Abstract
A series of N-isopropylacrylamide (NIPAM)-acrylic acid–ethyl methacrylate terpolymers with varied monomer compositions was prepared by radical polymerization. The solution behavior of these polymers was studied in dilute aqueous solution using spectrophotometry, fluorescence spectroscopy and high-sensitivity differential scanning calorimetry. The results obtained revealed that the lower critical solution temperatures depend strongly on the copolymer composition, solution pH and ionic strength. At a high pH, the ionization of acrylic acid (AA) units leads to an increase in solution cloud points (Tc). Solutions of polymers containing 10% or less of AA display a constant Tc for pH above 5.5, with 15% there is a continuous increase in Tc with pH and, for higher AA contents, no clouding was observed within the studied temperature range. Fluorescence probe studies were conducted by following the I 1/I 3 ratio of pyrene vibronic bands and the emission of anilinonaphtalene sulfonic acid, sodium salt (ANS), both approaches revealing the existence of hydrophobic domains for polymers with higher ethyl methacrylate content at temperatures lower than Tc, suggesting some extent of aggregation and/or a coil-to-globule transition. Scanning calorimetry measurements showed an endothermic transition at temperatures agreeing with the previously detected cloud points. Moreover, the transition curves became broader and with a smaller transition enthalpy, as both the AA content and the solution pH were increased. These broader transitions were interpreted to be the result of a wider molecular distribution upon polymer ionization, hence, displaying varied solution properties. The decrease in transition enthalpy was rationalized as a consequence of reminiscent hydration of NIPAM units, even after phase separation, owing to the presence of electric charges along the polymer chain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermosensitive polymers have been the subject of many recent investigations owing to their potential utilization as drug delivery systems [1]. In particular, poly(N-isopropylacrylamide) (PNIPAM) and N-substituted polyacrylamides are potential candidates for this purpose because they exhibit a lower critical solution temperature (LCST) type of behavior [2]. These polymers are soluble (below the LCST, which is ca. 32 °C for aqueous solutions of PNIPAM [3]) mainly due to hydrogen bonding between the water molecules and the amide groups of the macromolecular chain. As the temperature increases, phase separation takes place due to the breaking of these hydrogen bonds, associated with a coil-to-globule transition for the polymer chain and the dominance of NIPAM–NIPAM interaction [4–6].
In recent years, a variety of strategies have been employed to tailor the temperature-induced phase transition to temperatures appropriate for use in drug delivery systems, and also to obtain polymers capable of responding to an environmental stimulus such as pH [7]. The development of systems that fulfill these requirements with respect to phase transition and pH sensitivity has been achieved by random and block copolymerization employing hydrophobic and hydrophilic monomers containing ionizing groups. Following this strategy, NIPAM has been copolymerized with different monomers to change their phase transition temperatures [8]. When hydrophilic monomers are copolymerized with NIPAM, the LCST of the obtained polymer shifts to higher temperatures, while the copolymerization with hydrophobic monomers leads to more hydrophobic polymers and, hence, the LCST moves to lower temperatures [9]. The copolymerization with ionizing monomers is an interesting approach because it makes it possible to adjust the thermosensitivity close to that of human body temperature [10]. Acrylic acid (AA) is the most commonly employed monomer for this purpose, but it restricts the resulting thermosensitivity to a limited pH range, because at a high pH the carboxylic groups are ionized and the polymer becomes rather hydrophilic, decreasing its thermosensitivity. Besides the copolymerization with ionizing monomers [11], the grafting of preformed polymers is also used as an alternative method to impart pH sensitivity to the polymeric system [12, 13]. This procedure has been used by Chen and Hoffman [14] to prepare PNIPAM grafted with poly(acrylic acid). The polymer that is obtained maintains the ability to phase separate at physiological pH (7.4), and its cloud temperature remains at 32 °C [14].
Apart from the capability to display a temperature response that is close to that of the human body, the solubilization of hydrophobic drugs is an additional desirable property since many pharmacologically active compounds employed in drug delivery systems are amphiphilic or hydrophobic molecules [15]. The synthesis of polymers containing hydrophobic monomers is an alternative to obtain amphiphilic systems that could effectively encapsulate hydrophobic substances. N-alkyl methacrylate monomers have been employed for a long time as attractive alternatives in designing these systems because of their inexpensiveness and facility of large-scale production. Methyl methacrylate, for example, has been employed to design drug carriers for doxorubicin [16], propanolol [17] and nanoparticles for intravenous administration [18]. Ethyl and tert-butyl methacrylate have recently been employed by Jones et al. [19] to synthesize amphiphilic polymers for oral administration of hydrophobic drugs [19, 20]. Chiu et al. [21] have synthesized polymers displaying potential application as drug delivery systems employing AA, hexadecyl methacrylate and two other monomers. These polymers were capable of self-assembling in aqueous solution forming hydrophobic aggregates whose sizes depend on the polymer composition. Self-assembling has also been observed for copolymers of NIPAM with methacrylate monomers derived from cholic acid [22] and octadecylacrylate [23]. Kujawa et al. [24] have also prepared hydrophobically modified copolymers of NIPAM with N-glycine acrylamide, which form micellar aggregates with pH-dependent phase separation temperatures. For the above-mentioned systems, in addition to pH, which can have a large effect on their aggregation, the presence of salts, surfactants and other additives was also shown to affect considerably the LCST of these polymers with magnitudes depending on both the polymer structure and the nature of the additive [25, 26].
In earlier investigations, we used AA and ethyl methacrylate (EMA) to prepare amphiphilic copolymers which, in aqueous solution, exhibited hydrophobic domains surrounded by ionized carboxylic groups depending on the amount of EMA incorporated into the copolymer and on pH [27, 28]. For a composition of less than 20% of EMA, these features were detected at a relatively high pH (6.0). The present work aims at assessing the properties of the aqueous solutions of polymers obtained by copolymerization of the monomers mentioned earlier (AA and EMA) with the addition of NIPAM, in an attempt to achieve thermosensitive systems. Polymers containing increasing amounts of AA and EMA were synthesized and their phase separation and solution properties were investigated using spectrophotometry, fluorescence and scanning calorimetric measurements. The dependence of their thermosensitivity on different conditions of pH and ionic strength has been discussed taking into account the polymer structures and their hydrophobic/hydrophilic balances.
Experimental methods
Materials
N-isopropylacrylamide, from Sigma, was purified by recrystallization from hexane. AA, from Aldrich, was distilled twice under reduced pressure. EMA, from Merck, was washed twice with 5% aqueous NaOH and twice with water, then dried with MgSO4 and distilled under reduced pressure. Pyrene (Aldrich) was purified by two recrystallizations from ethanol.
Polymers synthesis and characterization
The terpolymers used in this work were prepared by thermal polymerization of appropriate proportions of the monomers NIPAM, EMA and AA in 1,4-dioxane, using 4,4-azobis (4-cyanovaleric acid) (AIBNC) as the initiator. As an example, the synthesis of T9055 is briefly described: 8.7 mg of AIBN was added to a mixture of NIPAM (2.69 g, 23.8 mmol), AA (0.09 ml, 1.30 mmol) and EMA (0.16 ml, 1.30 mmol) in dioxane (20 ml). The reaction was stirred in an N2 atmosphere at 60 °C for 16 h. After the polymerization, the mixture was cooled to room temperature and the polymer was precipitated by the addition of hexane. Finally, the polymer was purified through two cycles of reprecipitation from hexane and kept under reduced pressure in a desiccator with P2O5.
H1 NMR spectra were recorded in a Bruker AC200 MHz spectrometer, at 25 °C. The homo- and terpolymers were examined in about 10% (w/v) solutions of deuterated DMSO. The amount of AA in the terpolymers was determined by conductimetric titration with freshly prepared NaOH in 50:50 (v/v) methanol/water. The NIPAM content was determined from the results of elemental analysis for nitrogen. The terpolymer molar masses were determined by gel permeation chromatography using a Shimadzu C-R7A chromatograph with refractive index detection. These analyses were performed with N-methylpyrrolidone as solvent, using poly(styrene sulfonate) standards. The contents of AA, NIPAM, EMA and the molar masses of the copolymers are shown in Table 1.
Fluorescence measurements
Fluorescence measurements, using pyrene 10−6 mol l−1, were obtained using a Hitachi 4500 fluorescence spectrometer. Pyrene from a stock solution (1×10−3 mol l−1, in methanol) was added to the polymer solutions (1.0 g l−1), vigorously shaken and allowed to equilibrate for 24 h before measurements were obtained. The I1/I3 ratio of pyrene fluorescence spectra was used to measure the local environment polarity, I1 and I3 being the intensities of the first (372 nm) and the third (383 nm) vibronic peaks of pyrene fluorescence [27, 28]. The probe 1,8-anilinonaphtalene sulfonic acid sodium salt (ANS) was also used for the same purpose. Pyrene and ANS were excited at 310 and 377 nm, respectively, and their emission spectra recorded from 350 to 650 nm. The Hitachi F4500 instrument, with excitation and emission wavelength set to 377 nm, was used to measure the scattered light at 90° from the polymers solution as a function of temperature. These light scattering experiments were carried out to check possible intermolecular interactions upon heating of the solutions. The temperature of the water-jacketed cell holder was controlled with a circulating Fisher Scientific bath (±0.1 °C) and monitored with a thermistor probe inside the cell.
Cloud point determinations
Cloud point measurements were carried out in a Cary 100 spectrophotometer equipped with a Peltier temperature control system. A thermistor probe continuously monitored the temperature inside the cell and all experiments were carried out under stirring. The temperature of the polymers solutions (1.0 g l−1) was increased from 15 to 80 °C in increments of 0.5–1.0 °C and the transmittance was monitored at 450 nm. All measurements were performed 10 min after each temperature was reached, to ensure thermal equilibrium. The cloud point was determined as the onset of the decrease in transmittance plots as a function of temperature. The pH of these solutions was controlled by using phosphate and citric acid buffer systems (10 mM l−1), whose ionic strength was adjusted to 0.5 mol l−1 with KCl.
Differential scanning calorimetry measurements
These measurements were obtained using a VP-DSC (MicroCal, Northampton, MA, USA) high-sensitivity differential scanning calorimeter equipped with 0.542 ml twin cells for the reference and sample solutions. Terpolymer solutions at a concentration of 0.1 wt%, and dissolved in the appropriate buffer with ionic strength of 0.5 mol l−1, were used. All measurements were performed with a scan rate of 30° h−1, in which the transition was assumed to occur close to equilibrium conditions. The enthalpy change associated with each transition was calculated from the integration of the calorimetric curves using the equipment software (MicroCal Origin, version 5.0).
Results and discussion
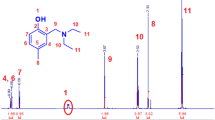
The NMR spectrum shown in Fig. 1 confirms the formation of the terpolymers containing the employed monomers. The peaks at δ3.9 and 7.2 ppm are assigned to protons in the isopropyl [(Me)2C H–] and amide (–CONH–) groups, respectively. The peaks of the main chain of the three monomers are shown in the δ1.0–2.0 ppm range, except for the methyl group in EMA [–CH2–(CH3)C(R)–], which appears at δ0.85 ppm. Peaks centered at 12.0 and 3.8 correspond to the protons of the carboxylic group and to the methylene group in –OC H2CH3, respectively [29]. The considerable overlap observed for the peaks of the three units does not allow their accurate characterization by NMR. Table 1 shows the results obtained for the characterization of the terpolymers by conductimetric and elemental analysis, confirming the successful preparation of copolymers with increasing contents of AA and EMA.
The thermosensitivity of PNIPAM aqueous solutions is attributed to changes in the degree of hydration of the polymer chain in solution. There is strong evidence that both the carbonyl and the hydrogen from the amide group are hydrogen bound to water, being responsible for holding the polymer chain in aqueous solution [30]. The increase in temperature leads to disruption of these hydrogen bonds and, ultimately, to phase separation. Therefore, the hydrophilic/hydrophobic balance plays an important role in determining its thermosensitivity. Figure 2 shows a representative plot of changes in transmittance at 450 nm as a function of temperature, at pH=6.0 and ionic strength 0.5 mol l−1. For solutions of the homopolymer NIPAM, the observed cloud point was 23 °C, which is consistent with the recently published study of Bokias et al. [31], who found a depression of the LCST with the increase of ionic strength of the solution. Shild and Tirrell [32] also verified a similar behavior in an earlier work, where it was proposed that the physical origin for the LCST depression could differ depending on the salt type. This result has been more recently explained on the basis of the capacity of these ions to subtract water molecules from the polymer chain strengthening the hydrophobic interactions [33]. Figure 2 illustrates that the phase transition temperature (Tc) for the terpolymers is progressively shifted to temperatures higher than those observed for PNIPAM. EMA should be less favorably solvated by water than NIPAM, as suggested by the much smaller solubility of homopolymers of EMA with respect to PNIPAM. The results shown in Fig. 2 reveal that, even if the terpolymer hydrophobicity is increased by adding EMA units, the presence of AA monomers overcomes this decrease in hydration resulting in an increased solubility of the terpolymer. This is due to the ionization of AA carboxylic groups, which start to become partially charged for pH values above 4.5. However, in contrast to NIPAM-AA copolymers, which exhibit phase separation only at low pH values (pH<4.5) [34–36], the presence of EMA monomer in the polymers balances to some extent the hydrophilic character of AA and the phase separation for the terpolymers can take place at higher pH values. Figure 3 shows the effect of pH on Tc, and makes it possible to evaluate the contribution of AA to the solution behavior of these polymers. Tc does not change for the homopolymer PNIPAM but, for all terpolymers, increases as the pH is raised. This effect is more pronounced the higher the AA content is. At lower extents of AA ionization, the polymer chain should adopt more compact conformations, associated with more effective intramolecular hydrogen bonds [31], thereby increasing the polymer hydrophobicity and decreasing its solubility in water.
For the copolymers with 5 and 10% of AA, the cloud points increase, reaching plateaus at 30 and 40 °C, respectively, in the pH range of 6–7.4, probably reflecting the high ionization degree of their AA units. The terpolymer containing 15% of AA displays a more pronounced increase of Tc with pH, with cloud points reaching temperatures above 70 °C for pH higher than 6. In the copolymers with 20 and 30% AA, no phase separation was observed within the studied temperature range, for pH above 6, and for lower pH they were insoluble in the buffer conditions. This general behavior can be explained by considering that, upon AA ionization, the polymer chain becomes more hydrophilic, adopting a more extended conformation owing to repulsion between the charges [27, 28].
Although no phase separation was observed for solutions of the polymers with higher contents of acrylic acid, this does not imply that their solution behavior is temperature-independent. Fluorescence measurements using pyrene and ANS were used to verify whether the polymer chains experience conformational changes and/or aggregation at temperatures below their cloud points. Figure 4 shows the results obtained for the different terpolymers at different pH values in the presence of pyrene. In general, the I1/I3 ratio decreases with increasing temperature, which indicates changes in the polarity of the probe surroundings. Pyrene, in the presence of the polymer solutions of PNIPAM and T80:5:15, exhibits an abrupt decrease in the ratio I1/I3 at the same temperature in which the transmittance is decreased, i.e., at 23 and 29 °C, respectively. At low temperatures (at pH=7.4) the I1/I3 ratios for the probe in the presence of PNIPAM and T80:5:15 remain at about 1.9–1.8, denoting an aqueous environment, probably associated with more expanded conformations for these polymers. The abrupt decrease of the I1/I3 values can be attributed to pyrene being solubilized into a globular form of the polymer chain, i.e., an apolar microenvironment is formed due to the coil–globule transition of the terpolymers [6]. On the contrary, with the terpolymers, T70:10:20 and T60:15:25, between pH 5 and 6, pyrene exhibits a decrease in the I1/I3 ratio starting before the detection of the phase separation, which indicates an intramolecular coil collapse and/or intermolecular aggregation of the polymer chains. The same behavior was also observed in experiments with the probe ANS. The fluorescence quantum yields and ANS fluorescence spectra are changed when this probe moves from a hydrophilic to a hydrophobic microenvironment [37, 38]. In an aqueous environment, the probe exhibits a maximum fluorescence at 515 nm and as the temperature increases both a blue shift and an increase in the fluorescence emission are observed. The new band exhibits a maximum emission at 465 nm corresponding to the probe solubilized in a hydrophobic microenvironment. The I515/I465 ratio was plotted as a function of temperature for the various polymers at pH=6.0 and the curves obtained are very similar to those obtained for pyrene (Fig. 5). The ratio I515/I465 for ANS in the presence of PNIPAM and T80:5:15 exhibited a break at their cloud points, 23 and 29 °C, respectively. On the other hand, for the terpolymers T70:10:20 and T60:15:25 the I515/I465 values decrease before clouding takes place, which can reflect either intramolecular interaction or aggregation of individual chains. These interactions provide hydrophobic microenvironments to which ANS may migrate, thus, having its fluorescence spectra displaced to shorter wavelengths.
Changes in the I1/I3 ratio of the pyrene vibronic bands as a function of temperature for a PNIPAM; b T80:5:15; c T70:10:20; d T60:15:25; e T55:20:25; f T40:30:30; at pH=7.4 (open triangle); pH=6.0 (open circle) and pH=5.0 (filled square) and ionic strength of 0.5 mol l−1. The vertical arrows indicate the cloud points determined by transmittance measurements.
I 523/I 474 ratio of ANS (1×10−5 mol l−1) fluorescence intensity as a function of temperature, at pH=6.0, for PNIPAM (filled square); T80:5:15 (filled circle); T70:10:20 (open triangle); T60:15:25 (filled inverted triangle). The vertical arrows indicate the cloud points determined by transmittance measurements.
In solutions of T55:20:25 and T40:30:30, the fluorescence spectra of pyrene and ANS did not change significantly, with I1/I3 (Fig. 4e, f) and I515/I465 (data not shown) remaining almost constant. It is known that the I1/I3 ratio of the pyrene emission spectrum is slightly affected by the temperature, which could explain the small decrease observed for these two polymers, as shown in Fig. 4e, f. Owing to the high AA and EMA content of these polymers, their chains may self-assemble to form hydrophobic microenvironments even at lower temperatures. However, despite the small temperature dependence of the probe’s fluorescence spectra, the light scattering at 90° (377 nm) for the solution of T70:10:20, T60:15:25, T55:20:25 and T40:30:30 increases continuously with temperature, as shown in Fig. 6, which can be interpreted as a sign of an aggregation process taking place in these solutions. The intramolecular interactions for these terpolymers are strengthened due to the polymer dehydration, increasing their tendency to interchain aggregation [39].
Variation of the intensity of scattered light as a function of temperature for solutions of the polymers T70:10:20 (open triangle); T60:15:25 (filled inverted triangle); T55:20:25 (filled diamond); T40:30:30 (open circle), at pH=6.0 and ionic strength of 0.5 mol l−1. The vertical arrows indicate the cloud points determined by transmittance measurements.
The ionic strength has a marked effect on the cloud points for all the polymer solutions studied. Figure 7 shows the Tc values determined for all polymers at pH 7.4 as a function of ionic strength. The cloud points for PNIPAM solutions decreased linearly with the ionic strength from 0.15 to 1.0 mol l−1. A similar result for PNIPAAm has recently been reported by Maeda et al. [40, 41] based on turbidimetric and FTIR spectroscopy studies. For solutions of the polymers T80:5:15 and T70:10:20 a more pronounced decrease in Tc was observed in the same ionic strength range (0.15–1.0 mol l−1). At low ionic strength these polymers should adopt more expanded conformations due to electrostatic repulsion between the charged carboxylic groups. As the ionic strength is increased, the added ions may shield the electrostatic repulsion and these polymers tend to assume more compact conformations, increasing the intramolecular and hydrophobic interactions. Moreover, upon electrolyte addition, water becomes a worse solvent, due to their salting out behavior [30]. These two processes explain the behavior depicted in Fig. 7.
The general behavior derived from the spectrophotometric and fluorescence probe studies was confirmed by the scanning calorimetric measurements. The enthalpy changes in these polymer solutions can be attributed to the disruption of the hydrogen bonds between the polymer and water molecules. In addition to this contribution, the destruction of water–water hydrogen bonds in the hydrophobic hydration shell around the isopropyl group has also been indicated as important for the whole process [41]. These two processes account for the endothermic transition observed in the calorimetric curves, as shown in Fig. 8.
a High-sensitivity DSC curves for 0.66% aqueous solutions of PNIPAM, T80:5:15 and T70:10:20, at pH=6.0 and ionic strength of 0.5 mol l−1. b Scanning calorimetric curves for 0.66% solutions of T80:5:15 at increasing pH. c Scanning calorimetric curves for 2.5% solutions at increasing ionic strengths. (insert: ΔH as a function of the ionic strength)
Figure 8a shows the calorimetric curves for the homopolymer PNIPAM and terpolymers T80:5:15 and T70:10:20, at pH 6 and ionic strength of 0.5 mol l−1. The observed phase transitions agree well with the temperatures determined with the other techniques, although the onset of transitions determined by high-sensitivity DSC (HSDSC) is always smaller due to the higher sensitivity of this technique [46, 47]. This finding also confirms that polymer conformational changes may start to occur prior to macroscopic phase separation, as suggested by our fluorescence measurements. One additional information provided by HSDSC measurements is about the shape of the transition curves. For PNIPAM, the phase separation transition displays a width at half-height of 1.6 degrees in agreement with other investigations [42]. On the other hand, the terpolymers show broader phase transitions, as can be seen in Fig. 8a. This kind of enlargement of the curves—an asymmetric shape characterized by a sharp leading edge followed by a gradually declining tail—is indicative of an association transition. This behavior has also been reported by Leharne and co-workers [43] working on aqueous solutions of poloxamine and by Kunugi et al. [44] with copolymers of poly(N-vinylisobutyramide-it co-vinylamine). In both works, the enlargement of the transition curves was observed as the pH of the aqueous solution was changed from basic to acid conditions, i.e., by increasing the number of ionic residues on the polymer chain. Therefore, the broadness of the transitions revealed by HSDSC is related to the Coulombic repulsion arising from the charged carboxylic groups on the polymer chain, which will prevent the aggregation [43, 44]. The chemical diversity of polymer chains may also affect the shape of the transition curves. This can be understood if one considers that the different monomers should be statistically distributed among the formed polymer chains, leading to a sample that contains not only macromolecules of different molar masses, but also displays monomer compositions that follow a statistical distribution. The energies involved in these phase transitions may be compared to the different polymers investigated taking, as a basis of comparison, the number of moles of NIPAM monomers [45], which are the monomers most significantly removed from the aqueous environment. Following this assumption, it makes sense, as the curves of Fig. 8a point out, that the enthalpies of transition decrease as other monomers are incorporated into the terpolymer. The transition enthalpies, calculated on the basis of moles of NIPAM, are 1.95, 1.52 and 0.77 kcal mol−1, respectively, for pure PNIPAM, T80:5:15 and T70:10:20, revealing a decrease of transition energy as the NIPAM content decreases. The value obtained for PNIPAM agrees well with previous measurements reported by Shild and Tirrell [32] and Kujawa et al. [24] and Loh et al. [42]. The trend displayed by these results can be rationalized considering the effect of the ionized AA groups present in the terpolymers, giving rise to repulsive forces hindering the complete collapse and aggregation of the polymer chains. As a consequence, it is likely that, the higher the AA content, the more NIPAM units remain somehow hydrated, even above the solution cloud point.
The effect of pH changes, which should result in different degrees of ionization for the AA units, is shown in Fig. 8b for the terpolymer T80:5:15. These results show that, upon increased ionization, cloud points are shifted to higher temperatures, as already verified by results with the other techniques. Moreover, there is a clear broadening of the transition peaks, associated with a marked decrease in transition enthalpies, both effects being more pronounced when pH is changed from 5 to 6, and less significant between pH 6 and 7.4. The extent of these changes within the studied pH range agrees with the trend displayed by the spectrophotometrically determined cloud points (shown in Fig. 3), confirming that, for this terpolymer, a more significant change in solution behavior occurs between pH 5 and 6, probably due to a larger extent of AA ionization. The widening of the transition peak and the decrease in transition enthalpy may be explained following the same arguments presented to interpret the results of Fig. 8a. The electrical charges associated with the ionization of the AA units are statistically distributed among the different polymer chains that compose this sample, widening its chemical diversity and, consequently, the range of transition temperatures leading to a broader peak. The presence of extra negative charges will also prevent some NIPAM–NIPAM contacts, with more units remaining hydrated after phase separation, resulting in a less endothermic transition process [44].
The effect of adding more electrolytes to a T80:5:15 solution at pH 7.4 is shown in Fig. 8c. These results reveal that by increasing the ionic strength, there is a depression in the cloud points, in agreement with the results shown in Fig. 7, and also that the transition peaks become narrower and are associated with larger enthalpy changes. These results follow the reverse trend displayed in Fig. 8b, owing to increased ionization of AA units. In fact, the addition of electrolytes should result in a shielding effect reducing repulsion between the charged carboxylic groups, which in turn increases the dehydration of the polymer chain leading to larger enthalpy changes displayed in Fig. 8c.
Conclusions
The copolymerization of the monomers AA and EMA with NIPAM allowed the preparation of terpolymers displaying varied aqueous solution properties. The phase transition temperatures for these terpolymer solutions are progressively shifted to temperatures higher than those observed for PNIPAM, and their solution behavior is highly dependent on both the solution pH and ionic strength. These results confirmed the possibility of tailoring polymer solutions to display a desired phase transition temperature by controlling their composition or solutions parameters. For some terpolymers, the formation of hydrophobic domains was detected at certain solution conditions, which confers additional importance on these polymer solutions allowing the incorporation of hydrophobic substances. Scanning calorimetric measurements revealed further insights into these transition processes in terms of increased transition broadness and decreased enthalpy changes upon the increase of electrical charges in the polymer chains.
References
Ichikawa H, Fukumori Y (1997) STP Pharma Sci 7:529
Liu HY, Zhu XX (1999) Polymer 40:6985
Maeda Y, Nakamura T, Ikeda I (2002) Macromolecules 35:10172
Winnik FM (1990) Polymer 31:2125
Wang X, Qiu X, Wu C (1998) Macromolecules 31:2972
Chee CK, Rimmer S, Soutar I, Swanson L (2001) Polymer 42:5079
Langer R (1998) Nature 392:5
Spafford M, Polozova A, Winnik F M (1998) Macromolecules 31:7099
Kurisawa M, Yokoyama M, Okano T (2000) J Control Release 68:1
Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A (2001) J Control Release 74:213
Ganorkar CR, Liu R, Baudys M, Kim SW (1999) J Control Release 59:287
Durand A, Hourdet D (1999) Polymer 40:4941
Durand A, Hourdet D (2000) Polymer 41:545
Chen G, Hoffman AS (1995) Nature 373:49
Schreier S, Malheiros SVP, Paula E (2000) Biochim Biophys Acta 1508:210
Inoue T, Chen G, Nakamae K, Hoffman AS (1998) J Control Release 51:221
Nujoma YN, Kim C-J (1996) J Pharm Sci 85:1091
Passarin C, Barratt G, Devissaguet J-P, Labarre D (1998) Pharm Res 15:1046
Jones MC, Ranger M, Leroux J-C (2003) Bioconj Chem 14:774
Dufresne MH, Garrec DL, Sant V, Leroux JC, Ranger M (2004) Int J Pharm 277:81
Chiu HC, Chern C-S, Lee C-K, Chang H-F (1998) Polymer 39:1609
Benrebouh A, Avoce D, Zhu XX (2001) Polymer 42:4031
Shi X, Li J, Sun C, Wu S (2000) Coll Surf A 177:41
Kujawa P, Goh CCE, Calvet D, Winnik FM (2001) Macromolecules 34:6387
Freitag R, Flaudy GF (2002) Langmuir 18:3434
da Silva RC, Loh W (1998) J Colloid Interface Sci 202:385
Oliveira VA, Tiera MJ, Neumann MG (1996) Langmuir 12:607
Tiera VAO, Tiera MJ, Gehlen MH, Neumann MG (1996) Photochem Photobiol 63:779
Demirelli K, Kaya I, Coskun M (2001) Polymer 42:5181
Maeda Y, Higuchi T, Ikeda I (2000) Langmuir 16:7503
Bokias G, Staikos G, Iliopoulos I (2000) Polymer 41:7399
Shild HG, Tirrell DA (1990) J Phys Chem 94:4352
Maeda Y, Higuchi T, Ikeda I (2001) Langmuir 17:7535
Jones MS (1999) Eur Polym J 35:795
Yoo MK, Sung YK, Lee YM, Cho CS (1998) Polymer 39:3703
Yoo MK, Sung YK, Cho CS, Lee YM (1997) Polymer 38:2759
Slavík J (1982) Biochim Biophys Acta 694:1
Vieira NAB, Moscardini MS, Tiera VAO, Tiera MJ (2003) Carboh Polym 53:137
Qiu X, Kwan MS, Wu C (1997) Macromolecules 30:6090
Maeda Y, Yamamoto H, Ikeda I (2001) Langmuir 17:6855
Maeda Y, Nakamura T, Ikeda I (2001) Macromolecules 34:1391
Loh W, Teixeira LAC, Lee LTJ (2004) J Phys Chem B 108:3196
Armstrong JK, Chowdry BZ, Snowden MJ, Dong J, Leharne SA (2001) Int J Pharm 229:57
Kunugi S, Tada T, Yamazaki Y (2000) Langmuir 16:2042
Principi T, Goh CCE, Liu CW, Winnik FM (2000) Macromolecules 33:2958
Fujishige S, Kubota K, Ando I (1989) J Phys Chem 93:3311
Boutris C, Chatzi EG, Kiparissides C (1997) Polymer 38:2567
Acknowledgements
The Brazilian agency Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) is gratefully acknowledged for financial support to M. J. T. (Grant 98/13960-0) and to W. L. (Grant 00/06635-8) and for the doctoral and postdoctoral fellowships of G. R. S. and R. C. S. W. L. thanks CNPq for a research fellowship. M. J. T. also extends his thanks to Fundação para o Desenvolvimento da UNESP (FUNDUNESP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiera, M.J., dos Santos, G.R., Tiera, V.A.d. et al. Aqueous solution behavior of thermosensitive (N-isopropylacrylamide-acrylic acid-ethyl methacrylate) terpolymers. Colloid Polym Sci 283, 662–670 (2005). https://doi.org/10.1007/s00396-004-1198-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1198-9