Abstract
The inflammatory sequelae of ischemia–reperfusion injury (IRI) are a major causal factor of tissue injury in various clinical settings. MicroRNAs (miRs) are short, non-coding RNAs, which regulate protein expression. Here, we investigated the role of miR-155 in IR-related tissue injury. Quantifying microRNA-expression levels in a human muscle tissue after IRI, we found miR-155 expression to be significantly increased and to correlate with the increased expression of TNF-α, IL-1β, CD105, and Caspase3 as well as with leukocyte infiltration. The direct miR-155 target gene SOCS-1 was downregulated. In a mouse model of myocardial infarction, temporary LAD ligation and reperfusion injury resulted in a smaller area of necrosis in miR-155−/− animals compared to wildtype animals. To investigate the underlying mechanisms, we evaluated the effect of miR-155 on inflammatory cell recruitment by intravital microscopy and on the generation of reactive oxygen species (ROS) of macrophages. Our intravital imaging results demonstrated a decreased recruitment of inflammatory cells in miR-155−/− animals during IRI. The generation of ROS in leukocytic cells of miR-155−/− animals was also reduced. RNA silencing of the direct miR-155 target gene SOCS-1 abrogated this effect. In conclusion, miR-155 aggravates the inflammatory response, leukocyte infiltration and tissue damage in IRI via modulation of SOCS-1-dependent generation of ROS. MiR-155 is thus a potential target for the treatment or prevention of IRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemia reperfusion injury (IRI) describes a harmful inflammatory process in every clinical setting in which blood flow is restored after a critical time of ischemia, such as acute myocardial infarction, stroke, and transplantation surgery. While inflammation is an important component of the host defence reaction against external pathogens and injury, the unrestricted post-ischemic inflammatory response can have detrimental effects [41, 55], contributing to cell death and even exceeding the initial ischemic injury. This is mediated by increased recruitment of circulating cells and local generation of reactive oxygen species, driving a local inflammatory reaction that can be responsible for more than 50 % of infarct size in acute coronary syndromes [55] and is also a crucial determinant of transplant survival of organs and surgical-free tissue transfer.
Many attempts to modulate this harmful inflammatory response to prevent or reduce tissue injury were made in the past. However, the basic regulatory principles of IRI remain little understood and it seems that the inhibition of an individual component of the inflammatory cascade, e.g., by a single antibody or small molecule inhibitor may not be enough to achieve a significant effect on IRI and cell survival in the injured tissue [15, 17].
Recently, microRNAs (miRNAs) have been identified as important regulators of gene expression in a wide range of biological processes. MiRNAs are short, non-coding RNA molecules of 17–23 nucleotides length, that regulate protein expression by recognizing sequences mostly located in the 3′-untranslated regions of target-messenger RNAs (mRNAs) via imperfect base pairing, thereby inducing translational repression or mRNA degradation [1].
There is emerging evidence that miRNAs also control vascular inflammation [14] and modulate leukocyte adhesion to endothelial cells.
In how far miRNAs are involved in IRI remains largely unclear: While first studies suggest a potential modulatory function of these small RNAs, reporting both aggravating as well as protective effects [16, 19, 53], other studies argue against an important pathophysiological role of miRNAs in IRI and ischemic postconditioning in larger mammals [3].
In the present study, we now hypothesized that miRNAs modulate the inflammatory response after ischemia–reperfusion and that differential miRNA expression contributes to tissue injury. We identified miR-155 as an upregulated miRNA in human biopsy samples obtained before and after IRI of free muscle grafts for plastic reconstruction of tissue defects and went on to characterize its effect on leukocyte recruitment, generation of ROS, and IRI in a mouse model of myocardial infarction. In summary, we discovered that miR–155 deficient mice are relatively protected from IRI, suggesting inflammatory miRNAs as potential therapeutic targets for tissue protection from ischemia and reperfusion injury.
Patients, materials, and methods
The study was approved by the ethics committee of the University of Freiburg Medical Centre. The study is registered as DRKS00007906 at the “Deutsche Register Klinischer Studien”, a WHO registered clinical registration platform.
Patient samples and tissue preparation
Human striated muscle biopsies of 0.5 × 0.5 × 0.5 cm were obtained from patients receiving free muscle flap surgery (latissimus dorsi, gracilis or rectus abdominis flap). Informed consent was obtained from each patient.
Biopsies were taken during the surgery before interruption of the blood supply to the flap and 5 days after surgery [7, 8]. Samples for RNA extraction were stored in RNA-later (Qiagen, Hilden, Germany) for 24 h at 4 °C and at −80 °C until use [7]. Samples for histological examination were embedded in Tissue Tek® OCT™ and snap-frozen in liquid nitrogen, then stored at −80 °C for at least 24 h and cut into 10-µm thick slices using a cryotome.
Immunofluorescence staining
For immunofluorescence staining of human and murine samples, slices were processed as described before [13] and incubated with antibodies against human CD68 (Dako, Hamburg, Germany), murine Mac-3 (BD Pharmingen, San Diego, USA), and murine Ly-6G (Abcam, Cambridge, United Kingdom), respectively. Subsequently, the sections were incubated with appropriate Cy3-conjugated secondary antibodies (Jackson Immunoresearch, Baltimore Pike, USA and Chemicon Merck®, Billerica, USA) and Hoechst 33258 (Sigma, Steinheim, Germany) or DAPI (Sigma, Steinheim, Germany) and embedded in moviol. Sections treated with the same protocol but without a primary antibody were used as negative controls. The sections were analyzed using an Axiovert microscope and an Axiocam digital camera (Leica, Wetzlar, Germany). For evaluation of post-ischemic transmigration of monocytes and macrophages in human striated muscle, the number of CD68 positive cells was normalized to the number of muscle cells in the same area. Transmigrated monocytes/macrophages were quantified by blinded examiners. In murine sections, an area of interest within the muscle tissue was defined and the number of Mac-3 positive cells was normalized to the size of the area of interest.
RNA isolation
Isolation of RNA from human striated muscle was performed using TriPure® modified phenol–chloroform-extraction (Applied Biosystems ™Roche, Branchburg, USA) according to the manufacturer’s protocol. RNA was isolated from murine bone marrow derived macrophages (BMDM) using the miRNeasy® Mini Kit (Quiagen, Hilden, Germany) according to the manufacturer’s protocol. The concentration of mRNA was measured using the Spectrophotometer ND-1000 (Thermo Fischer Scientific, Wilmington, USA) and individually diluted in RNAse-free water to achieve equal mRNA concentrations in all samples.
MicroRNA TaqMan RT-PCR
Expression of miR-155 in human striated muscle was measured using the TaqMan® MicroRNA Assay (Qiagen, Hilden, Germany) according to the manufacturer’s protocol with specific primers for the reverse transcriptase reaction and TaqMan probes for the real-time PCR. The expression of miR-155 was normalized to rnu19.
cDNA synthesis
1 µg RNA of each sample was transcribed to cDNA using the iScript™ cDNA-synthesis kit (Bio-Rad, Munich, Germany) according to the manufacturer’s protocol.
mRNA expression with quantitative real-time PCR
Quantitative real-time PCR was performed using either TaqMan probes or SYBR-Green. SYBR-Green PCR was performed as previously described [42] using iQ™ SYBR®-Green Supermix (Bio-Rad, München, Germany) and the following primers: hsa-Interferone γ (forward: ACTGACTTGAATGTCCAACGCA, reverse: ATCTGACTCCTTTTTCGCTTCC), hsa-caspase 3 (forward: AGAACTGGACTGTGGCATTGAG, reverse:GCTTGTCGGCATACTGTTTCAG), mmu-Interleukine 1β (forward: CAACCAACAAGTGATATTCTCCATG, reverse: GATCCACACTCTCCAGCTGCA). Ct values were normalized to that of the housekeeping gene hrpII (forward: GCACCACGTCCAATGACAT, reverse: GTGCGGCTGCTTCCATAA).
TaqMan PCR was performed as described previously [7] using ABsolute QPCR ROX Mix (Thermo Scientific, Epsom, Surrey, UK). Ct values were normalized to that of the housekeeping gene GAPDH. TaqMan probes including primer sets for IL-1β, TNF-α, Endoglin, and GAPDH were obtained commercially (Applied Biosystems, Foster City, USA).
Assays were performed in either triplicates or duplicates. Negative controls were run using diethylpyrocarbonate-H2O. Relative expression was calculated using the ΔΔC t-method.
Murine cremaster model
Male wildtype (WT) (B57BL/6J) and miR-155−/− (B6.Cg-Mirn155tm1.1Rsky/J) mice were purchased from Charles River (Sulzfeld, Germany). All experiments were performed according to the “Guide for Care and Use of Laboratory Animals“(National Research Council, 2010).
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) (Intervet, Unterschleißheim, Germany) and xylazine (2 mg/kg) (Bayer, Leverkusen, Germany). The left jugular vein was cannulated for injection of a rhodamine 6 G solution (1 mg/ml) (Sigma, Steinheim, Germany) for intravenous leukocyte staining. The cremaster muscle was prepared for intravital microscopy as described previously [4]. Thereby, the cremaster muscle was exposed by ventral incision of the scrotum and opened ventrally, using careful electric cautery to stop bleeding. The testis and epididymis were detached from the cremaster muscle and placed into the abdominal cavity. The cremaster muscle was spread on a custom made Plexiglas pedestal und continuously perfused throughout the procedure with warm 0.9 % saline. The body temperature of the mice was maintained using a heating pad. Intravital microscopy was performed using a Zeiss Axio Tec Vari microscope (Carl Zeiss, Jena, Germany) with a green filter connected to a Zeiss Axiocam MRm digital camera (Carl Zeiss, Jena, Germany). All videos were recorded and evaluated using Zeiss Axiovision 4.8.2 software (Carl Zeiss, Jena, Germany). Venules of 20–40 µm diameter were observed and rolling and adhering leukocytes were quantified by blinded investigators.
Following intravital microscopy, the cremaster muscle was surgically removed, embedded in Tissue Tek® OCT™ (Sakura Finetek, Alphen, Netherlands), snap-frozen in liquid nitrogen, stored at −80 °C for at least 24 h, and cut into 7 µm thick slices using a Leica cryotome CM 1510 S (Leica, Wetzlar, Germany).
Murine LAD ligation model
Murine LAD ligation was performed as previously desribed [27] in WT and miR-155−/− mice. Samples were taken from the unaffected myocardium and the area of infarction 48 h after induction of myocardial infarction by surgical ligation of the left anterior coronary artery (LAD) following reperfusion 30 min after the ligation (IR). The area at risk (AAR) was identified by perfusing the heart with phthalocyanine blue. A cannula was fixed in the aortic stump with the tip laying distal of the aortic valve. The ligation of the LAD prevented the perfusion of the area distal of the ligation. Thus, the area, referred to as AAR, remained unstained whereas the unaffected myocardium was stained blue. For the measurement of the infarct size, the hearts were cut into 2 mm thick slices and incubated in 1 % triphenyltetrazolium chloride (TTC) in 0.9 % NaCl at 37 °C for 10 min, which stained non-necrotic areas red, while the area of necrosis (AON) remained unstained. Slices were photographed and areas were manually countered using ImageJ. The ratios of area at risk/total area (AAR/TA) and AON/AAR were obtained by dividing the sum of the measurements of all slices of each individual heart.
Murine bone marrow derived macrophages
Bone marrow cells were isolated from the femur bones of WT (B57BL/6 J) and miR-155−/− (B6.Cg-Mirn155tm1.1Rsky/J) mice. Therefore, the bone marrow was flushed with 0.9 % NaCl. The obtained suspension was centrifuged and erythrocytes were lysed using lysis buffer. The remaining cells were again centrifuged, resuspended in RPMI medium (Invitrogen, Carlsbad, USA) with 10 % fetal bovine serum (FBS) (Invitrogen, Carlsbad, USA), 1 % non-essential amino acids PAN™ Biotech, Aidenbach, Germany), and 2 % Penicillin/Streptomycin (Carl Roth, Karlsruhe, Germany) and sown in 6 well plates to 1 × 106 cells per well. The cells were differentiated to macrophages using 30 ng/ml monocyte colony stimulating factor 1 (MCSF-1) (PreProTech, Rocky Hill, USA) for 72 h. The success of the procedure was verified using fluorescence-activated cell sorting (FACS) (FACS Canto II, BD Bioscienences, San Jose, USA) after the cells were labeled with antibodies against F4/80 (AbD Serotec, Oxford, UK) or, as a negative control, IgG2b (Dako, Hamburg, Germany).
Transfection
BMDMs were transfected with SOCS-1 siRNA using Lipofectamin™ RNAiMAX (all by Invitrogen™, Carlsbad, USA) as described previously [12].
THP1 cells transfected with premiR-155, premiR-scrambled, antimiR-155, or antimiR-scrambled using 5 µl Lipofectamin™ RNAiMAX (all by Invitrogen™, Carlsbad, USA) as described previously [12].
Measurement of ROS production
For measurement of ROS production in native miR-155−/− and WT BMDMs, as well as miR-155−/− BMDMs transfected with SOCS-1 siRNA or scrambled siRNA, the cells were stimulated using 100 ng/ml PMA for 20 min, simultaneously incubated with 10 µM Dichloro-dihydro-fluorescein-diacetat (DCFH-DA) and detached using Accutase (invitrogen™, Carlsbad, USA). ROS production was finally measured by flow cytometry using FACS Canto II (BD Biosciences, San Jose, USA). The negative control was only incubated with DCFH-DA.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of Interleukin-1β (IL-1β) was determined in native protein lysates of lipopolysaccharide (LPS) (Sigma, Hilden, Germany) stimulated (10 ng/ml, 24 h) BMDMs using a murine IL-1β ELISA kit (Preprotech, Hamburg, Germany). Prior to measurement of IL-1β mRNA expression, the BMDMs were stimulated for 24 h using 10 ng/ml LPS.
Statistical analysis
All values are indicated as standard error of the mean. Gaussian distribution could not be verified due to small sample size, hence either Mann–Whitney U test or Wilcoxon log-rank test were conducted. P < 0.05 was considered statistically significant.
Results
Human studies
Free microsurgical tissue transfer induces significant ischemia–reperfusion injury
Striated muscle tissue showed a significant inflammatory response after ischemia/reperfusion that was attributable to IRI and led to the recruitment of inflammatory cells (Fig. 1a, b). Expression of the pro-inflammatory cytokines TNF-α, Interferon γ and IL-1β was significantly increased (Fig. 1c–e). Furthermore, Caspase 3 expression as parameter of apoptotic cell death and therefore surrogate parameter for the degree of tissue injury and Endoglin (CD105) as parameter for angiogenesis were significantly upregulated after IRI (Fig. 1f–g).
a CD68 immunofluorescence detection of leucocyte invasion in human striated muscle tissue 5 days after free-flap surgery. Typical results are shown. b Quantification of immunofluorescence results. Number of transmigrated leucocytes increased significantly 5 days after free-flap surgery (n = 11). c–g Relative mRNA expression of Tumor necrosis factor α (n = 10), Interferon γ (n = 10), Interleukin-1β (n = 10), Caspase 3 (n = 12), and Endoglin (CD105) (n = 10) were significantly increased in human striated muscle tissue 5 days after free-flap surgery. Values represent mean ± SEM (*P < 0.05)
Ischemia–reperfusion injury induces the upregulation of miR-155 in human striated muscle tissue
In 10 human muscle tissue samples of different patients, we found a significant upregulation of miR-155 5 days after ischemia and reperfusion by TaqMan PCR of miR-155 expression levels when compared to expression levels before ischemia (Fig. 2a). Interestingly, expression of suppressor of cytokine signaling 1 (SOCS-1) was significantly downregulated after IRI (Fig. 2b).
a Relative expression of miR-155 in human striated muscle tissue increased significantly 5 days after free-flap surgery (n = 14). b Relative expression of SOCS-1 decreased significantly 5 days after free-flap surgery (n = 14). c Relative miR-155 expression increased in hearts of mice after ligation of the left anterior coronary artery (LAD) for 30 min and recovery (I/R) for 5 days after induction of myocardial infarction (n = 4). d Relative miR-155 expression increased in the CD11b positive cell fraction in hearts of mice after ligation of the LAD for 30 min and recovery (I/R) for 5 days after induction of myocardial infarction (n = 4). Values represent mean ± SEM, *P < 0.05
Studies with mouse models
Following these interesting results in human muscle biopsie samples, we went on to investigate the expression of miR-155 in a model of ischemia reperfusion in mice. In analogy to the results obtained in human tissue, we found a significant increase of miR–155 expression level in animals undergoing temporary LAD occlusion compared to sham-operated animals 5 days after reperfusion (Fig. 2c). To determine the cell fraction which contributed to enhanced miR-155 expression level following reperfusion, we used antibody-coupled magnetic beads to isolate the CD11b positive cell fraction from sham and IR injured hearts and determined miR-155 expression by stemloop-based TaqMan PCR. MiR-155 expression was significantly increased in CD11b positive cells under reperfusion conditions (Fig. 2d).
MiR-155 deficiency protects from IRI-induced tissue necrosis and attenuates the infiltration of inflammatory cells in a mouse model of temporary LAD occlusion
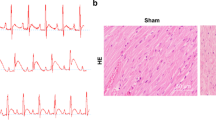
The clinical consequence of miR-155 in IRI was investigated in a mouse model of myocardial infarction using temporary LAD occlusion (Fig. 3a). Exemplary pictures of stained and sliced hearts of WT and miR-155−/− mice with the unaffected myocardium (blue), the area at risk (AAR, red), and the necrotic tissue (white, exemplary highligted by dashed lines in the third section) are shown in Fig. 3b. Next, we compared the AAR and the necrotic tissue area in WT mice to miR-155−/− animals by planimetry after phthalocyanine blue and TTC staining (Fig. 3c). We found a small, albeit significant reduction of the area of necrosis (AON) to AAR ratio in miR–155−/− animals (Fig. 3e), indicating a protective effect of miR-155 deficiency, whereas the AAR remains similar (Fig. 3d). Infiltration of neutrophils and macrophages in the AAR was visualized by Ly-6G and Mac-3 staining, respectively (Fig. 4a–c). In miR-155−/− hearts, we found a significant decrease in the number of infiltrating neutrophils and macrophages (Fig. 4b–d) compared to WT hearts.
Reduced area of necrosis in miR-155 knock out hearts after ligation of the left anterior coronary artery (LAD) for 30 min and recovery (I/R) for 48 h after induction of myocardial infarction. a Exemplary picture of a ligated heart with the affected tissue distal of the ligation (white area right panel) and the unaffected tissue (back side left panel). b Representative pictures of sliced miR-155−/− and wildtype hearts after I/R injury from the base to the apex. Unaffected tissue is stained blue by phthalocyanine blue infiltration. By incubating sliced hearts in TTC, non-necrotic areas of the area at risk (AAR) were stained red while the area of necrosis (AON) remained unstained. c Midventricular slice with schematic of manual infarct planimetry. AAR (non-blue area 19 % of total area) and AON (white area 40 % of the AAR) were manually contoured. d No significant difference could be detected in the size of the AAR relative to the total area (TA) (n ≥ 12). e The size of the AON relative to the AAR is significantly reduced in miR-155 deficient mice 48 h post myocardial I/R injury compared to WT mice (n = 14). Values represent mean ± SEM, *P < 0.05
Neutrophils and macrophage abundance in miR-155−/− hearts after I/R injury (30 min LAD ligation and ensuing 48 h reperfusion). A series of heart sections was stained with anti Ly-6G antibody to detect neutrophils (a red) and with anti Mac-3 antibody to visualize macrophages (c red), nuclei were stained with DAPI (blue). For each heart, the number of neutrophils (b) and macrophages (d) was quantified on 5 serial sections starting from the point of ligation and progressing toward the apex (n ≥ 4). Less infiltrating neutrophils and macrophages were detected in miR-155 deficient hearts compared to wildtype hearts. Values represent mean ± SEM, *P < 0.05
Leukocyte rolling and adhesion in IRI is significantly reduced in miR-155−/− compared to WT animals
We analyzed the impact of miR-155 expression on inflammatory cell recruitment by intravital microscopy in an established IRI model of the cremaster muscle [45] (Fig. 5). A schematic timeline of the course of these experiments is given in Fig. 5h. 30 min of ischemia lead to a significant increase in rolling and adherent leukocytes in WT animals as a hallmark of IRI (Fig. 5b–e). These pro-inflammatory effects were markedly reduced in miR-155−/− animals compared to WT controls (Fig. 5c–f), with a most prominent effect at 15 min (rolling) and 30 min (adhesion) after reperfusion (Fig. 5d–g).
a Intravital microscopy of leucocyte–endothelium interaction in postcapillary venules before and after 30 min of ischemia and 15 min of reperfusion or 30 min of ischemia and 30 min of reperfusion in murine cremaster muscle. Intravenous staining of leucocytes with rhodamine. Representative results are shown. Quantitative results of leucocyte–endothelium interaction before and after 30 min of ischemia (n ≥ 6). IRI lead to increased numbers of rolling (b) and adherent (e) leucocytes on the endothelium. The increase of rolling (c) and adherent (f) cells is attenuated in miR-155−/− mice. d The number of rolling leukocytes is significantly higher in WT mice compared to miR-155−/− mice at 15 min of reperfusion. g The number of adherent leucocytes is significantly higher in WT mice compared to miR-155−/− mice at 30 min of reperfusion. h Flow chart of the experimental procedure. Values represent mean ± SEM, *P < 0.05
Leukocyte transmigration in IRI is significantly reduced in miR-155−/− compared to WT animals
After intravital microscopy, the cremaster muscle was analyzed by immunohistochemistry for leukocyte transmigration (Fig. 6a). In line with the findings obtained for leukocyte rolling and adhesion, WT animals showed an increase in infiltrating leukocytes after IRI as detected by Mac-3 staining. Tissue positive for Mac-3 was significantly reduced after IRI in miR-155−/− animals as confirmed by quantitative analysis of Mac-3 positive cells/100.000 μm2 (Fig. 6b). Importantly, there was no difference in the number of tissue resident Mac-3 positive cells at baseline between the two groups in the sham-operated animals.
a Mac-3 immunofluorescence detection of leucocytes invasion in murine cremasteric muscle after 30 min of ischemia and 120 min of reperfusion. Typical results are shown. b Quantification of immunofluorescence results. Number of transmigrated leucocytes increased significantly after 30 min of ischemia and 120 min of reperfusion in WT mice. Number of transmigrated leucocytes did not increase after 30 min of ischemia and 120 min of reperfusion in miR-155−/− mice (n ≥ 5). Values represent mean ± SEM, *P < 0.05
Pro-inflammatory cytokine/chemokine production is impaired in miR-155 deficient BMDM
As our initial characterization of the IRI in free-flap tissue had shown a prominent increase of IL-1β during IRI, we analyzed whether the expression of this pro-inflammatory mediator was altered in bone marrow derived macrophages (BMDM) of miR-155 deficient mice. While baseline expression was similar to wildtype cells, we detected a significant attenuation of IL-1β expression in miR-155−/− cells in response to LPS-stimulation on both mRNA (Fig. 7a) and protein level (Fig. 7b), indicating that miR-155 is directly modulating the post-ischemic inflammatory response detected in post-ischemic tissues. This is in good correspondence with our findings in human biopsy samples after IRI, where we found both miR-155 and IL-1β to be increased. Additionally, we observed an impaired TNF-alpha as well as MCP-1 expression under pro-inflammatory PMA stimulation in BMDM if miR-155 was lacking (Fig. 7c–d). Interestingly, we could rescue this impairment by transfection of miR-155-specific precursor molecules, indicating the dependency of pro-inflammatory cytokine/chemokine expression on miR-155 expression (Fig. 7e–f).
Expression of pro-inflammatory cytokines and chemokines in bone marrow derived macrophages (BMDM). a Relative mRNA expression in miR-155−/− BMDM was significantly reduced compared to WT BMDM after stimulation with LPS (n = 12). b ELISA analysis of IL-1β showed significantly lower expression in miR-155−/− BMDM compared to WT BMDM after stimulation with LPS (n = 4). Relative mRNA expression of TNF-α (c) and MCP-1 (d) is impaired under pro-inflammatory PMA stimulation (n = 6). The disturbed MCP-1 (e) and TNF-α (f) expression in miR-155−/− BMDM could be rescued by transfection of miR-155-specific precursor molecules (n = 9). g Increased ROS generation after PMA stimulation in BMDMs derived from either WT or miR-155−/− animals, detected by DCFH-DA staining in flow cytometry. This effect was significantly reduced in miR-155−/− animals (n = 7). Values represent mean ± SEM, *P < 0.05
miR-155 enhances generation of ROS in inflammatory cells
Generation of ROS in inflammatory cells is a major mechanism of tissue injury in the inflammatory response. In BMDMs derived from either WT animals or miR-155−/− animals stimulation with PMA led to a significantly increased ROS generation as detected by DCFH-DA staining in flow cytometry. This effect was significantly reduced in miR-155−/− animals (Fig. 7g).
miR-155 mediates its effects in circulating cells by suppressing SOCS-1
Besides the diminished cytokine/chemokine and ROS production in BMDMs lacking miR-155, we found that SOCS-1 expression was not significantly effected following PMA stimulation compared to basal culture conditions (Fig. 8a), suggesting the negative regulator of JAK/STAT signaling as a possible mediator of the observed miR-155 effects. Indeed, we found highly increased SOCS-1 expression level in miR-155 deficient BMDM compared to wildtype cells on mRNA (Fig. 8b) and protein expression level (Fig. 8c–d). We went on to investigate if silencing of SOCS-1 expression level could rescue the phenotype of BMDM lacking miR-155. Using an established transfection protocol, we could achieve a significicant downregulation of SOCS-1 on mRNA (Fig. 8e) and protein expression level (Fig. 8f–g) following transfection of SOCS-1-specific siRNAs. Indeed, RNA silencing of SOCS-1 in miR-155−/− BMDM led to an increase of ROS production, suggesting that miR-155 negatively regulates SOCS-1 that in turn is modulating ROS generation presumably via cytokine expression (Fig. 8h).
miR-155 suppresses SOCS-1 expression in BMDM. a Relative mRNA expression of SOCS-1 is disturbed in miR-155−/− BMDM stimulated with PMA (n = 6). Increased SOCS-1 mRNA expression (b) and protein level (c) in miR-155 deficient cells compared to WT cells under basal conditions (n = 6). d Quantification of SOCS-1 protein level observed in (c). Silencing of SOCS-1 by transfection with SOCS-1-specific siRNAs could rescue the phenotype of BMDM lacking miR-155 at the mRNA level (e) and the protein level (f) (n ≥ 3). g Quantification of SOCS-1 protein level observed in (f). h RNA silencing of SOCS-1, together with PMA stimulation, led to an increase of ROS production in miR-155−/− BMDM compared to cells silenced with control siRNA (n = 6). Values represent mean ± SEM, *P < 0.05
Discussion
Major findings
Here, we identify the upregulation of the inflammation-related microRNA miR-155 during IRI in a unique human sample collection and characterize the role of miR-155 in the pathogenesis of IRI. Our results point to miR-155 as a potential therapeutic target based on the following findings: (1) In ischemically challenged human striated muscle tissue after free-flap transfer miR-155 expression is significantly upregulated and correlates with markers of inflammation, tissue injury, and leukocyte infiltration. (2) In a mouse model of myocardial infarction, miR-155 deficiency significantly reduces the area of necrosis, indicating a protective effect of the lack of miR-155. (3) MiR-155 modulates leukocyte recruitment in IRI and genetic deficiency for miR-155 leads to decreased rolling, adhesion, and infiltration of inflammatory cells into the post-ischemically inflamed tissue. (4) MiR-155 aggravates cytokine expression and ROS expression in inflammatory cells, a major contributing factor to tissue injury in IRI, partially via suppression of the direct miR-155 target gene SOCS-1.
Selection of miR-155 as target miR
With the aim to analyze inflammation-specific miR expression in ischemia–reperfusion injury, we performed a stem-loop TaqMan PCR quantification of miR-155 using striated muscle tissue derived from reconstructive free-flap surgery. In this surgical procedure, striated muscle tissue is transplanted to other parts of the body and the blood supply is reestablished via microsurgical vessel anastomosis. This tissue represent an attractive human surrogate tissue to investigate the mechanisms of IRI, as the tissue undergoes reproducible times of ischemia and is easily accessible for biopsies and further investigations without risk for the patient. This technique has already been used by us and others to investigate potential treatment concepts aimed to reduce IRI [7, 8, 46]. As these samples represent striated muscle tissue, care has to be taken when extrapolating results obtained in this tissue to the myocardium. However, it is an ideal surrogate model of IRI in human tissue as painless biopsies can be taken on the body surface without the need of invasive procedures.
We focused on miR-155, as this miR has been previously described as a miR with potential pro-inflammatory properties [48], but to the best of our knowledge has not been investigated in the context of ischemia–reperfusion injury so far. MiR-155 is expressed from an evolutionary broadly conserved structure in the B cell Integration Cluster (BIC) gene on chromosome 21. It is involved in a broad spectrum of biological processes including tumor development, inflammation, and immunity. In the cardiovascular field, Martin and coworkers first described miR-155 in 2007 as a translational repressor of angiotensin II type 1 receptor (AGTR1) expression [28]. Recent work has shown that miR-155 is induced in macrophages by cytokines such as TNF-α and IFN-β [33, 47] and that it contributes to physiological granulocyte/monocyte expansion during inflammation [32]. Although miR-155 has previously been shown to have a broad expression pattern in a wide variety of cell types, our analysis of mir-155 expression in isolated CD11b positive cells from the LAD-ligation model confirmed that the increased expression of miR-155 in this infiltrating cell population at least contributes to the overall regulation observed in our initial experiment. In addition, miR-155 is required for B and T lymphocyte dendritic cell function [37, 50]. Mechanistically, the transcription factor Pu.1 has been identified as direct target of miR-155 in B cells [50]. Thus, miR-155 seemed to be a promising candidate miRNA for investigation in the context of IRI-induced inflammation.
Regulation of inflammation by miR-155
Our results do not only identify miR-155 as a contributing factor to tissue injury in IRI and myocardial infarct, but also provides the mechanisms by which these detrimental effects are regulated. In the inflammatory sequelae leading to tissue injury after ischemia reperfusion, the major role of pathologically altered leukocyte–endothelial interaction [10, 24] and the generation of ROS [36, 58] are well documented and were therefore in the focus of our interest.
The results from our current study revealed an attenuation of inflammatory cell infiltration during acute IRI under conditions of miR-155 deficiency. This is in good agreement with previous reports on chronic vascular inflammation: in the setting of cardiovascular disease miR-155 promotes atherosclerosis by repressing Bcl6 in macrophages [31]. This was confirmed in an Apo E−/− model of atherogenesis in which miR-155 deficiency resulted in decreased macrophage inflammation and attenuated atherogenesis [6]. Interestingly, Nazari–Jahantigh and coworkers also observed a reduced macrophage count in lesions of miR-155−/− animals in this model of chronic inflammation. Thus, the influence of miR-155 on inflammation in the setting of atherogenesis seems to be predominantly due to its effects on leukocytes. This is further emphasized by a recent study demonstrating a reduced cardiac inflammation and reduced hypertrophic growth in miR-155−/− animals upon chronic pressure overload [40]. In line with the aforementioned findings, these protective effects are dependent on the lack of mir-155 expression in macrophages, again highlighting the role of this leukocyte lineage in mediating pro-inflammatory miR-155 effects.
In contrast to the situation in atherogenesis and cardiac hypertrophy by pressure overloading, which both present chronic inflammatory processes, the acute cardiac ischemia by LAD occlusion and our cremasteric IRI model as model of post-surgical IRI represent models of acute inflammation. This is a major novelty of our study, as our results demonstrate a protective effect of miR-155 deficiency already in the early acute phase of the inflammatory response to injury. In addition, our data also point toward a critical role of miR-155 in non-monocytic leukocyte populations in this setting, with a particular focus on rolling and adhesion and ROS generation as crucial events in this phase of the inflammatory cascade.
MiR-155 is a multifunctional miRNA with a broad spectrum of validated or predicted target genes in multiple cell types [9]. The identification of a single target gene mediating the effects of miR-155 in IRI seems therefore unlikely. We focused on SOCS-1, as the upregulation of miR-155 during IRI in human free-flap tissue, is mirrored by a corresponding downregulation of this intrinsic anti-inflammatory regulator, suggesting a functional interaction in human disease.
This negative regulator of the JAK/STAT signaling pathway harbors a highly conserved binding site for miR-155 in its 3′UTR. Its suppression by miR-155 was recently identified as the mediating mechanism for the pro-inflammatory effects of this microRNA in a variety of diseases including rheumatoid arthritis [25], lung injury [35], and malignancy [57]. Our finding that the reduced generation of ROS in BMDMs derived from miR-155−/− animals is restored upon silencing of (SOCS-1) shows that the suppression of this direct miR-155 target is an essential component of the miR-155-mediated increase in ROS-production. The specificity of our findings of a decreased pro-inflammatory capacity of miR-155−/− BMDM is further supported by the successful rescue of cytokine generation in miR-155−/− BMDM, if miR-155 is overexpressed by transfection with premiR-155 oligonucleotides.
miR-155 in the cardiovascular system
While the functional role of miR-155 in the setting of myocardial infarction has so far remained unclear, several observatory clinical studies have already pointed toward a harmful and potentially important role of miR-155 in human patients with acute coronary syndrome: Matsumoto and coworkers recently found that serum levels of miR-155 were significantly elevated in patients who died after admittance for myocardial infarction compared to patients who survived up to one year [29]. Xie et al. published observational data that suggest a partial mediation of the protective effects of statin treatment in acute coronary syndromes via the suppression of miR-155/SHIP-1 signaling pathway [54]. As miR-155 is a multifunctional miRNA with a broad expression pattern across cell types [9], these studies do not allow a conclusive interpretation of miR-155 function in the ischemically challenged heart. In cardiomyocyte progenitor cells, miR-155 even seems to offer a relative protection from necrotic cell death by targeting receptor interacting protein 1 [26], whereas miR-155 expression in murine cardiac fibroblasts promotes fibrotic remodeling after myocardial infarction [23]. Heymans and coworkers recently showed that miR-155 promotes cardiac hypertrophy and heart failure in mice and that this phenotype is dependent on miR-155 expression in macrophages [18]. To our knowledge, our study is the first to directly assess the functional effects of miR-155 in the setting of experimental myocardial infarction in mice, demonstrating an overall harmful effect of miR-155 on myocardial infarct size. Although our genetic model of global miR-155 deficiency does not allow the direct identification of the responsible cell population, our histological analysis as well as our mechanistic experiments demonstrate that a reduction of inflammatory cell infiltration by attenuation of leukocyte–endothelial interaction is at least one of the contributing mechanisms.
In addition, we found that miR-155 expression correlates with the upregulation of IL-1β expression both in our human biopsy samples obtained before and after IRI as well as during inflammatory activation of BMDMs in culture, suggesting the modulation of pro-inflammatory cytokine signaling as a miR-155-dependent factor. IL-1β is a major contributing factor to IRI in the murine myocardium [44] and has been shown to directly correlate with the extent of IRI in human free muscle flaps [8].
Previously published studies on the modulation of inflammatory cytokine expression by miR-155 reported partly contradictory results: while a recent study reported a negative modulation of TNF-α and IL-1β by miR-155 in monocyte-derived dendritic cells and oxLDL-treated macrophages [5], another study described a positive regulation of both cytokines by miR-155 in PBMCs [25]. Our own results showed an increased expression of IL-1β upon LPS stimulation in WT BMDMs compared to miR155−/−-derived BMDMs. These differences between studies might be explained by the different cell types and different ways of stimulation used.
In comparison with other studies on miRNA expression in acute myocardial infarction, the upregulation of miR-155 detected in our experiment is relatively modest [38, 49]. However, this finding is in good correspondence with the previous reported oscillatory change in expression of miR-155 in monocytes and macrophages during initiation of the innate immune response, in which LPS or TNF-α stimulation of mouse macrophages lead to only moderate miR-155 upregulation [47] despite significant functional effects. This together with the finding that miR155-levels are upregulated in response to interferon-beta in human monocytes [33] indicates that miR-155 plays a central role in regulating the acute inflammatory response to a wide range of extrinsic pathogens. Our work is for the first time showing that miR-155 upregulation is also an acute response to intrinsic tissue injury, e.g., in IRI.
Our intravital imaging results demonstrate comparable leukocyte rolling and adhesion between miR-155−/− and wildtype mice at rest, in good correspondence with previous characterizations of miR-155−/− mice showed that these animals generate normal leukocyte populations under resting conditions [21, 43]. The protective phenotype of miR-155 deficient animals only becomes apparent after induction of IRI, showing a significant reduction of leukocyte rolling and adhesion as well as tissue infiltration. Finally, a reduced necrotic cardiac tissue area in miR-155−/− animals compared to WT mice could be observed. This correlated directly with a reduced inflammatory cell infiltration in good agreement with a recent report which showed that the reduced infiltration of neutrophils in toll-like receptor 4 deficient mice results in decreased infarction after IRI [34].
In our mouse model of ischemia/reperfusion, we observed a significant increase as early as 30 min after reperfusion. This is in line with the observations of Ayhan and coworkers in cremasteric ischemia/reperfusion [2] and is also found in ischemia/reperfusion injury of other organs, such as the liver [51]. The time of ischemia used by us (30 min) was chosen, as this amount of time does not significantly alter microcirculatory blood flow that can confound results [20] and ischemia time, as well as kinetics of leukocyte adhesion after reperfusion are similar to the findings of Johns and coworkers in a similar model of ischemia/reperfusion injury [20]. However, the increase of leukocyte rolling and adhesion over the first 2 h after reperfusion observed in our animal model might only represents the initial steps of the inflammatory cascade as in other animal models of IRI it has been shown that the most prominent increase in leukocyte infiltration can be detected on day five after ischemia and reperfusion [56].
Limitations of the study
While our findings clearly demonstrate the functional role of miR-155 in the mediation of the acute inflammatory response, definite conclusions about the long-term effects of miR-155 deficiency, or therapeutic miR-155 inhibition cannot be drawn from our data. A limited inflammatory response is also part of the physiologic healing process of myocardial injury [11, 30] and our model of a relatively short ischemic period and a small resulting infarct size does not allow the valid assessment of long-term effects of miR-155 deficiency. The DCFH-DA-method, as all fluorescence-based techniques for ROS-quantification, has several substrate-specific limitations that need to be taken into account when interpreting our findings, including the fact that DCFH does not directly react with H2O2 but is oxidized to DCF by several one-electron oxidizing species [22].
In addition, the potential therapeutic inhibition of miR-155 is hampered by the multifunctional role of this microRNA and could result in multiple unwanted side effects such as immunosuppression [39] and tumor growth [52].
Conclusion
Our findings identify a causal role for miR-155 during IRI. MiR-155 modulates leukocyte recruitment in the microvasculature and contributes to ROS-production in inflammatory cells via suppression of its target gene SOCS-1, thereby aggravating post-ischemic tissue injury. Although specific oligonucleotide-based microRNA-inhibitors are on the way to clinical application, the therapeutic potential of miR-155 is limited by its multifunctional role and its involvement in host defence and neoplastic growth. However, our findings demonstrate that microRNAs could represent promising targets for the modulation of IRI or other acute inflammatory conditions, where the a bolus treatment with a microRNA-inhibitor could result in a sustained anti-inflammatory effect.
Abbreviations
- BMDM:
-
Bone marrow derived macrophages
- IRI:
-
Ischemia–reperfusion injury
- ROS:
-
Reactive oxygen species
- SOCS-1:
-
Suppressor of cytokine signaling 1
References
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355. doi:10.1038/nature02871
Ayhan S, Tugay C, Norton S, Araneo B, Siemionow M (2003) Dehydroepiandrosterone protects the microcirculation of muscle flaps from ischemia-reperfusion injury by reducing the expression of adhesion molecules. Plast Reconstr Surg 111:2286–2294. doi:10.1097/01.PRS.0000060242.85268.8F
Baars T, Skyschally A, Klein-Hitpass L, Cario E, Erbel R, Heusch G, Kleinbongard P (2014) microRNA expression and its potential role in cardioprotection by ischemic postconditioning in pigs. Pflugers Arch 466:1953–1961. doi:10.1007/s00424-013-1429-3
Baez S (1973) An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res 5:384–394
Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P (2009) MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A 106:2735–2740. doi:10.1073/pnas.0811073106
Du F, Yu F, Wang Y, Hui Y, Carnevale K, Fu M, Lu H, Fan D (2014) MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 34:759–767. doi:10.1161/ATVBAHA.113.302701
Eisenhardt SU, Schmidt Y, Karaxha G, Iblher N, Penna V, Torio-Padron N, Stark GB, Bannasch H (2012) Monitoring molecular changes induced by ischemia/reperfusion in human free muscle flap tissue samples. Ann Plast Surg 68:202–208. doi:10.1097/SAP.0b013e3181f77ba5
Eisenhardt SU, Schmidt Y, Thiele JR, Iblher N, Penna V, Torio-Padron N, Stark GB, Bannasch H (2012) Negative pressure wound therapy reduces the ischaemia/reperfusion-associated inflammatory response in free muscle flaps. J Plast Reconstr Aesthet Surg 65:640–649. doi:10.1016/j.bjps.2011.11.037
Faraoni I, Antonetti FR, Cardone J, Bonmassar E (2009) miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta 1792:497–505. doi:10.1016/j.bbadis.2009.02.013
Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H (1995) Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol 57:368–374
Frantz S, Hofmann U, Fraccarollo D, Schafer A, Kranepuhl S, Hagedorn I, Nieswandt B, Nahrendorf M, Wagner H, Bayer B, Pachel C, Schon MP, Kneitz S, Bobinger T, Weidemann F, Ertl G, Bauersachs J (2013) Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 27:871–881. doi:10.1096/fj.12-214049
Grundmann S, Hans FP, Kinniry S, Heinke J, Helbing T, Bluhm F, Sluijter JP, Hoefer I, Pasterkamp G, Bode C, Moser M (2011) MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation 123:999–1009. doi:10.1161/CIRCULATIONAHA.110.000323
Grundmann S, Hoefer I, Ulusans S, van Royen N, Schirmer SH, Ozaki CK, Bode C, Piek JJ, Buschmann I (2005) Anti-tumor necrosis factor-{alpha} therapies attenuate adaptive arteriogenesis in the rabbit. Am J Physiol Heart Circ Physiol 289:H1497–1505. doi:10.1152/ajpheart.00959.2004
Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ (2008) MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA 105:1516–1521. doi:10.1073/pnas.0707493105
Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM (2010) Translating novel strategies for cardioprotection: the Hatter workshop recommendations. Basic Res Cardiol 105:677–686
He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW (2011) Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci 18:22. doi:10.1186/1423-0127-18-22
Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943
Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stoger L, Wijnands E, Janssen BJ, Creemers EE, Pinto YM, Grimm D, Schurmann N, Vigorito E, Thum T, Stassen F, Yin X, Mayr M, de Windt LJ, Lutgens E, Wouters K, de Winther MP, Zacchigna S, Giacca M, van Bilsen M, Papageorgiou AP, Schroen B (2013) Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation 128:1420–1432. doi:10.1161/CIRCULATIONAHA.112.001357
Hsieh CH, Jeng JC, Jeng SF, Wu CJ, Lu TH, Liliang PC, Rau CS, Chen YC, Lin CJ (2010) MicroRNA profiling in ischemic injury of the gracilis muscle in rats. BMC Musculoskelet Disord 11:123. doi:10.1186/1471-2474-11-123
Johns DG, Ao Z, Eybye M, Olzinski A, Costell M, Gruver S, Smith SA, Douglas SA, Macphee CH (2005) Rosiglitazone protects against ischemia/reperfusion-induced leukocyte adhesion in the zucker diabetic fatty rat. J Pharmacol Exp Ther 315:1020–1027
Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R, Meinl E (2009) MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 132:3342–3352. doi:10.1093/brain/awp300
Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ 2nd, Ischiropoulos H (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52:1–6. doi:10.1016/j.freeradbiomed.2011.09.030
Kishore R, Verma SK, Mackie AR, Vaughan EE, Abramova TV, Aiko I, Krishnamurthy P (2013) Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS One 8:e60161. doi:10.1371/journal.pone.0060161
Lehr HA, Guhlmann A, Nolte D, Keppler D, Messmer K (1991) Leukotrienes as mediators in ischemia-reperfusion injury in a microcirculation model in the hamster. J Clin Invest 87:2036–2041
Li X, Tian F, Wang F (2013) Rheumatoid arthritis-associated microRNA-155 targets SOCS1 and upregulates TNF-alpha and IL-1beta in PBMCs. Int J Mol Sci 14:23910–23921. doi:10.3390/ijms141223910
Liu J, van Mil A, Aguor EN, Siddiqi S, Vrijsen K, Jaksani S, Metz C, Zhao J, Strijkers GJ, Doevendans PA, Sluijter JP (2012) MiR-155 inhibits cell migration of human cardiomyocyte progenitor cells (hCMPCs) via targeting of MMP-16. J Cell Mol Med 16:2379–2386. doi:10.1111/j.1582-4934.2012.01551.x
Luo X, Cai H, Ni J, Bhindi R, Lowe HC, Chesterman CN, Khachigian LM (2009) c-Jun DNAzymes inhibit myocardial inflammation, ROS generation, infarct size, and improve cardiac function after ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 29:1836–1842. doi:10.1161/ATVBAHA.109.189753
Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ, Chotani M, Feldman DS, Schmittgen TD, Elton TS (2007) The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microRNA-155 binding. J Biol Chem 282:24262–24269. doi:10.1074/jbc.M701050200
Matsumoto S, Sakata Y, Nakatani D, Suna S, Mizuno H, Shimizu M, Usami M, Sasaki T, Sato H, Kawahara Y, Hamasaki T, Nanto S, Hori M, Komuro I (2012) A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem Biophys Res Commun 427:280–284. doi:10.1016/j.bbrc.2012.09.039
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204:3037–3047. doi:10.1084/jem.20070885
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A (2012) MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest 122:4190–4202. doi:10.1172/JCI61716
O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D (2008) Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 205:585–594. doi:10.1084/jem.20072108
O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A 104:1604–1609. doi:10.1073/pnas.0610731104
Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T (2004) Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109:784–789. doi:10.1161/01.CIR.0000112575.66565.84
Rao R, Rieder SA, Nagarkatti P, Nagarkatti M (2014) Staphylococcal enterotoxin B-induced microRNA-155 targets SOCS1 to promote acute inflammatory lung injury. Infect Immun 82:2971–2979. doi:10.1128/IAI.01666-14
Rashid MA, William-Olsson G (1991) Are leukocytosis and lipid peroxidation involved in ischemic or reperfusion injury in cardiac surgery? Thorac Cardiovasc Surg 39:193–195
Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A (2007) Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611. doi:10.1126/science.1139253
Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK (2009) MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res 82:21–29. doi:10.1093/cvr/cvp015
Seddiki N, Brezar V, Ruffin N, Levy Y, Swaminathan S (2014) Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology 142:32–38. doi:10.1111/imm.12227
Seok HY, Chen J, Kataoka M, Huang ZP, Ding J, Yan J, Hu X, Wang DZ (2014) Loss of MicroRNA-155 protects the heart from pathological cardiac hypertrophy. Circ Res 114:1585–1595. doi:10.1161/CIRCRESAHA.114.303784
Silvestre JS, Mallat Z, Tedgui A, Levy BI (2008) Post-ischaemic neovascularization and inflammation. Cardiovasc Res 78:242–249. doi:10.1093/cvr/cvn027
Sluijter JP, Smeets MB, Velema E, Pasterkamp G, de Kleijn DP (2004) Increased collagen turnover is only partly associated with collagen fiber deposition in the arterial response to injury. Cardiovasc Res 61:186–195. doi:10.1016/j.cardiores.2003.09.028
Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 58:1001–1009. doi:10.1002/art.23386
Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH (2001) Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation 104:I303–I308. doi:10.1161/hc37t1.094871
Thiele JR, Goerendt K, Stark GB, Eisenhardt SU (2012) Real-time digital imaging of leukocyte-endothelial interaction in ischemia-reperfusion injury (IRI) of the rat cremaster muscle. J Vis Exp (66):e3973. doi:10.3791/3973
Thiele JR, Habersberger J, Braig D, Schmidt Y, Goerendt K, Maurer V, Bannasch H, Scheichl A, Woollard KJ, von Dobschutz E, Kolodgie F, Virmani R, Stark GB, Peter K, Eisenhardt SU (2014) Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation 130:35–50. doi:10.1161/CIRCULATIONAHA.113.007124
Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179:5082–5089
Urbich C, Kuehbacher A, Dimmeler S (2008) Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res 79:581–588. doi:10.1093/cvr/cvn156
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105:13027–13032. doi:10.1073/pnas.0805038105
Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KG, Rada C, Enright AJ, Toellner KM, Maclennan IC, Turner M (2007) microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 27:847–859. doi:10.1016/j.immuni.2007.10.009
Vollmar B, Menger MD, Glasz J, Leiderer R, Messmer K (1994) Impact of leukocyte-endothelial cell interaction in hepatic ischemia-reperfusion injury. Am J Physiol 267:G786–793
Wang J, Yu F, Jia X, Iwanowycz S, Wang Y, Huang S, Ai W, Fan D (2015) MicroRNA-155 deficiency enhances the recruitment and functions of myeloid-derived suppressor cells in tumor microenvironment and promotes solid tumor growth. Int J Cancer 136:E602–613. doi:10.1002/ijc.29151
Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC (2010) MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 122:1308–1318. doi:10.1161/CIRCULATIONAHA.110.964684
Xie W, Li P, Wang Z, Chen J, Lin Z, Liang X, Mo Y (2014) Rosuvastatin May Reduce the Incidence of Cardiovascular Events in Patients with Acute Coronary Syndromes Receiving Percutaneous Coronary Intervention by Suppressing miR-155/SHIP-1 Signaling Pathway. Cardiovasc Ther 32:276–282. doi:10.1111/1755-5922.12098
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135. doi:10.1056/NEJMra071667
Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, De Broe ME (2000) Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant 15:1562–1574
Zhao XD, Zhang W, Liang HJ, Ji WY (2013) Overexpression of miR -155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. PLoS One 8:e56395. doi:10.1371/journal.pone.0056395
Zimmerman BJ, Granger DN (1994) Mechanisms of reperfusion injury. Am J Med Sci 307:284–292
Acknowledgments
Funding received for this work: this work was supported by the “Else-Kröner-Fresenius Stiftung” Grant #2011_A191 to SUE and SG.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. U. Eisenhardt and J. B.W. Weiss contributed equally to this work.
Rights and permissions
About this article
Cite this article
Eisenhardt, S.U., Weiss, J.B.W., Smolka, C. et al. MicroRNA-155 aggravates ischemia–reperfusion injury by modulation of inflammatory cell recruitment and the respiratory oxidative burst. Basic Res Cardiol 110, 32 (2015). https://doi.org/10.1007/s00395-015-0490-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0490-9