Abstract
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that contributes a crucial role in protection against ischemia (ISC)-reperfusion (REP) injury by driving expression of anti-apoptotic and anti-oxidant genes. STAT3 is also present in the mitochondria, where it modulates the activity of the electron transport chain (ETC) and the permeability transition pore. Transgenic mice that overexpress a mitochondrial-targeted, transcriptionally inactive STAT3 in cardiomyocytes (MLS-STAT3E mice) exhibit a persistent, partial blockade of electron transfer through complex I that uniquely did not lead to tissue dysfunction at baseline, yet increased mitochondrial ischemic tolerance. The direct contribution of non-transcriptional, mitochondria-localized STAT3 to protection during ISC-REP remains to be established. We hypothesized that the enhanced mitochondrial tolerance to ischemia present in MLS-STAT3E mice would decrease cardiac injury during ISC-REP. In the isolated buffer-perfused heart model, MLS-STAT3E hearts exhibit a decreased infarct size compared to non-transgenic littermate hearts. Contractile recovery, expressed as a percent of LV developed pressure before ISC, is improved in MLS-STAT3E mice. Mitochondria isolated at the end of 60 min. of REP from MLS-STAT3E hearts show attenuated ROS release. The partial and persistent blockade of complex I present in MLS-STAT3E mice decreases cardiac injury during REP, in part via a persistent decrease in ROS production and attenuation of mitochondrial permeability transition pore opening at the onset of REP. In vivo, MLS-STAT3E hearts exhibit substantially higher postoperative survival rate and a substantial decrease in myocardial infarct size. STAT3 mediates cardioprotection not only via canonical action as a transcription factor, but also as a modulator of ETC activity directly in the mitochondria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondria sustain progressive damage during myocardial ischemia (ISC) [25]. ISC disrupts the mitochondrial electron transport chain (ETC), leading to increased generation of reactive oxygen species (ROS) [9], mitochondrial permeabilization [12], release of pro-apoptotic factors (e.g., cytochrome c [7, 25] and apoptosis-inducing factor (AIF) [32]) and activation of apoptosis [6], resulting in tissue injury during reperfusion (REP). A blockade of ETC before ISC with a reversible inhibitor of complex I, amobarbital, results in ETC protection, decreased ROS generation and reduced infarct size measured after REP [8]. These data suggest that partial and transient blockade of the ETC can decrease cardiac injury.
Signal transducer and activator of transcription 3 (STAT3), a component of the JAK/STAT signaling pathway, is a transcription factor that regulates the expression of genes encoding proteins involved in multiple processes, including inflammation, apoptosis, angiogenesis, cellular signaling, and cell stress responses [13, 26]. The role of STAT3 in the protection of myocardium against ISC-REP injury has been documented for ischemic preconditioning [38], ischemic postconditioning [2] and action of JAK ligands, such as TNF-α [23], leptin [37], and insulin [15]. Until recently, the cardioprotective function of STAT3 has only been attributed to the regulation of expression of antiapoptotic (e.g. Bcl-2, Bcl-xL) [26] and antioxidant genes (e.g., manganese superoxide dismutase, metallothioneins) [33, 34].
STAT3 is also present in the mitochondria, where it modulates activity of the ETC in a non-transcriptional fashion [42]. It interacts with matrix-localized cyclophilin D, a target of mitochondrial permeability transition pore (MPTP) inhibitor cyclosporine A [4, 18]. The genetic ablation of STAT3 results in deleterious impairment of complex I and II activities [42] and increased sensitivity to calcium overload that leads to MPTP opening, causing mitochondrial permeabilization [4].
In order to investigate the role of mitochondrial STAT3 during ISC-REP, a transgenic mouse was generated that overexpressed a mitochondrial-targeted, transcriptionally inactive form of STAT3 (MLS-STAT3E) in the heart [40]. MLS-STAT3E did not affect the expression of STAT3-dependent genes at baseline or under conditions of ISC and following exposure to leukemia inhibitory factor (LIF), a member of the IL-6 family of STAT3 activators [40]. Mitochondria with MLS-STAT3E exhibited modestly decreased complex I and II activities at baseline, whereas the mitochondrial membrane potential and baseline ROS production were not affected [40]. That observation was in contrast with the STAT3 knock-out model that showed profound decreases in complex I dependent oxidative phosphorylation and complex I activity [22, 36, 40]. Interestingly, the partial blockade of complex I by MLS-STAT3E protected complex I against ischemic damage, attenuated ROS release and inhibited the release of cytochrome c during ISC [40]. Thus, the MLS-STAT3E response during ISC is reminiscent of approaches using pharmacologic blockade of complex I, which result in protection of mitochondria and the myocardium against ischemic injury [30]. The contribution of the non-transcriptional, mitochondria-localized STAT3 to cardioprotection during ISC-REP was addressed in this study using the MLS-STAT3E mouse model.
Materials and methods
Reagents
The following antibodies were used: AIF, GAPDH (Cell Signaling, Danvers, MA); cytochrome c (BD Biosciences, San Diego, CA). All chemicals were purchased from Sigma–Aldrich (St. Louis, MO) unless indicated otherwise.
Animals
Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals under the protocols approved by Virginia Commonwealth University Institutional Animal Care and Use Committee and the McGuire VA Medical Center Institutional Animal Care and Use Committee. These studies were thus performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Generation and phenotypic characterization of MLS-STAT3E mice on the 129X1/SvJ background was described previously [40].
Isolated heart perfusion and functional measurement
Mice were anesthetized with pentobarbital (90 mg/kg; i.p.), and the hearts were quickly excised and mounted on the Langendorff perfusion system. Hearts were perfused with modified Krebs-Henseleit buffer (in mM: 115 NaCl, 4.0 KCl, 2.5 mM CaCl2·2H2O, 26 NaHCO3, 1.1 MgSO4·7H2O, 0.9 KH2PO4, 5.5 glucose) bubbled with 95 % O2 and 5 % CO2 to adjust pH to 7.35–7.45 [8]. Hearts were paced at 420 beats per min. The cardiac function was monitored with a balloon inserted into the left ventricle, and data was recorded digitally with Powerlab (AD Instruments, Colorado Springs, CO). Non-transgenic littermate (WT) or MLS-STAT3E mouse hearts were subjected to 35 min of global ISC and 60 min of REP. Hearts were harvested at the end of experiment for infarct size measurement or mitochondrial isolation. C57Bl/6 mice were subjected to 35 min of global ISC and 60 min REP for measurement of infarct size. The isolated mitochondria were used to measure the rate of oxidative phosphorylation, ETC-driven ROS release and the susceptibility to MPTP opening. For infarct size measurement [20], hearts were frozen at −20 °C, sectioned into 2 mm thick slices, incubated in 1 % 2,3,5-triphenyl tetrazolium chloride (TTC) at 37 °C for 20 min and stored in 10 % formalin for 24 h. Infarct size, expressed as a percentage of the left ventricle, was calculated using BIOQUANT imaging software (BIOQUANT Image Analysis Corp, Nashville, TN).
Isolation of mitochondrial and cytosolic fractions
Hearts were studied at the end of 60 min reperfusion and ventricles were used to isolate a single population of cardiac mitochondria. Tissue was briefly washed in a modified Chappell-Perry (CP) buffer (buffer CP1 at pH 7.4: 100 mM KCl, 50 mM MOPS, 1 mM EGTA, 5 mM MgSO4·7H2O, 1 mM ATP), dried with Whatman filter paper, weighed, then placed in glass beaker, and thoroughly minced. Ventricular tissue was homogenized in 3 ml of CP1 buffer using a polytron tissue blender (Kinematica, Bohemia, NY) for 2.5 s at a rheostat setting of 10,000 rpm. The polytron homogenate was centrifuged at 6000g for 10 min at 4 °C and supernatant was saved as a crude cytosol for further purification. The homogenate pellets were re-suspended in 3 ml of CP1 buffer supplemented with 5 mg/g (wet weight) trypsin (#T0303, Sigma–Aldrich), incubated with stirring for 15 min at 4 °C followed by addition of 3 ml of CP2 buffer (CP1 buffer containing 0.2 % BSA to attenuate trypsin activity). Digested tissue was further homogenized by two strokes using digital steady-stirring tight Teflon pestle/glass tube homogenizer set at 600 rpm (Fisher Scientific, Pittsburgh, PA). Undigested tissue and heavier cell fractions in the remaining volume were pelleted by centrifugation at speed 500g for 10 min at 4 °C. The mitochondria-containing supernatant was centrifuged at 3000g for 10 min at 4 °C. The mitochondrial pellet was washed with 2 ml of KME buffer, pH 7.4 (100 mM KCl, 50 mM MOPS, 0.5 mM EGTA). Mitochondria were re-suspended in 80–120 μl of KME and used within 4 h after isolation or frozen. Crude cytosolic fraction was supplemented with protease and phosphatase inhibitor cocktails (Roche, Indianapolis, IN) and purified by ultra-centrifugation at 100,000g for 1 h at 4 °C (Thermo Scientific, Waltham, MA). The protein concentration was measured by Lowry [27] using BSA as a standard and sodium deoxycholate as a detergent.
SDS-PAGE and immunoblotting
Proteins were separated using 12 % Tris–glycine gels (Biorad, Hercules, CA) according to the manufacturer’s protocol. Gels were transferred to Immobilon-P PVDF membrane (Millipore) using semi-dry transfer (Bio-Rad). The blots were incubated for 1 h at room temperature in 5 % (w/v) non-fat dry milk (Bio-Rad) in TBS-T buffer (10 mM Tris pH 7.5, 150 mM NaCl, 0.1 % Tween20) followed by the overnight incubation at 4 °C with primary antibody. After 1 h incubation at room temperature with a 1:10,000 dilution of HRP-conjugated anti-mouse or anti-rabbit IgG F(ab)2 (GE Healthcare Life Sciences, Piscataway, NJ), blots were developed using ECL Plus Western Blotting Detection Reagents (GE Healthcare Life Sciences).
Detection of H2O2 production
The enzymatic reduction of mitochondria-generated H2O2 by horseradish peroxidase (HRP) was coupled with the oxidation of the fluorogenic indicator Amplex Red (Invitrogen) to the fluorescent resorufin as described previously [10]. Intact mitochondria (200 μg) were incubated with 25 μM Amplex Red and 0.20 units/ml HRP in chelex-treated buffer, pH 7.4 (150 mM KCl, 5 mM KH2PO4, 1 mM EGTA) in the presence or absence of 2.5 μM rotenone, a complex I-specific inhibitor.
Calcium retention capacity
CRC was evaluated in mitochondria incubated in CRC buffer (in mM: 150 sucrose, 50 KCl, 2 KH2PO4, 5 succinic acid, 20 Tris/HCl, pH 7.4) by sequential pulses of calcium (5 nmol). Extra-mitochondrial Ca2+ concentration was recorded with 0.5 µM Calcium Green-5 N and fluorescence monitored with excitation and emission wavelengths set at 500 and 530 nm, respectively [35].
Model of in vivo regional ISC-REP
Mice were anesthetized with pentobarbital (70 mg/kg; i.p.). A left thoracotomy was performed at the fourth intercostal space and the heart was exposed by stripping the pericardium. The left anterior descending coronary artery was identified and occluded for 30 min followed by 24 h of REP. At the onset of REP, the air was expelled from the chest. The animals were extubated and then received intramuscular doses of analgesia (buprenex; 0.01 mg/kg; i.m. every 12 h). Post-operative survival was evaluated as % of animals that survived the 24 h period of REP. Only mice that recovered from ISC surgery were taken into account. After 24 h of REP, the hearts of surviving animals were quickly excised and mounted on a Langendorff apparatus. Blood was washed out from coronary arteries by 0.9 % NaCl with 2.5 mM CaCl2, left anterior descending artery was re-occluded and 10 % Evans blue dye was injected as a bolus into the aorta until most of the heart turned blue. After saline washing, the hearts were removed, frozen, and cut into transverse slices. The slices were incubated in 10 % TTC at room temperature for 30 min. The areas of infarcted tissue, the risk zone, and the whole left ventricle were determined by computer morphometry with BIOQUANT imaging software. Infarct size was expressed as a percentage of the ischemic risk area, which was determined as a percentage of the left ventricle.
Statistical analysis
Results consisting of two groups were analyzed by two-tailed Student’s t test. In case of more than two groups (WT vs. MLS-STAT3E in context of time control and ISC-REP), results were subjected to two-way ANOVA with Holm-Sidak post test (pairwise multiple comparison procedures) for multiple groups. All analyses were executed using Prism (GraphPad Software Inc., La Jolla, CA). P < 0.05 was considered statistically significant.
Results
MLS-STAT3E decreases cardiac injury
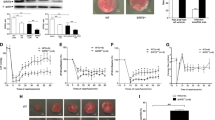
MLS-STAT3 exerted a partial and persistent blockade of electron transport through complex I, and also attenuated damage to complex I during ISC [40]. Thus, we designed a set of experiments that allowed us to test whether MLS-STAT3E mediates cardioprotection against ISC-REP-driven injury. In the isolated buffer-perfused WT hearts, ISC-REP led to an infarct size of 34 ± 2 % of the left ventricle (LV) (Fig. 1a). Infarct size was similar in C57Bl6 mice following ISC-REP to the WT 129X1/SvJ (46 ± 3 and 34 ± 2 % in C57Bl6 and 129X1/SvJ, respectively). MLS-STAT3E hearts are more resistant to ISC-REP injury, with infarct size decreased by approximately one-third. Contractile recovery, expressed as a percent of LV developed pressure (LVDP) before ISC, is improved in MLS-STAT3E mice compared to WT (10 ± 2 % WT vs. 21 ± 3 % MLS-STAT3E; Fig. 1b). These results suggest that STAT3 targeted to the mitochondria in the MLS-STAT3E mice attenuates injury during acute, in vitro ISC-REP.
Hearts expressing MLS-STAT3E are more resistant to ISC-REP injury. Hearts excised from MLS-STAT3E mice and WT littermates were subjected to ex vivo Langendorff model of 35 min ISC and 60 min REP. a Infarct size, expressed as a percentage of the left ventricle, was measured using 2,3,5-triphenyl tetrazolium chloride (TTC), which is turned to red 1,3,5-triphenyl formazan in viable tissue containing active dehydrogenases. b Contractile recovery was expressed as a percent of left ventricular developed pressure after ISC-REP. All results are mean ± SEM, n = 7. *P < 0.05 vs. WT
MLS-STAT3E attenuates mitochondrial generation of ROS during ISC-REP
Ischemic damage to the mitochondrial ETC, particularly complex I, leads to increased leakage of electrons onto oxygen and formation of superoxide anions [9], which are dismutated to H2O2. ROS generation from ETC, measured as a net release of H2O2 from intact WT mitochondria respiring on complex I substrates, was substantially increased due to ISC-REP-driven mitochondrial damage (Fig. 2a). However, the presence of MLS-STAT3E abrogated ISC-REP-mediated H2O2 net release from complexes I and III (Fig. 2a). The addition of rotenone quantified the maximal rate of ROS release from complex I alone. Similar to the conditions without rotenone, MLS-STAT3E attenuated maximal H2O2 net release from the mitochondria after ISC-REP compared to WT (Fig. 2b).
MLS-STAT3E attenuates ETC-dependent ROS release from mitochondria subjected to ISC-REP. MLS-STAT3E and WT hearts were subjected to ex vivo Langendorff model of 35 min ISC and 60 min REP or perfusion only without ISC (time control) followed by mitochondria isolation and assessment of mitochondrial H2O2 net release using HRP/Amplex Red protocol. a Mitochondria were incubated with glutamate + malate to measure ROS production dependent on electron flow through complex I and complex III. b Rotenone was added to glutamate + malate to establish the maximal capacity of complex I to generate ROS. Results are mean ± SEM, n = 6. *P < 0.05 ISC vs. corresponding time control, # P < 0.05 MLS-STAT3E vs. corresponding WT. The dotted bars reflect the data obtained under ISC-REP conditions
There were no differences in the CRC between WT and MLS-STAT3E mitochondria at the end of 60 min REP, indicating that the susceptibility to calcium-mediated MPTP was similar at this later time of reperfusion (Fig. 3). Two major pro-apoptotic proteins normally located within the mitochondria, cytochrome c and apoptosis-inducing factor (AIF), were present in the cytosol to the same extent in MLS-STAT3E and WT at the end of 1 h of REP (Fig. 4a, b). In addition, an anti-PAR antibody was used to measure poly (ADP-ribose) chains (PAR polymers) as an indication of activated Poly (ADP-ribose) polymerase (PARP) enzyme as another marker of apoptosis. PAR polymers were equally detected in both MLS-STAT3E and WT cytosolic extracts (Supplemental Fig. 1), in agreement with the cytochrome c and AIF results.
MLS-STAT3E does not affect MPTP at 60 min of REP. MLS-STAT3E and WT hearts were subjected to ex vivo Langendorff model of 35 min ISC and 60 min REP or perfusion only (time control) followed by mitochondria isolation and assessment of calcium retention capacity (CRC). CRC was evaluated in mitochondria by sequential pulses of calcium (5 nmol) in a presence of calcein green indicator. Results are mean ± SEM, n = 6. *P < 0.05 ISC vs. corresponding time control. The dotted bars reflect the data obtained under ISC-REP conditions
At later reperfusion, MLS-STAT3E does not alter release of proapoptotic peptides from mitochondria. Equal amounts of cytosolic extracts from ISC-REP (IR) and time control (TC) hearts from WT and MLS-STAT3E mice were resolved by SDS-PAGE, transferred to PVDF membrane and probed for cytochrome c (a) and AIF (b). GAPDH was used as protein loading control. Densitometry was measured using ImageJ (NIH, Bethesda) and expressed as ratio of probed protein signal to GAPDH signal and normalized to TC set as 1. Results are mean ± SEM, n = 6. *P < 0.05 vs. corresponding TC. One representative immunoblot for each protein evaluated is shown. The dotted bars reflect the data obtained under ISC-REP conditions
MLS-STAT3E mice have an improved post-operative survival rate and sustain less injury after in vivo ISC-REP
MLS-STAT3E mice exhibited higher post-operative procedure survival rate compared to WT littermates (Fig. 5a). Unexpectedly, the survival rate of WT littermates was decreased to 24 %, in contrast to our previous studies using the C57BL/6 mice, where the survival rate was 70–80 % [41]. MLS-STAT3E and WT littermates were established on 129X1/SvJ strain background [40]. Nonetheless, even in this apparently higher risk 129X1/SvJ strain, survival was improved in the MLS-STAT3E mice compared to WT littermates (Fig. 5a). In order to measure infarct size in our in vivo model of ISC-REP, we increased the number of WT animals to total of 25 (compared to ten MLS-STAT3E mice). On average, WT mice on 129X1/SvJ genetic background displayed an infarct size of approximately 50 % of the area at risk (AAR) in surviving animals (Fig. 5b). The infarct size in C57Bl6 mice was also approximately 50 % of the area at risk (Fig. 5b), in line with previous results. A larger infarct size in the 129X1/SvJ does not clearly support the decreased survival rate. MLS-STAT3E mice sustained considerably less injury, exhibiting 23.4 ± 0.8 % infarct size after ISC and 24 h of REP. Nonetheless, the in vivo model of ISC-REP corroborated the initial ex vivo results (Fig. 1a).
MLS-STAT3E mice sustain less ISC-REP injury and display increased post-operative survival. Mice were anesthetized with pentobarbital and left thoracotomy was performed at the fourth intercostal space to expose the heart. The left anterior descending coronary artery was identified and occluded for 30 min followed by 24 h of REP. Eight C57BL/6J WT mice, 25 129X1/SvJ WT mice and ten 129X1/SvJ MLS-STAT3E mice were used in in vivo experiments. a Post-operative survival was evaluated as % of animals that survived 24 h period of REP. Only mice that recovered from ISC surgery were taken into account. b Hearts from survived animals were subjected to evaluation of infarction. Infarct size, expressed as a percentage of the ischemic area at risk (AAR), which was determined as a percentage of the left ventricle, was measured using 10 % TTC and analyzed by BIOQUANT imaging software. All results are mean ± SEM, n = 8 for C57BL/6J WT, n = 7 for 129X1/SvJ WT and n = 4 for 129X1/SvJ MLS-STAT3E. *P < 0.05 vs. WT
Discussion
Ischemia and reperfusion injury causes mitochondrial oxidative stress, which leads to cardiac injury. Numerous reports have indicated the involvement of the canonical JAK2/STAT3 transcriptional signaling pathway in cardioprotection against ISC-REP injury [3]. A non-canonical role for mitochondrial STAT3 has been shown to contribute to attenuation of ischemic damage to the mitochondria [40]. However, the contribution of mitochondrial STAT3 in the physiological response of the heart to ISC-REP-mediated stress has remained to be investigated.
Only recently, it has been shown that the ischemic postconditioning in an in vivo porcine model of regional myocardial ISC-REP leads to the phosphorylation of mitochondrial STAT3, preservation of complex I respiration, increased calcium tolerance, and ultimately to reduced tissue injury [21]. This postconditioning-mediated protection was attenuated by an in vivo administration of AG490, an inhibitor of the JAK/STAT pathway [21]. However, the experimental design in such models does not differentiate the relative contributions to cardioprotection due to mitochondrial mechanisms in contrast to STAT3 transcriptional activity. Based on available reports, it is unlikely though that the role of STAT3 in myocardial protection during ISC-REP is attributed solely to its action as a nuclear transcription factor, given the short period after STAT3 activation at the time of REP by use of ischemic postconditioning [21], TNF-α [23] or leptin [37] was sufficient to reduce tissue injury. The unique MLS-STAT3E model of mitochondrial-targeted and transcriptionally inactive STAT3 utilized in the current study separates transcriptional and non-transcriptional mechanisms of protection. The information obtained from the studies presented in this manuscript advances our understanding of the contribution of mitochondrial-localized STAT3 to the overall cardioprotection mediated by STAT3. We report herein that MLS-STAT3E-mediated protection of mitochondria during ISC [40] leads to attenuation of myocardial injury during early period of REP through inhibition of ETC-driven ROS release. The in vivo mouse model of ISC-REP further confirms the cardioprotective role of MLS-STAT3E during prolonged periods of REP by significantly augmenting the survival rate of animals subjected to the surgery and substantially decreasing the extent of damage to the myocardium. Thus, MLS-STAT3E may be a genetic equivalent of protective pharmacologic complex I blockade [40].
MLS-STAT3E mice exhibit attenuated mitochondrial ROS release measured at 60 min of REP (Fig. 2), a period selected based on infarction data. This is likely a result of STAT3 attenuating mitochondrial ROS generation through protection of complex I from ischemic damage [40], which further translates into the REP phase [8]. Interestingly, MLS-STAT3E-mediated regulation of ROS production from the ETC has also been implicated in breast cancer progression. MLS-STAT3E regulates breast cancer growth through modulation of ROS accumulation in the mitochondria [44]. Cells expressing MLS-STAT3E with constitutively active serine (Ser727Asp) show enhanced complex I activity, resulting in decreased production of ROS, inhibition of the primary tumor growth and the metastasis potential of breast cancer cells. These results highlight the importance of serine 727 of MLS-STAT3E and confirm a novel role for MLS-STAT3E in regulation of mitochondrial ROS production through its action on the ETC. In our experiments, we are unable to detect serine 727 phosphorylated STAT3 (P-Ser727-STAT3) in the mitochondria after 60 min REP (Supplemental Fig. 2). Previous work to detect P-Ser727-STAT3 utilized initial immunoprecipitation of STAT3 [4]. Our approach to directly probe mitochondria with the antibody to P-Ser727-STAT3 was unsuccessful.
The MPTP is a non-selective pore in the mitochondrial inner membrane. MPTP opening is a crucial contributor to cardiac ISC-REP injury [19]. MPTP opening is favored at the onset of REP due to increased oxidative stress, normalization of intracellular pH, and mitochondrial calcium overload [11, 17]. While ischemic damage to the ETC increases ROS production during re-oxygenation phase, the prevention of ischemic damage decreases ROS generation during REP and decreases the susceptibility of mitochondria to undergo MPTP during early REP [1]. Therefore, ischemic damage to the ETC may contribute to cardiac injury during REP through mitochondrial ROS generation, which facilitates MPTP opening. Recently, it has been reported that the inhibition of mitochondrial STAT3 by Stattic augments cardiac mitochondrial MPTP opening through enhanced ROS formation [5]. However, in our model, despite having decreased ROS generation from preserved ETC, MLS-STAT3E mitochondria do not exhibit a reduced susceptibility to MPTP at the more prolonged REP period of 60 min (Fig. 3). Interestingly, previous work has shown that protection of the ETC during ISC attenuates susceptibility to MPTP opening during early REP [1]. Thus, consistent with our previous observations contrasting very early and later periods of REP [43], although the susceptibility to MPTP is not different between MLS-STAT3E and WT mice at the 60 min REP, opening of MPTP during early REP is likely attenuated by STAT3 overexpression. Mitochondria from STAT3 heart knock-out mice and Stattic-treated mitochondria are more susceptible to calcium-mediated MPTP opening [4], providing further support that overexpression of STAT3 within mitochondria blunts the MPTP opening during early REP. STAT3 has been reported to interact with cyclophilin D, the target of MPTP inhibitor cyclosporine A. Our results, which show lack of effect of overexpressed mitochondrial STAT3 on MPTP at end of 60 min REP, are likely due to the prolonged REP period. These results indicate that the relationship between STAT3 and cyclophilin D is very likely a dynamic interaction that while playing a crucial role during early minutes of REP, it may be less critical as REP continues. Cardiac protection has been achieved through the mechanism of complex I partial inhibition at the onset of REP by treatment of hearts with amobarbital [39] or extracellular acidification [43] during the first 5 min of REP that in turn attenuate MPTP opening. Since MLS-STAT3E mitochondria sustain less damage during ISC [40], the possible differences between genotypes might be more apparent at the onset of the REP and gradually disappear during 60 min of REP. For instance, the preserved mitochondrial membrane potential in MLS-STAT3E at the onset of REP could lead to attenuated Parkin recruitment [31], resulting in inhibition of mitophagy, a process that can be injurious to the cardiac tissue when over-activated [24, 29].
Additional experiments were performed where MLS-STAT3E mice were buffer-perfused and subjected to 35 min global ISC and only 10 min REP followed by isolation of mitochondria. CRC and cytosolic cytochrome c content were determined. Comparison of CRC in MLS-STAT3E mitochondria sampled at early and later REP time showed a decrease in CRC with longer REP time (Supplemental Fig. 3a). However, the CRC is greater in mitochondria from MLS-STAT3E mice compared to WT at 10 min REP (Supplemental Fig. 3b). These results support that overexpression of STAT3 in the mitochondria decreases the susceptibility of mitochondria to undergo permeability transition pore opening during early REP. In order to address the potential impact from mitochondrial-driven injury during early REP, the cytochrome c content in cytosol at 10 min REP was assessed. The cytochrome c content in cytosol from MLS-STAT3E mice following 10 min REP was lower compared to WT (Supplemental Fig. 4). These results indicate that overexpression of STAT3E in the mitochondria will potentially decrease apoptosis during REP.
The relevance of mitochondrial STAT3-mediated cardioprotection during ISC-REP translates into longer periods of REP, as confirmed by our results from in vivo model of infarction. MLS-STAT3E mice exhibit almost twice higher post-surgical survival rate compared to WT littermates (Fig. 5a). The survival rate in WT group at the level of 24 % is unexpected and should likely be attributed to the genetic background of the mice used in our model. Both MLS-STAT3E and WT littermates have been backcrossed to 129X1/SvJ background for over 10 generations. In contrast, WT mice on C57BL/6 J genetic background, which our group has used in other projects, exhibit 70–80 % survival rate in the same experimental setting of in vivo ISC-REP. The infarct size in WT C57BL/6 J and 129X1/SvJ strains was similar (Fig. 5b). Thus, factors other than extent of infarction appear to contribute to the strain differences in survival with ISC-REP. The results confirm previous report showing that genetic variables can alter the myocardial response to ISC-REP tissue injury depending on the mouse strain used in the study [16]. The issue of genetic background of animal models in molecular biology studies [14, 28] has far-reaching implications. Nevertheless, our results suggest that the increased survival rate after in vivo regional ischemia, evident even in this high-risk for ISC-REP 129X1/SvJ strain, is likely attributed to the presence of MLS-STAT3E in cardiac mitochondria.
The dramatically low survival rate observed in WT littermates complicates further plans for in vivo studies, including assessment of cardiac function, characterization of mitochondrial function after longer periods of REP. Despite the low survival rate of WT littermates, we were able to assess infarct size following 24 h of REP by increasing number of animals in the WT littermate group. MLS-STAT3E hearts sustain 50 % less damage to the myocardium compared to WT littermates. These data clearly demonstrate the MLS-STAT3E-mediated protection against ISC-REP-induced damage even following the prolonged period of REP. These results also confirm the initial ex vivo observations.
In conclusion, the myocardial injury is one of the major causes of morbidity and mortality in western nations. MLS-STAT3E mice are used here as an experimental tool to investigate the relationship between a persistent, partial blockade of mitochondrial electron transport during ISC-REP and the decreased myocardial tissue injury. Results presented in this manuscript indicate that mitochondrial STAT3 contributes to cardioprotection during ISC-REP, potentially through the attenuation of MPTP opening and cytochrome c release at early REP and decreased mitochondrial ROS generation during more prolonged periods of REP. A greater understanding of the role of STAT3 in cardioprotection will aid the development of novel molecular and pharmacological strategies to attenuate ISC-REP injury. Based on the mechanistic insights from transgenic MLS-STAT3E mouse model, a pharmacological approach to harness endogenous STAT3 in non-transgenic mitochondria to exhibit comparable protective effect as observed in MLS-STAT3E should be considered as an area of future studies. The use of a pharmacologic agent to augment mitochondrial STAT3 potential, while at the same time activating also the transcriptional activity of STAT3, is likely to provide a novel cardioprotective strategy that utilizes the acute mitochondrial modulation in concert with transcriptional regulation to decrease myocardial ISC-REP injury and improve the prognosis of patients suffering from myocardial infarction. The search for such an agent is ongoing.
References
Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK (2008) Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res 77:406–415. doi:10.1016/j.cardiores.2007.08.008
Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R (2008) Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102:131–135. doi:10.1161/CIRCRESAHA.107.164699
Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R (2008) The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120:172–185. doi:10.1016/j.pharmthera.2008.08.002
Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R (2010) Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol 105:771–785. doi:10.1007/s00395-010-0124-1
Boengler K, Ungefug E, Heusch G, Schulz R (2013) The STAT3 inhibitor stattic impairs cardiomyocyte mitochondrial function through increased reactive oxygen species formation. Curr Pharm Des 19:6890–6895. doi:10.2174/138161281939131127115940
Borutaite V, Brown GC (2003) Mitochondria in apoptosis of ischemic heart. FEBS Lett 541:1–5. doi:10.1016/S0014-5793(03)00278-3
Borutaite V, Budriunaite A, Morkuniene R, Brown GC (2001) Release of mitochondrial cytochrome c and activation of cytosolic caspases induced by myocardial ischaemia. Biochim Biophys Acta 1537:101–109. doi:10.1016/S0925-4439(01)00062-X
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2006) Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319:1405–1412. doi:10.1124/jpet.106.110262
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ (2008) Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294:C460–C466. doi:10.1152/ajpcell.00211.2007
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031. doi:10.1074/jbc.M304854200
Cohen MV, Yang XM, Downey JM (2007) The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation 115:1895–1903. doi:10.1161/CIRCULATIONAHA.106.675710
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249. doi:10.1016/S0022-2828(03)00043-9
Darnell JE Jr (1997) STATs and gene regulation. Science 277:1630–1635. doi:10.1126/science.277.5332.1630
Doetschman T (2009) Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol 530:423–433. doi:10.1007/978-1-59745-471-1_23
Fuglesteg BN, Suleman N, Tiron C, Kanhema T, Lacerda L, Andreasen TV, Sack MN, Jonassen AK, Mjos OD, Opie LH, Lecour S (2008) Signal transducer and activator of transcription 3 is involved in the cardioprotective signalling pathway activated by insulin therapy at reperfusion. Basic Res Cardiol 103:444–453. doi:10.1007/s00395-008-0728-x
Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, Bolli R (2012) Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarct size in 1074 mice. Basic Res Cardiol 107:288. doi:10.1007/s00395-012-0288-y
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 61:372–385. doi:10.1016/S0008-6363(03)00533-9
Halestrap AP, Connern CP, Griffiths EJ, Kerr PM (1997) Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174:167–172. doi:10.1023/A:1006879618176
Hausenloy DJ, Ong SB, Yellon DM (2009) The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol 104:189–202. doi:10.1007/s00395-009-0010-x
Hedayati N, Schomisch SJ, Carino JL, Timothy Sherwood J, Lesnefsky EJ, Cmolik BL (2003) Cardioprotection by St Thomas’ solution is mediated by protein kinase C and tyrosine kinase. J Surg Res 113:121–127. doi:10.1016/S0022-4804(03)00146-X
Heusch G, Musiolik J, Gedik N, Skyschally A (2011) Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109:1302–1308. doi:10.1161/CIRCRESAHA.111.255604
Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128:589–600. doi:10.1016/j.cell.2006.12.036
Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, Opie LH (2005) Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase). Circulation 112:3911–3918. doi:10.1161/CIRCULATIONAHA.105.581058
Lee Y, Lee HY, Gustafsson AB (2012) Regulation of autophagy by metabolic and stress signaling pathways in the heart. J Cardiovasc Pharmacol 60:118–124. doi:10.1097/FJC.0b013e318256cdd0
Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL (1997) Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol 273:H1544–H1554
Levy DE, Lee CK (2002) What does Stat3 do? J Clin Invest 109:1143–1148. doi:10.1172/JCI15650
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marques SM, Campos PP, Castro PR, Cardoso CC, Ferreira MA, Andrade SP (2011) Genetic background determines mouse strain differences in inflammatory angiogenesis. Microvasc Res 82:246–252. doi:10.1016/j.mvr.2011.08.011
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922. doi:10.1161/01.RES.0000261924.76669.36
Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS (2009) In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol 46:960–968. doi:10.1016/j.yjmcc.2009.01.012
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803. doi:10.1083/jcb.200809125
Natarajan SK, Becker DF (2012) Role of apoptosis-inducing factor, proline dehydrogenase, and NADPH oxidase in apoptosis and oxidative stress. Cell Health Cytoskelet 2012:11–27. doi:10.2147/CHC.S4955
Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K (2001) Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104:979–981. doi:10.1161/hc3401.095947
Oshima Y, Fujio Y, Nakanishi T, Itoh N, Yamamoto Y, Negoro S, Tanaka K, Kishimoto T, Kawase I, Azuma J (2005) STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res 65:428–435. doi:10.1016/j.cardiores.2004.10.021
Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M (2009) Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol 46:902–909. doi:10.1016/j.yjmcc.2009.02.017
Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, Sofroniew MV (2010) Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One 5:e9532. doi:10.1371/journal.pone.0009532
Smith CC, Dixon RA, Wynne AM, Theodorou L, Ong SG, Subrayan S, Davidson SM, Hausenloy DJ, Yellon DM (2010) Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol 299:H1265–H1270. doi:10.1152/ajpheart.00092.2010
Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, Sack MN (2004) Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res 63:611–616. doi:10.1016/j.cardiores.2004.06.019
Stewart S, Lesnefsky EJ, Chen Q (2009) Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res 153:224–231. doi:10.1016/j.trsl.2009.02.003
Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC (2011) Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem 286:29610–29620. doi:10.1074/jbc.M111.226209
Toldo S, Das A, Mezzaroma E, Chau VQ, Marchetti C, Durrant D, Samidurai A, Van Tassell BW, Yin C, Ockaili RA, Vigneshwar N, Mukhopadhyay ND, Kukreja RC, Abbate A, Salloum FN (2014) Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circulation Cardiovasc Genet 7:311–320. doi:10.1161/CIRCGENETICS.113.000381
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC (2009) Function of mitochondrial Stat3 in cellular respiration. Science 323:793–797. doi:10.1126/science.1164551
Xu A, Szczepanek K, Maceyka MW, Ross T, Bowler E, Hu Y, Kenny B, Mehfoud C, Desai PN, Baumgarten CM, Chen Q, Lesnefsky EJ (2014) Transient complex I inhibition at the onset of reperfusion by extracellular acidification decreases cardiac injury. Am J Physiol Cell Physiol 306:C1142–C1153. doi:10.1152/ajpcell.00241.2013
Zhang Q, Raje V, Yakovlev VA, Yacoub A, Szczepanek K, Meier J, Derecka M, Chen Q, Hu Y, Sisler J, Hamed H, Lesnefsky EJ, Valerie K, Dent P, Larner AC (2013) Mitochondrial localized Stat3 promotes breast cancer growth via phosphorylation of serine 727. J Biol Chem 288:31280–31288. doi:10.1074/jbc.M113.505057
Acknowledgments
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (to E.J.L.), the American Heart Association Postdoctoral Fellowship Award (to K.S.), the American Heart Association Scientist Development Grant (to Q.C.), American Heart Association Scientist Development Grant and Grant in Aid (to F.N.S.) and the Pauley Heart Center, Virginia Commonwealth University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2015_509_MOESM1_ESM.tif
Supplementary material 1 Supplemental Fig.S1 At later reperfusion, MLS-STAT3E does not alter PAR polymers formation. Equal amounts of cytosolic extracts from ISC-REP and time control hearts from WT and MLS-STAT3E mice were resolved by SDS-PAGE, transferred to PVDF membrane and probed for PAR (Merck Millipore, Billerica, MA). GAPDH was used as protein loading control. Densitometry was measured using ImageJ (NIH, Bethesda) and expressed as ratio of probed protein signal to GAPDH signal and normalized to time control set as 1. Results are mean ± SEM, n = 6. *P < 0.05 vs. corresponding time control. The dotted bars reflect the data obtained under ISC-REP conditions. (TIFF 465 kb)
395_2015_509_MOESM2_ESM.tif
Supplementary material 2 Supplemental Fig.S2 STAT3 serine 727 phosphorylation status in the mitochondria from MLS-STAT3E and WT hearts subjected to 35 min ISC and 60 min REP. 30 μg of mitochondrial protein from WT hearts and 5 μg of mitochondrial protein from MLS-STAT3E hearts were separated by SDS-PAGE, transferred to PVDF membrane and probed for phosphorylated STAT3 serine 727 (p-Ser727-STAT3) and total STAT3. Porin was used as protein loading control. A representative immunoblot of three independent experiments is shown. (TIFF 1688 kb)
395_2015_509_MOESM3_ESM.tif
Supplementary material 3 Supplemental Fig.S3 MLS-STAT3E attenuates MPTP during early REP. MLS-STAT3E and WT hearts were subjected to ex vivo Langendorff model of 35 min ISC and 10 or 60 min REP followed by mitochondria isolation and assessment of Calcium Retention Capacity (CRC). CRC was evaluated in mitochondria by sequential pulses of calcium (5 nmol) in a presence of calcein green indicator. Results are mean ± SEM, n = 4. *P < 0.05 vs. 10 min REP, #P < 0.05 vs. WT. (TIFF 483 kb)
395_2015_509_MOESM4_ESM.tif
Supplementary material 4 Supplemental Fig.S4 MLS-STAT3E attenuates cytochrome c release into cytosol during early REP. Equal amounts of cytosolic extracts from WT and MLS-STAT3E hearts subjected to ex vivo Langendorff model of 35 min ISC and 10 REP were resolved by SDS-PAGE, transferred to PVDF membrane and probed for cytochrome c. GAPDH was used as protein loading control. Densitometry was measured using ImageJ (NIH, Bethesda) and expressed as ratio of probed protein signal to GAPDH signal. Results are mean ± SEM, n = 4. *P < 0.05 vs. WT. (TIFF 776 kb)
Rights and permissions
About this article
Cite this article
Szczepanek, K., Xu, A., Hu, Y. et al. Cardioprotective function of mitochondrial-targeted and transcriptionally inactive STAT3 against ischemia and reperfusion injury. Basic Res Cardiol 110, 53 (2015). https://doi.org/10.1007/s00395-015-0509-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0509-2