 75:1971
Muller N, Birkhahn RH (1968) J Phys Chem 72:583
Svens R, Rosenholm B (1966) J Colloid Interface Sci 21:634
Stigter D (1974) J Phys Chem 78:2480
Muller M, Pellerín JM, Chen WW (1972) J Phys Chem 76:3012
Drakenberg T, Lindman B (1973) J Colloid Interface Sci 44:184
Walker T (1973) J Colloid Interface Sci 45:372
Griffith GH, Dehlinger PH, Van SP (1974) J Membrane Biol 15:159
Acknowledgements
This research was funded by the Spanish Ministry of Science and Technology (Project MAT2002-00608, European FEDER funds included) and Xunta de Galicia (Project PXIC20615PN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Fontán, J.L., Sarmiento, F. & Schulz, P.C. The aggregation of sodium perfluorooctanoate in water. Colloid Polym Sci 283, 862–871 (2005). https://doi.org/10.1007/s00396-004-1228-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1228-7