Abstract
Recent reports indicate that elevating DNA glycosylase/AP lyase repair enzyme activity offers marked cytoprotection in cultured cells and a variety of injury models. In this study, we measured the effect of EndoIII, a fusion protein construct that traffics Endonuclease III, a DNA glycosylase/AP lyase, to the mitochondria, on infarct size in a rat model of myocardial ischemia/reperfusion. Open-chest, anesthetized rats were subjected to 30 min of occlusion of a coronary artery followed by 2 h of reperfusion. An intravenous bolus of EndoIII, 8 mg/kg, just prior to reperfusion reduced infarct size from 43.8 ± 1.4 % of the risk zone in control animals to 24.0 ± 1.3 % with no detectable hemodynamic effect. Neither EndoIII’s vehicle nor an enzymatically inactive EndoIII mutant (K120Q) offered any protection. The magnitude of EndoIII’s protection was comparable to that seen with the platelet aggregation inhibitor cangrelor (25.0 ± 1.8 % infarction of risk zone). Because loading with a P2Y12 receptor blocker to inhibit platelets is currently the standard of care for treatment of acute myocardial infarction, we tested whether EndoIII could further reduce infarct size in rats treated with a maximally protective dose of cangrelor. The combination reduced infarct size to 15.1 ± 0.9 % which was significantly smaller than that seen with either cangrelor or EndoIII alone. Protection from cangrelor but not EndoIII was abrogated by pharmacologic blockade of phosphatidylinositol-3 kinase or adenosine receptors indicating differing cellular mechanisms. We hypothesized that EndoIII protected the heart from spreading necrosis by preventing the release of proinflammatory fragments of mitochondrial DNA (mtDNA) into the heart tissue. In support of this hypothesis, an intravenous bolus at reperfusion of deoxyribonuclease I (DNase I) which should degrade any DNA fragments escaping into the extracellular space was as protective as EndoIII. Furthermore, the combination of EndoIII and DNase I produced additive protection. While EndoIII would maintain mitochondrial integrity in many of the ischemic cardiomyocytes, DNase I would further prevent mtDNA released from those cells that EndoIII could not save from propagating further necrosis. Thus, our mtDNA hypothesis would predict additive protection. Finally to demonstrate the toxicity of mtDNA, isolated hearts were subjected to 15 min of global ischemia. Infarct size doubled when the coronary vasculature was filled with mtDNA fragments during the period of global ischemia. To our knowledge, EndoIII and DNase are the first agents that can both be given at reperfusion and add to the protection of a P2Y12 blocker, and thus should be effective in today’s patient with acute myocardial infarction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite continuous advances in treatment, loss of contractile myocardium following acute myocardial infarction continues to be a serious medical problem. Although a number of strategies have been devised to quickly restore blood flow to ischemic myocardium, it can seldom be done before some irreversible damage to the ventricular muscle has occurred. Animal studies indicate that preservation of myocardium could still be accomplished by interventions that block specific processes that contribute to infarction of ischemic heart muscle [19]. Mitochondria have recently emerged as central players in the ischemia/reperfusion injury process. Ischemic preconditioning is now thought to protect by inhibiting the formation of permeability transition pores that destroy ischemically injured mitochondria when they are reoxygenated [12, 15]. However, formation of transition pores may not be the only treatable process by which cardiac cells are killed during ischemia/reperfusion. Reintroduction of oxygen to mitochondria that have experienced a prolonged period of hypoxia can generate a burst of reactive oxygen species (ROS) which can oxidize bases and the sugar/phosphate backbone in the mitochondrial DNA (mtDNA) [6, 11]. If this damage is incompletely repaired, DNA nicks and abasic sites accumulate which block transcription and replication of mtDNA [2, 3, 32].
Mitochondrial base excision repair machinery normally mitigates oxidative mtDNA damage, but its capacity can be overwhelmed by the intense ROS burst such as that seen in ischemia/reperfusion. The repair of oxidative damage to bases in mtDNA requires both glycosylase and AP lyase activity. The latter cleaves the sugar/phosphate backbone at abasic (AP) sites on the 3′ side of the lesion. While enzymes are present to repair oxidative damage to the mtDNA, the only known lyase in mitochondria is DNA polymerase γ which has weak lyase activity [28]. Stable overexpression of the nuclear glycosylase/AP lyase, 8-oxoguanine DNA glycosylase (OGG1), in the mitochondrial matrix renders HeLa cells resistant to oxidative damage inflicted by the mitochondrial redox cycler menadione [8, 20, 31], hydrogen peroxide [4] or generated nitric oxide [30] and increases survival of pulmonary artery endothelial cells exposed to ROS from xanthine oxidase enzymatic activity [7] suggesting that lyase activity may be a rate-limiting step in repairing mtDNA oxidative damage. Similarly, augmenting mitochondrial OGG1 activity has resulted in protection against a variety of injurious insults including ventilator injury of the lung [13], ROS attack in H9C2 cells [39], oxidant exposure of lung adenocarcinoma (A549) cells [27], and, most recently, infarction in a mouse heart model of ischemia/reperfusion [25].
OGG1 primarily excises oxidized purines from DNA. Other glycosylases exist that preferentially target oxidized pyrimidines. One such enzyme is the bacterial DNA glycosylase, Endonuclease III, but both OGG1 and Endonuclease III share a common lyase activity which cleaves apurinic/apyrimidinic (AP) sites on the 3′ side of the lesion. When Endonuclease III targeted to mitochondria was stably overexpressed in HeLa cells, the cells became more tolerant of oxidative stress, had less mitochondrial DNA damage, and displayed increased survival [29]. Thus, mitochondrially targeted OGG1 or EndoIII seems to protect against lethal oxidative mtDNA damage.
Ventricular myocardium becomes necrotic following membrane failure within an hour or two of an ischemic insult which is faster than would be expected if mtDNA damage interfered only with protein synthesis. One intriguing possibility is that increasing mitochondrial DNA glycosylase/AP lyase prevents release of fragments of mtDNA into the tissue. Recent studies suggest that these fragments can act as danger-associated molecular patterns (DAMPs) which the tissue “mistakes” for bacterial DNA and mounts an inflammatory response which can inappropriately attack otherwise viable tissue [43]. Released fragments of mtDNA could cause a wave of necrosis across the reperfused myocardium.
In the present study, we created and tested a fusion protein (EndoIII) containing Endonuclease III which was engineered to allow its rapid uptake into the matrix of mitochondria. In a preliminary report, this protein reduced infarct size in a mouse model of coronary occlusion/reperfusion [26]. For the present work, this protein was tested in rat hearts to determine whether systemic administration could protect the heart from infarction resulting from myocardial ischemia/reperfusion and to provide novel information concerning its mechanism of action.
Methods
In situ heart model
Male Sprague–Dawley rats (Charles River) weighing approximately 500 g were anesthetized by intraperitoneal injection of 25 mg of sodium pentobarbital, and then intubated through a tracheotomy and ventilated under positive pressure with 100 % oxygen. Catheters were placed in a carotid artery for measurement of blood pressure and a jugular vein for administration of supplemental anesthesia and intravenous preparations. The heart was exposed by a left thoracotomy, and a suture was placed under the left anterior descending coronary artery. The suture was threaded through a short length of tubing to form a snare. Regional ischemia was caused by tightening the snare. After 30 min of ischemia, the snare was loosened and the artery was allowed to reperfuse for 2 h after which the heart was removed for analysis. Arterial blood pressure and heart rate were monitored throughout the procedure and anesthesia was supplemented with pentobarbital as needed.
Isolated heart model
Rats were prepared as above. After the ligature was passed under the coronary branch, the heart was quickly removed by cutting the great vessels. The heart was mounted on a Langendorff apparatus and the aorta was retroperfused under constant pressure with oxygenated Krebs buffer. A fluid-filled balloon was placed in the left ventricular lumen and ventricular pressure was recorded. After 20 min of equilibration, the coronary branch was occluded for 30 min and then reperfused for 2 h. In other isolated hearts, the ligature around the coronary branch was omitted and the entire heart was made ischemic by arresting aortic perfusion for 15 min and then resuming aortic perfusion for 2 h. In the latter group, the entire ventricular mass became the risk zone.
Measurement of infarct size
At the end of the study, in situ hearts were excised, mounted on a Langendorff apparatus, and briefly retroperfused with saline through the aortic root to flush out all blood. In all mounted hearts subjected to regional myocardial ischemia, the coronary branch was then reoccluded and fluorescent microspheres were infused into the heart to stain the non-ischemic tissue. For those isolated hearts subjected to global ischemia in which the entire left ventricle represented the risk region, injection of fluorescent microspheres was not necessary. The heart was sliced from apex to base into 2-mm-thick slices which were then incubated in warmed triphenyltetrazolium chloride (TTC) solution to visualize the infarct. Infarcted regions (TTC-negative tissue) and risk zone (non-fluorescent myocardium in hearts with fluorescent microspheres and the total left ventricle in hearts subjected to global ischemia) were traced onto plastic overlays, and infarct size was expressed as a percent of the risk zone. Normalized infarct sizes for groups are presented as mean ± SEM. A picture of a sliced and stained heart from each of the 19 groups can be seen in the online supplement.
Preparation of mtDNA fragments
Exogenous mtDNA was generated from freshly harvested rat liver placed in an iced beaker containing 0.9 % sodium chloride. Mitochondria were isolated from liver using the Mitochondria Isolation Kit for Tissue (Thermo Scientific, Rockford, IL, USA) according to the company’s protocol. DNA was immediately isolated from mitochondria using the DNeasy Blood and Tissue Kit (Qiagen, Santa Clarita, CA, USA) following manufacturer’s instructions. Eluted DNA was immediately sonicated 10 times with a Vibra-Cell sonicator (Sonics and Materials, Newtown, CT, USA), each sonication lasting 30 s and separated from the next by a 30-s sonication-free interval, to produce mtDNA fragments of 50–150 base pairs. Aliquots were stored at −20 °C.
Compartment analysis of EndoIII in hearts and blood
Two rats were prepared as described above for the in situ heart model. After 25 min of ischemia, an arterial blood sample was collected and then 8 mg/kg EndoIII was injected intravenously. Two min later, a second arterial blood sample was collected. Three min later, the coronary artery was reperfused for 15 min after which another arterial blood sample was collected and the heart was rapidly removed and placed in iced saline. The left ventricle was separated into ischemic and normally perfused regions. Those samples were homogenized and separated into cytosolic, mitochondrial and nuclear fractions by differential centrifugation as described earlier [5]. These fractions were subjected to polyacrylamide gel electrophoresis (30 µg of protein per lane). The resulting blots were probed with an antibody to the HA tag on EndoIII. To determine the effectiveness of the differential centrifugation, we also probed with an antibody to the mitochondrial enzyme ATP synthase.
Statistics
Data are presented as mean ± SEM. To determine whether there were any significant group differences, mean blood pressure and heart rate at baseline were compared for all 19 in vivo groups by one-way ANOVA with Student–Newman–Keuls post hoc testing. For each individual group, changes of blood pressure and heart rate over time were analyzed by ANOVA for repeated measures. For analysis of infarct size in the in vivo groups, ANOVA with Student–Newman–Keuls post hoc testing was performed on all 19 groups together. The in vivo control group is large (n = 12) because untreated animals were periodically infarcted during the course of the duration of the study to determine whether the vulnerability to infarction might have changed. Since no time-related trends were detected, we concluded that this was a valid control group for all of the other 18 groups. For presentation of the data in figures, groups were combined by theme, but indicated significance values are based on the analysis of the entire 19 groups. In some figures, groups presented in an earlier figure are reproduced to assist the reader in comparison between those groups and the new groups. When this was done, the data points of the reproduced groups are segregated at the left of the figure and appear in a shade of gray. p < 0.05 denoted significance. All animals that survived the protocol contributed data and there were no exclusions. The number of animals in each of the 19 in situ groups is indicated in Table 1.
Chemicals
The repair enzyme comprised the core of a fusion protein which was a generous gift from the Exscien Corporation. The EndoIII protein includes a mitochondrial import sequence and a protein transduction domain appended to Endonuclease III. An HA tag was also included. The protein was expressed in E. coli strain C41 (DE3) and purified by metal chelate affinity chromatography. Purified protein was collected by buffer exchange on a gel-filtration column. An enzymatically inactive K120Q mutant was generated by site-directed mutagenesis using overlap extension [17]. The P2Y12 receptor inhibitor cangrelor was a generous gift from The Medicines Company (Parsippany, NJ, USA). Cangrelor was given as a 60 µg/kg loading intravenous bolus followed by an infusion of 6 µg/kg/min starting 10 min before reperfusion and continuing until its end. In some rats, signaling antagonists PD98059 (MEK 1/2 and, therefore, ERK 1/2 inhibitor), wortmannin (phosphatidylinositol-3 [PI3] kinase inhibitor), or 8-(p-sulfophenyl)theophylline (SPT) (non-selective adenosine receptor inhibitor) were injected as an intravenous bolus 5 min prior to EndoIII at doses (0.3 mg/kg, 60 µg/kg, and 7.5 mg/kg, respectively) previously determined to block protection from cangrelor [42]. All signaling antagonists were obtained from Sigma Aldrich (St. Louis, MO, USA). For DNase I studies, we used DNase I type IV (Sigma Aldrich catalogue # D5025) which has specific activity of approximately 2,000 Kunitz units per mg of protein. For western blots, antibody to HA tag was obtained from Sigma Aldrich. Antibody to ATP synthase (complex V, subunit α) was obtained from Invitrogen (Carlsbad, CA, USA).
Results
Hemodynamics for the 19 in vivo groups are presented in Table 1. Mean blood pressure and heart rate were comparable in all groups at baseline. Temporally there was a tendency for blood pressure to decrease during the 30-min coronary occlusion, although this decline was not significant in most groups. Heart rate changed little during the course of the experiments. Intravenous injection of EndoIII had no measurable effect on blood pressure or heart rate.
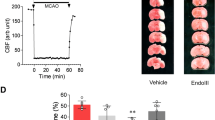
Figure 1 shows EndoIII’s effect on infarct size in the open-chest rat. Pretreatment with a bolus of 4 mg/kg 5 min prior to ischemia reduced infarct size from 43.8 ± 1.4 % of the risk zone in untreated rats to only 23.6 ± 1.1 %. It is standard practice to treat patients presenting with acute myocardial infarction with a loading dose of a P2Y12 receptor inhibitor prior to recanalization. P2Y12 inhibitors not only prevent thrombus formation by suppressing platelet aggregation but also have a direct anti-infarct effect [33, 42]. Animal studies indicate that P2Y12 receptor inhibitors including clopidogrel, ticagrelor, and cangrelor have a similar potency against infarction indicating a class effect [41, 42]. Cardioprotective interventions that have shown great potency in animal models have yielded disappointing results in the clinic and one proposed explanation is that cardioprotective agents including platelet inhibitors that are given to these patients but are not present in the animal models might be masking their protection [16]. For any anti-infarct intervention to be of clinical value, it would have to be capable of producing added salvage of myocardium when used in conjunction with a platelet anti-aggregatory drug. We, therefore, tested EndoIII in the presence of cangrelor, a parenteral P2Y12 blocker. Cangrelor had similar potency to that of EndoIII and the combination of the 2 drugs further reduced infarct size to 15.1 ± 0.9 % which was significantly smaller than that seen with either cangrelor or EndoIII alone.
Infarct size as a % of risk zone in open-chest rats. Open circles represent individual experiments, and closed circles represent group mean ± SEM. Endonuclease III (Endo III) was administered before ischemia (pretreat), while cangrelor infusion was begun 10 min before reperfusion. M Endo is an inactive EndoIII mutant. *p < 0.001 vs Control **p < 0.005 vs Cangrelor and p < 0.01 vs EndoIII Pretreat
Figure 1 also reveals that the vehicle for EndoIII had no effect on infarct size in otherwise untreated animals. Finally, we tested the enzymatically inactive K120Q mutant of EndoIII (MEndo in Fig. 1). The modified molecule had no effect on infarct size suggesting that EndoIII’s protection was related to its glycosylase/lyase activity rather than a non-specific effect of the protein.
In the above studies, we administered the drug prior to the onset of coronary occlusion, thus enabling it to target both ischemic and reperfusion injury. Unfortunately, pretreatment is seldom an option when treating patients with acute myocardial infarction. Therefore, we determined if EndoIII would still be protective when given just prior to reperfusion. Figure 2 reveals that while a 4 mg/kg bolus 2 min prior to reperfusion significantly reduced infarct size below that seen in control animals, this regimen was not as protective as when given as pretreatment (Fig. 1). It is possible that an important component of the injury targeted by EndoIII had already occurred during ischemia prior to starting the drug thus causing an apparent loss of effectiveness. An alternative explanation could be that the injury was still occurring after the onset of reperfusion but the drug simply did not have enough time to reach a therapeutic concentration in the mitochondria to prevent all of the reperfusion injury. To test the latter hypothesis, we doubled the dose which should have caused a more rapid rise in the mitochondrial drug concentration. Figure 2 confirms that the 8 mg/kg dose at reperfusion was as protective as the 4 mg/kg pretreatment dose in Fig. 1 supporting the pharmacokinetic hypothesis. Higher doses of EndoIII had no additional effect (data not shown). These results indicate that EndoIII protects against a reperfusion injury. Combining EndoIII at reperfusion with cangrelor again yielded additive protection. The last group of hearts in Fig. 2 reveals that administering EndoIII even after 10 min of reperfusion still offered significant protection.
Infarct size as a % of risk zone in open-chest rats. Open circles represent individual experiments, and closed circles represent group mean ± SEM. Endonuclease III (EndoIII) was administered either 2 min before or 10 min following the onset of reperfusion (Reper), and cangrelor infusion was commenced 10 min before reperfusion. Data points in gray represent groups that have been presented in a previous figure, and are reproduced in this figure to facilitate comparison with new groups. *p < 0.001 vs Control **p < 0.001 vs Cangrelor or EndoIII 4 mg/kg and p < 0.002 vs EndoIII 8 mg/kg at Reper
We next explored the mechanism of EndoIII’s protection. We tested whether 3 key signaling components (ERK, PI3 kinase and adenosine receptors) used by cangrelor [42] might also be involved in EndoIII’s protection. Figure 3 reveals that the ERK inhibitor PD98059 completely blocked EndoIII’s protection. Yet the blocker had no effect on infarct size in the untreated heart indicating that it selectively blocked EndoIII’s protection which might suggest that EndoIII and cangrelor share a common mechanism. However, activation of PI3 kinase and binding of adenosine receptors are also vital steps in cangrelor’s protective pathway [42]. But neither wortmannin nor SPT had any effect on EndoIII’s protection. We did not test these two blockers in control rats in the present study since we have previously shown that neither SPT nor wortmannin alters infarct size in untreated rats [41]. These observations suggest that EndoIII’s mechanism differs from that of cangrelor which would explain why combining the two treatments provides additive effects.
Infarct size as a % of risk zone in open-chest rats. Open circles represent individual experiments, and closed circles represent group mean ± SEM. Endonuclease III (EndoIII) was administered before ischemia (Pre). The signaling antagonist PD98059 or wortmannin or adenosine receptor blocker 8-(sulfophenyl)theophylline (SPT) was infused 5 min before EndoIII. Data points in gray represent groups that have been presented in a previous figure, and are reproduced in this figure to facilitate comparison with new groups. *p < 0.001 vs Control **p < 0.001 vs Endo III Pre
We considered the possibility that EndoIII might protect ischemic myocardium by limiting the release of mtDNA fragments into the tissue. Those fragments could trigger a lethal inflammatory response which would kill adjacent cells and cause them in turn to release their mtDNA. This chain reaction could cause necrosis to spread through the ischemic zone. If that were the case, then eliminating extracellular DNA with an intravenous injection of DNase I should also be protective. Figure 4 shows that a bolus of DNase I, 16 units/kg, 5 min prior to reperfusion was very protective. Doubling the dose to 32 units/kg was no more protective indicating that a maximal effect had been achieved. The protection was similar to that seen with cangrelor, and combining DNase and cangrelor offered an additive effect similar to what was seen when cangrelor and EndoIII were combined (see Fig. 2). When DNase and EndoIII were combined, there was also an additive effect. Finally, unlike what was found with EndoIII, the ERK inhibitor PD98059 did not eliminate DNase’s protection.
Infarct size as a % of risk zone in open-chest rats. Open circles represent individual experiments, and closed circles represent group mean ± SEM. DNase I was infused 5 min before reperfusion (rep) and cangrelor infusion was commenced 10 min before reperfusion. PD98059 (PD) was administered 5 min before DNase I and Endo III (infused 2 min before reperfusion). Data points in gray represent groups that have been presented in a previous figure, and are reproduced in this figure to facilitate comparison with new groups. *p < 0.001 vs Control **p < 0.006 vs Cangrelor and < 0.02 vs DNase 16 and 32 U/kg # p < 0.008 vs DNase 16 and 32 U/kg and p < 0.01 vs Endo III
DNase was recently reported to reduce infarct size in a similar in situ heart model [35]. Savchenko et al. proposed that DNase’s protection derived from dissolving viscous DNA neutrophil extracellular traps (NETs) formed when hyperactivated neutrophils expel their chromatin as part of their immunological defense response. These NETs would occlude the heart’s microcirculation and hamper reoxygenation of the tissue. To test that hypothesis we administered DNase to isolated hearts perfused with cell-free perfusate. Figure 5 reveals that, after a 30-min coronary occlusion, including DNase I (333 units/L) in the Krebs buffer during the 2 h of reperfusion was just as protective as observed in the blood-perfused hearts thus arguing against the NET microemboli hypothesis and favoring the mtDNA hypothesis.
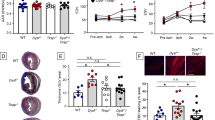
We devised a protocol to determine whether mtDNA fragments were toxic to ischemic rat myocardium. For these in vitro heart experiments, the entire heart was made ischemic by occluding the tubing perfusing the aortic root for 15 min. In control hearts with only 15-min no-flow ischemia/2-h reperfusion, 16.1 ± 1.7 % of the left ventricle infarcted (Fig. 6). In the experimental group of hearts, 100 μg of mtDNA fragments in 2 ml of buffer was injected into the aortic root immediately after cessation of aortic perfusion. Thus, this aliquot of mtDNA fragments filled the aortic root and coronary vasculature and was undisturbed for the next 15 min. Thereafter when aortic perfusion was resumed, the mtDNA fragments were washed out of the heart. Infarction averaged 36.5 ± 3.3 % in these hearts (Fig. 6). To confirm that it was indeed mtDNA fragments that were causing the increase in infarction, 8 Kunitz units of DNase I were added to the mtDNA fragments and were incubated at 37 °C for 20 min. Then the solution was heated to 65 °C for 15 min to denature the DNase before injection into the aortic root at 37 °C. The amount of DNase I was calculated to be more than sufficient to degrade 100 μg of DNA. Infarct size averaged only 17.7 % in the 2 hearts treated with degraded mtDNA (Fig. 6).
Infarct size in isolated rat hearts subjected to 15 min of global ischemia and 2 h of reperfusion. Open circles represent individual experiments, and closed circles represent group mean ± SEM. Infarction in slices of representative hearts in each of the three groups is shown. Including purified mitochondrial DNA fragments (mtDNA) in the perfusate at the onset of no-flow ischemia doubled infarct size. Degrading mtDNA with DNase prior to infusion rendered mtDNA innocuous. *p < 0.001 vs. 15′ Ischemia (Control)
Finally, we studied two rats to see whether EndoIII actually enters the heart’s mitochondria as we hypothesized. Figure 7 shows that in the tissue harvested from one heart there were strong bands in the mitochondrial fraction from the ischemic muscle and distinctly weaker bands in the mitochondrial fraction from normal myocardium. Similar changes were observed in the second heart (data not shown). We were surprised that in both hearts EndoIII was preferentially taken up by the ischemic myocardium. Both blots also showed some EndoIII in the nuclear fraction, but the nuclear fraction also showed appreciable ATP synthase content indicating mitochondrial contamination. Twenty minutes after injection, EndoIII was virtually absent from the plasma (Fig. 7) indicating that EndoIII quickly moves from the blood to the interior of the cells. The spot in the Base lane (plasma obtained prior to injection of EndoIII) in Fig. 7 was not seen in the other heart and is assumed to be encroachment from the 2′ lane which the Ponceau staining revealed was heavily overloaded.
Western blots of HA-tagged EndoIII in subcellular myocardial compartments and plasma. The left panel shows EndoIII in the total heart homogenate (Total), cytosolic (Cyt), mitochondrial (Mito), and nuclear (Nucl) fractions of normally perfused (N) and ischemic (I) ventricular myocardial samples collected 15 min after reperfusion. EndoIII was injected 5 min prior to reperfusion after 25 min of regional ischemia. Note that EndoIII is concentrated in mitochondria of the ischemic tissue. The right panel shows plasma content of EndoIII just prior to injection of EndoIII (Base), 2 min after injection (2′), and 15 min after reperfusion (15′). Twenty minutes after injection most of the EndoIII had been removed from the blood. The small spot in the Base lane is thought to be contamination from the 2′ lane as all 3 lanes were overloaded in an attempt to see the 15′ signal. ATP synthase is a mitochondria-specific marker. Little mitochondrial contamination is present in the cytosolic fraction but the nuclear fraction shows significant mitochondrial contamination
Discussion
These data reveal that EndoIII, a DNA glycosylase/AP lyase directed to mitochondria, has a strong anti-infarct effect in open-chest rats undergoing myocardial ischemia/reperfusion. Protection is observed when the drug is injected either before ischemia, just prior to reperfusion, or 10 min after reperfusion and occurs without any measurable hemodynamic changes. Protection from EndoIII could be added to that from cangrelor indicating that it would be expected to further reduce infarct size in patients treated with a P2Y12 blocker. Beneficial effects of cangrelor are lost when either ERK, PI3 kinase, or the adenosine receptor is blocked [42]. While an ERK inhibitor attenuates EndoIII’s anti-infarct effect, EndoIII’s protection was unaltered by blockade of PI3 kinase or adenosine receptors suggesting differing mechanisms. Giving cangrelor 10 min after reperfusion is not protective [42] but EndoIII is, again suggesting that there are fundamental differences between the mechanisms by which jeopardized myocardium is salvaged by cangrelor and EndoIII.
Because a loading dose of an anti-platelet drug prior to reperfusion therapy is currently standard of care, any viable candidate for treatment of patients with acute myocardial infarction must be capable of limiting infarct size beyond that caused by the anti-platelet drug. We found that hypothermia and sodium-hydrogen exchange blockers can add to cangrelor’s protection, but those two interventions protect best when given as a pretreatment prior to ischemia [22, 23, 38] which is usually not possible for treatment of patients with acute myocardial infarction. EndoIII and DNase 1 are the first agents we have tested that, when given at reperfusion, provide additional protection in the presence of a P2Y12 receptor inhibitor.
Although cangrelor is not available clinically, it was used because it is the only available P2Y12 blocker that can be given intravenously with immediate onset of action. We have tested oral ticagrelor [41] and clopidogrel [42] in rat and rabbit models, respectively, and find them equally protective against infarction indicating that this is a class effect. The oral agents are impractical for acute animal studies, however, because there is a delay of hours after oral dosing before platelet inhibition and cardioprotection are established.
Many recent publications suggest that augmenting DNA glycosylase/AP lyase activity in the cell or mitochondria can protect cells against a wide spectrum of injurious modalities indicating that the constitutive glycosylase or lyase activity may be a rate-limiting step that is critical to the survival of the cell suffering oxidative attack. Most studies have utilized mammalian OGG1 which primarily targets oxidized purines. Stably expressing a plasmid containing the OGG1 gene with an attached mitochondrial import sequence in HeLa cells concentrated the enzyme in mitochondria and protected cells from lethal oxidative stress [8]. Additionally, a transfected plasmid coding for OGG1 with a mitochondrial localization sequence enhanced the amount of OGG1 in mitochondria in pulmonary endothelial cells, reduced oxidant-induced mitochondrial DNA damage, increased cell viability [7], and preserved mitochondrial membrane potential [34]. Overexpressing OGG1 with a mitochondrial import sequence was more protective than a nuclear import sequence in HeLa cells exposed to ROS [4]. Overexpressing an inactive mutant of OGG1 with a mitochondrial import sequence actually made the cells more vulnerable to ROS, again suggesting that mitochondria are the vulnerable organelles. Protection from OGG1 was also seen in whole organ models. Introducing a fusion molecule similar to the one used in the present study but based on OGG1 rather than Endonuclease III into the perfusate protected isolated, whole lungs from elevated airway pressure-induced injury [13]. In mice when a mitochondrially directed form of OGG1 was expressed, hypertension-induced cardiac fibrosis was reduced [40]. When OGG1 was overexpressed in cardiac-derived H9C2 cells they were protected from menadione-induced oxidant stress [39]. OGG1 not only prevented cell death from oxidant stress, but damage to mtDNA was also reduced as was mitochondrial fragmentation. Most recently, a mitochondrial-targeted OGG1-based fusion protein made by Exscien Corporation reduced infarct size when given at reperfusion to an open-chest mouse model of ischemia/reperfusion [25].
While the above investigations studied OGG1, we elected to evaluate EndoIII which is based on a bacterial DNA glycosylase/AP lyase, Endonuclease III, and is directed to the mitochondria. Endonuclease III primarily excises oxidized pyrimidines as opposed to OGG1 which primarily excises oxidized purines. However, both have lyase activity. When an EndoIII-coding plasmid with a mitochondrial localization sequence was stably expressed in HeLa cells, EndoIII was targeted to the mitochondria and it protected the HeLa cells from oxidative stress with potency similar to that seen with OGG1 [29]. EndoIII as used here offered equal potency against ventilator-induced injury in the lung as OGG1 [14]. In a recent abstract, the same EndoIII molecule was administered to mice undergoing myocardial ischemia/reperfusion [26]. In that study, it reduced infarct size measured after 24 h of reperfusion. In the present study, protection was clearly related to the Endonuclease III enzymatic activity as an enzymatically inactive mutant offered no protection. EndoIII has distinct manufacturing advantages over OGG1 as the former, a bacterial product, can be produced more efficiently by E. coli.
In the present study, we administered a protein intravenously that was directed to the mitochondria rather than resorting to transfection which would not be appropriate for emergency use. Even when administered at reperfusion, Endo III found its way to the target tissue fast enough to attenuate infarction. Plasma measurements reveal that 20 min after injection EndoIII has essentially disappeared from the blood and can be found in the heart’s mitochondria. Interestingly, the mitochondrial uptake appeared to be more robust in reperfused myocardium than in those regions that were continuously well perfused. Appreciable uptake was also seen in the nucleus, but this uptake may be an artifact since our nuclear fraction is contaminated with some mitochondria.
The link between EndoIII’s repair of mtDNA and improved cell survival is incompletely understood. However, we have explored one possibility. Recent studies reveal that extracellular mtDNA can be pro-inflammatory [18, 21, 24, 43]. mtDNA strongly resembles bacterial DNA because both lack CpG methylation. Extracellular mtDNA can act as a DAMP and trigger a local inflammatory response. This inflammation can attack healthy tissue and contributes to the systemic inflammatory response syndrome which is seen in patients experiencing extensive tissue destruction as in crush and burn injury [36, 43]. These patients exhibit all the signs of sepsis, yet there is no infection.
After mitochondrial damage induced by oxidative stress, matrix DNA content decreased by 58 % [10]. Furthermore, when quantitated, DNA coding for mitochondrial genes is found outside the mitochondria. This effect is inhibited by cyclosporin A suggesting that mtDNA fragments following mitochondrial damage may be released through the mitochondrial permeability transition pore.
mtDNA serum levels rise in patients with acute myocardial infarction [9], and in those treated with primary coronary angioplasty the magnitude of elevation correlates with the peak troponin T level [1]. There is negligible elevation of mtDNA in patients with stable angina pectoris undergoing elective coronary angioplasty in the absence of infarction [1]. mtDNA is also elevated after a 1 h coronary occlusion followed by reperfusion in isolated, perfused murine hearts [1]. Extracellular mtDNA has also been implicated in heart failure. Oka et al. [24] found that deletion of DNase II which degrades mtDNA in lysosomes that remove defective mitochondria contributed to heart failure in mice. Furthermore, suppression of TLR9 which triggers the inflammatory response when it detects mtDNA fragments could prevent the progression to failure in these knockout mice [24]. mtDNA has also been implicated in heart failure resulting from hypertension [21].
It has been proposed that circulating mtDNA contributes to the systemic involvement associated with traumatic shock [43]. Those patients have a large amount of mtDNA released from their wounds. The amount of mtDNA released from a myocardial infarct is probably too little to have a systemic effect. Accurate measurement of small mtDNA fragments in body fluids is difficult because PCR of fragments smaller than 40 BP is problematic. In pilot studies, we looked for mitochondrial sequences in the plasma and found a rise after the thoracotomy but only a small increment after reperfusing the heart indicating the release into the plasma as a result of myocardial infarction was trivial compared to that from the surgical wound. On the other hand, the interstitial concentration of mtDNA in the heart muscle could be quite high. It has long been noted that myocardial infarcts resulting from a transient period of ischemia tend to be confluent rather than patchy. It is as if the necrosis spreads outward from a central core. The dying cells appear to release a toxin that poisons the surrounding cells. That toxin may be mtDNA triggering inflammatory cytokine release that attacks and kills injured but otherwise viable cells. Those killed cells would then release more mtDNA and this chain reaction could spread the necrosis through the previously ischemic zone. EndoIII could prevent the initial release of mtDNA by enhancing its repair and thus inhibiting its degradation, while DNase I could prevent accumulation of the mtDNA fragments by simply degrading them as they are released.
Mitochondrial DNA is toxic to cardiomyocytes. Necrosis of isolated adult mouse cardiomyocytes increases by 67 % after 24 h exposure to mtDNA, whereas exposure to nuclear DNA actually decreases cell death by 38 % [37]. Furthermore, following incubation of myocardial cells with mtDNA, the time to mitochondrial depolarization was decreased following laser-induced formation of reactive oxygen species. Exposing the cardiomyocytes to CPG-B, an agonist of the mtDNA receptor TLR9, also increased cell death. These observations in isolated cardiomyocytes are supported by experiments reported here in which mtDNA fragments increased infarct size (Fig. 6). We used a short coronary occlusion to minimize infarction, thus maximizing the chance of observing increased infarction induced by mtDNA fragments. These experiments indicate that mtDNA fragments do augment necrosis. Since it is impossible to estimate how many of these fragments actually entered the myocardium, the experiment is only qualitative in nature. But it should serve as a model for assessing potential treatments.
The finding that combining DNase I and EndoIII provides greater protection than either alone suggests that their actions are not identical. This is further indicated by the observation that EndoIII’s protection was ERK-dependent while that from DNase I was not. We hypothesize that enhanced repair induced by EndoIII minimizes DAMP production by keeping cells alive through mtDNA repair and possibly by activating protective signaling pathways in the cell which are ERK-related, while DNase I removes only mtDNA fragments that have been released from mitochondria that have already been destroyed. When the two proteins are combined, EndoIII would be expected to protect by preventing mtDNA release from many of the ischemically injured myocytes, while DNAse I would remove all mtDNA fragments released from those cardiomyocytes that EndoIII was unable to save. Hence an additive effect would be expected. DNase is currently available for human use and there is no reason to believe that EndoIII would not be safe. Thus, their combined use as a therapy for acute myocardial infarction appears feasible.
In summary, these data reveal that a mitochondrially targeted, bacterially-derived DNA repair enzyme, EndoIII, provides a potent anti-infarct effect in myocardial ischemia/reperfusion in open-chest rats. No adverse hemodynamic effects were seen; it was effective when given just before reperfusion, and its protection could be added to that from a platelet P2Y12 inhibitor. It may protect by preventing the release of pro-inflammatory mtDNA from the injured myocardium, and its protection was dependent on ERK-associated signaling pathways. EndoIII’s protection was enhanced when combined with DNase I. Thus, EndoIII’s potential for promoting myocardial salvage in the clinical arena merits further investigation.
References
Bliksøen M, Mariero LH, Ohm IK, Haugen F, Yndestad A, Solheim S, Seljeflot I, Ranheim T, Andersen GØ, Aukrust P, Valen G, Vinge LE (2012) Increased circulating mitochondrial DNA after myocardial infarction. Int J Cardiol 158:132–134. doi:10.1016/j.ijcard.2012.04.047
Boiteux S, Guillet M (2004) Abasic sites in DNA: repair and biological consequences in Saccharomyces cerevisiae. DNA Repair 3:1–12. doi:10.1016/j.dnarep.2003.10.002
Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR (1999) The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res 424:37–49. doi:10.1016/S0027-5107(99)00006-8
Chatterjee A, Mambo E, Zhang Y, DeWeese T, Sidransky D (2006) Targeting of mutant hogg1 in mammalian mitochondria and nucleus: effect on cellular survival upon oxidative stress. BMC Cancer 6:235. doi:10.1186/1471-2407-6-235
Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN (2011) Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am J Physiol 301:L892–L898. doi:10.1152/ajplung.00210.2011
Cuzzocrea S, Riley DP, Caputi AP, Salvemini D (2001) Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev 53:135–159
Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN (2002) Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol 283:L205–L210. doi:10.1152/ajplung.00443.2001
Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL (2000) Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J Biol Chem 275:37518–37523. doi:10.1074/jbc.M000831200
Fernández-Ruiz I, Arnalich F, Cubillos-Zapata C, Hernández-Jiménez E, Moreno-González R, Toledano V, Fernández-Velasco M, Vallejo-Cremades MT, Esteban-Burgos L, Pérez de Diego R, Llamas-Matias MA, García-Arumi E, Marti R, Boscá L, Andreu AL, López-Sendón JL, López-Collazo E (2014) Mitochondrial DAMPs induce endotoxin tolerance in human monocytes: an observation in patients with myocardial infarction. PLoS One 9:e95073. doi:10.1371/journal.pone.0095073
García N, Chávez E (2007) Mitochondrial DNA fragments released through the permeability transition pore correspond to specific gene size. Life Sci 81:1160–1166. doi:10.1016/j.lfs.2007.08.019
Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN (2001) Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol 280:L1300–L1308
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res 61:372–385. doi:10.1016/S0008-6363(03)00533-9
Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC (2013) Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol 304:L287–L297. doi:10.1152/ajplung.00071.2012
Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Wilson GL, Gillespie MN, Parker JC (2014) Mitochondrial targeted endonuclease III DNA repair enzyme protects against ventilator induced lung injury in mice. Pharmaceuticals 7:894–912. doi:10.3390/ph7080894
Hausenloy DJ, Yellon DM (2003) The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol 35:339–341. doi:10.1016/S0022-2828(03)00043-9
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175. doi:10.1016/S0140-6736(12)60916-7
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi:10.1016/0378-1119(89)90358-2
Ibrahim M, Strah H, Scozzi D, Huang HJ, Kreisel D, Krupnick A, Puyo C, Hachem RR, Alouch A, Trulock E, Gelman A (2014) Circulating mitochondrial DNA is elevated in lung transplant recipient patients with immediate early primary graft dysfunction. Am J Respir Crit Care Med 189:A1605
Kloner RA (2013) Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res 113:451–463. doi:10.1161/CIRCRESAHA.112.300627
Koczor CA, Shokolenko IN, Boyd AK, Balk SP, Wilson GL, LeDoux SP (2009) Mitochondrial DNA damage initiates a cell cycle arrest by a Chk2-associated mechanism in mammalian cells. J Biol Chem 284:36191–36201. doi:10.1074/jbc.M109.036020
Konstantinidis K, Kitsis RN (2012) Cardiovascular biology: escaped DNA inflames the heart. Nature 485:179–180. doi:10.1038/485179a
Miki T, Liu GS, Cohen MV, Downey JM (1998) Mild hypothermia reduces infarct size in the beating rabbit heart: a practical intervention for acute myocardial infarction? Basic Res Cardiol 93:372–383. doi:10.1007/s003950050105
Miura T, Ogawa T, Suzuki K, Goto M, Shimamoto K (1997) Infarct size limitation by a new Na+-H+ exchange inhibitor, Hoe 642: difference from preconditioning in the role of protein kinase C. J Am Coll Cardiol 29:693–701. doi:10.1016/S0735-1097(96)00522-0
Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K (2012) Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485:251–255. doi:10.1038/nature10992
Otsuka H, Bhushan S, King AL, Polhemus D, Wilson GL, Lefer DJ (2013) Mitochondrial targeted DNA repair enzyme 8-oxoguanene DNA glycosylase 1 (OGG1) attenuates myocardial infarct size. Circulation 128:A14115
Otsuka H, Bhushan S, King AL, Polhemus D, Wilson GL, Lefer DJ (2013) Mitochondrial targeted DNA repair enzyme, Endonuclease III (ENDO III), attenuates myocardial ischemia/reperfusion injury. Circulation 128:A14149
Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, de Souza-Pinto NC, Bohr VA, Budinger GRS, Schumacker PT, Weitzman SA, Kamp DW (2009) Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Rad Biol Med 47:750–759. doi:10.1016/j.freeradbiomed.2009.06.010
Pinz KG, Bogenhagen DF (2000) Characterization of a catalytically slow AP lyase activity in DNA polymerase γ and other family A DNA polymerases. J Biol Chem 275:12509–12514. doi:10.1074/jbc.275.17.12509
Rachek LI, Grishko VI, Alexeyev MF, Pastukh VV, LeDoux SP, Wilson GL (2004) Endonuclease III and endonuclease VIII conditionally targeted into mitochondria enhance mitochondrial DNA repair and cell survival following oxidative stress. Nucleic Acids Res 32:3240–3247. doi:10.1093/nar/gkh648
Rachek LI, Grishko VI, LeDoux SP, Wilson GL (2006) Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Rad Biol Med 40:754–762. doi:10.1016/j.freeradbiomed.2005.09.028
Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL (2002) Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem 277:44932–44937. doi:10.1074/jbc.M208770200
Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW (2008) Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol 294:C413–C422. doi:10.1152/ajpcell.00362.2007
Roubille F, Lairez O, Mewton N, Rioufol G, Ranc S, Sanchez I, Cung TT, Elbaz M, Piot C, Ovize M (2012) Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol 107:275. doi:10.1007/s00395-012-0275-3
Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi A-B, Gillespie MN (2005) Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol 288:L530–L535. doi:10.1152/ajplung.00255.2004
Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, Brill A, Wang Y, Wagner DD (2014) VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 123:141–148. doi:10.1182/blood-2013-07-514992
Simmons JD, Lee Y-L, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO (2013) Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 258:591–596. doi:10.1097/SLA.0b013e3182a4ea46
Stenslokken KO, Bilksoen M, Mariero L, Ytrehus K, Baysa A, Vaage JI, Valen G (2014) Extracellular mitochondrial DNA induces cell death in cardiomyocytes. Cardiovasc Res 103(Suppl 1):S122. doi:10.1093/cvr/cvu098.93
Tissier R, Hamanaka K, Kuno A, Parker JC, Cohen MV, Downey JM (2007) Total liquid ventilation provides ultra-fast cardioprotective cooling. J Am Coll Cardiol 49:601–605. doi:10.1016/j.jacc.2006.09.041
Torres-Gonzalez M, Gawlowski T, Kocalis H, Scott BT, Dillmann WH (2014) Mitochondrial 8-oxoguanine glycosylase decreases mitochondrial fragmentation and improves mitochondrial function in H9C2 cells under oxidative stress conditions. Am J Physiol 306:C221–C229. doi:10.1152/ajpcell.00140.2013
Wang J, Wang Q, Watson LJ, Jones SP, Epstein PN (2011) Cardiac overexpression of 8-oxoguanine DNA glycosylase 1 protects mitochondrial DNA and reduces cardiac fibrosis following transaortic constriction. Am J Physiol 301:H2073–H2080. doi:10.1152/ajpheart.00157.2011
Yang X-M, Cui L, Alhammouri A, Downey JM, Cohen MV (2013) Triple therapy greatly increases myocardial salvage during ischemia/reperfusion in the in situ rat heart. Cardiovasc Drugs Ther 5:403–412. doi:10.1007/s10557-013-6474-9
Yang X-M, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV (2013) Platelet P2Y12 blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther 18:251–262. doi:10.1177/1074248412467692
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107. doi:10.1038/nature08780
Acknowledgments
This study was funded in part by the National Institutes of Health grants PO1 HL66299 and OD010944 (MA), the National Institute of Environmental Health Sciences grant ES03456 (GW), and Heart, Blood and Lung Institute of the National Institutes of Health grants HL058234 and HL113614 (MNG).
Conflict of interest
GW and MNG are co-founders of Exscien, the manufacturer of Endo III.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, XM., Cui, L., White, J. et al. Mitochondrially targeted Endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Res Cardiol 110, 3 (2015). https://doi.org/10.1007/s00395-014-0459-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0459-0