Abstract
Matrix metalloproteinase (MMPs) are long understood to be involved in remodeling of the extracellular matrix. However, over the past decade, it has become clear that one of the most ubiquitous MMPs, MMP-2, has numerous intracellular targets in cardiac myocytes. Notably, MMP-2 proteolyzes components of the sarcomere, and its intracellular activity contributes to ischemia–reperfusion injury of the heart. Together with the well documented role played by MMPs in the myocardial remodeling that occurs following myocardial infarction, this has led to great interest in targeting MMPs to treat cardiac ischemic injury. In this review we will describe the expanding understanding of intracellular MMP-2 biology, and how this knowledge may lead to improved treatments for ischemic heart injury. We also critically review the numerous preclinical studies investigating the effects of MMP inhibition in animal models of myocardial infarction and ischemia–reperfusion injury, as well as the recent clinical trials that are part of the effort to translate these results into clinical practice. Acknowledging the disappointing results of past clinical trials of MMP inhibitors for other diseases, we discuss the need for carefully designed preclinical and clinical studies to avoid mistakes that have been previously made. We conclude that inhibition of MMPs, and in particular MMP-2, shows promise as a therapy to prevent the progression from ischemic injury to heart failure. However, it is critical that the full breadth of MMP-2 biology be taken into account as such therapies are developed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease is one of the leading causes of death in industrialized and developing countries. The first considerations of the involvement of matrix metalloproteinases (MMPs) in ischemic heart disease came from their roles in atherosclerotic plaque growth and stability, as well as the remodeling of the heart following myocardial infarct [41, 105]. At the time these hypotheses were made, MMPs were only considered to be secreted enzymes, activated by proteolytic removal of their propeptide domain, and their only considered targets were extracellular matrix proteins. This thinking was not only limited to cardiovascular medicine, as in the 1990s several pharmaceutical companies had MMP inhibitor programs developing anti-cancer and anti-arthritic drugs by virtue of the role of MMPs in tumour cell metastasis and joint inflammation/degradation, respectively. What has happened since then? It is now clear that MMP-2, a highly ubiquitous MMP, is also localized inside cardiac myocytes, can be directly and rapidly activated (within seconds) directly by peroxynitrite (Fig. 1), and has specific proteolytic targets within these cells (Fig. 2). In this review, we will, from this new perspective, interpret preclinical studies and clinical trials targeting MMPs in ischemic heart disease.
Activation of intracellular isoforms of MMP-2. The Zn2+ bound to the catalytic site of native MMP interacts with a cysteine residue in the N-terminal propeptide domain, inhibiting proteolytic activity. MMP-2 secreted from the cell can interact with MMP-14 and TIMP-2 which catalyze the proteolytic removal of the propeptide domain, yielding an active protease. The portion of MMP-2 which is not secreted, as well as the N-terminally truncated MMP-2NTT-50 and MMP-2NTT-76 isoforms lacking a signal peptide, can be exposed to RONS produced under pathophysiological conditions such as I/R injury. In the example here, peroxynitrite (ONOO−) and GSH interact with MMP-2, resulting in the S-glutathiolation of the propeptide cysteine residue leading to its dissociation from the catalytic Zn2+, and, therefore, allowing for proteolytic activity. MMP-2 is natively phosphorylated, and dephosphorylation by protein phosphatase 2A (PP2A) can further increase its proteolytic activity
Intracellular localization of MMP-2 and proteolytic targets relevant to ischemic heart injury. MMP-2 has been found to be associated with the cardiomyocyte sarcomere, mitochondria, mitochondria-associated membrane (MAM), cytoskeleton, nucleus, and caveolae, as well as a soluble protein in the cytosol. Proteins identified as substrates of MMP-2 within each cellular compartment are also indicated. Those substrates for which the relevance to ischemic heart injury has not been firmly established are indicated by a question mark
MMPs are a family of zinc metalloproteinases that were initially described primarily as extracellular proteases. The first members of what would eventually become known as the MMP family were discovered in 1962 as a collagenolytic activity released from tadpoles undergoing metamorphosis [50]. In 1975, a 33 kD protein with collagenolytic activity was purified from human tissue [120] and the sequence of human fibroblast collagenase, later termed MMP-1, was published in 1986 [47]. In the following years numerous proteins were found to share significant sequence homology with the catalytic domain of MMP-1 and were grouped into the MMP family, with 23 different human MMPs currently known [reviewed by 84]. Although they are sometimes referred to by their original names, referring to the extracellular matrix substrate with which they were associated (e.g., MMP-1 = collagenase 1, MMP-2 = gelatinase A), these names are misleading since, as will be described below, the different MMPs, and, in particular, MMP-2, have a wide repertoire of both intracellular and extracellular substrates that they are capable of proteolyzing.
MMP-2 has been widely studied due to its ubiquitous tissue distribution [31]. It is found in all cells of the heart, including cardiomyocytes [29, 118]. A large number of studies over the last decade have greatly enriched our knowledge of the functional roles of MMP-2, especially regarding the role of MMP-2 in the pathology of ischemic heart disease [4, 63]. From an experimental perspective, MMP-2 is also an attractive target since it is readily measurable in tissue samples using gelatin zymography.Footnote 1 We will, therefore, focus this review on the role of MMP-2 in ischemic heart injury and disease. As several excellent reviews cover the actions of MMPs in extracellular matrix remodeling in the heart [107, 115], here we will interpret the preclinical and clinical literature of MMP-2 with an eye towards elucidating the intracellular roles of MMP-2 in ischemic heart disease.
Regulation of MMP-2 activity and intracellular isoforms
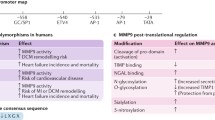
To prevent unwanted proteolytic activity, MMPs are tightly regulated at both levels of gene expression as well as via post-translational modifications and interactions [86]. MMP-2 was previously regarded as a constitutive enzyme due to its widespread tissue distribution, as well as the absence of proximal N-terminal promoter regions [26, 106]. However, MMP-2 gene expression is dynamically regulated, as it can be up-regulated by endothelin-1, angiotensin II and interleukin-1 beta, and its gene has a functional AP-1 element required for hypoxia-induced expression located 1 kb upstream from the MMP-2 N-terminus [2, 13].
MMPs are transcribed as an inactive zymogen consisting of an N-terminal signal sequence which can target proteins to the ER lumen for secretion, a propeptide domain containing an inhibitory cysteine residue, the eponymous Zn2+-binding metalloproteinase catalytic domain, a linker domain, and a hemopexin domain involved in substrate recognition. MMP-2 (and the closely related MMP-9) are distinguished from other MMPs by the presence of three fibronectin type II motif repeats in the catalytic domain. The activity of an MMP is controlled by a “cysteine switch” mechanism, in which Zn2+ in the catalytic domain can form a hydrogen bond with the propeptide cysteine sulphydryl residue, blocking the active site and preventing MMP activity [114]. The propeptide domain can be removed by plasmalemmal and extracellular proteases, often other MMPs, to yield an active protease. For example, membrane-bound MMP-14 (also known as MT1-MMP) cleaves the propeptide domain from MMP-2 [119].
The last decade of research has markedly expanded the view of MMP activation mechanisms beyond proteolysis (Fig. 1). It was recently discovered that the signal sequence of MMP-2 inefficiently targets the protein for secretion, such that nearly half of MMP-2 remains cytosolic [6]. We also found an N-terminally truncated splice variant lacking the first 50 amino acids, comprising the signal sequence as well as part of the N-terminal propeptide domain (MMP-2NTT-50) [6]. In addition, another MMP-2 isoform, MMP-2NTT-76, expressed only following oxidative stress, was identified in cardiac mitochondrial fractions [78]. Thus, there are at least three different intracellular MMP-2 isoforms: canonical MMP-2 (henceforth referred to as “MMP-2”), MMP-2NTT-50 and MMP-2NTT-76.
Original thought held that removal of the N-terminal propeptide domain was necessary for MMP activation (i.e. 72 kD MMP-2 being cleaved to a 64 kD enzyme). However, it was long recognized that any mechanism which disrupts the cysteine-Zn2+ bond results in MMP activity [114]. This is demonstrated in vitro via the SDS-mediated activation of MMP-2 that allows for 72 kD MMP-2 to be detectable with gelatin zymography. The cysteine switch of MMPs can also be triggered by endogenous stimuli, notably by reactive oxygen–nitrogen species (RONS) (Fig. 1) [51, 60, 88, 116]. Treating the zymogen form of MMP-2 with sub-micromolar concentrations of peroxynitrite, a highly reactive product of superoxide and nitric oxide, results in increased proteolytic activity. Conversely, higher levels of peroxynitrite (greater than approximately 100 µM) decreases its activity [116]. In the presence of glutathione, peroxynitrite S-glutathiolates the cysteine-switch motif in the propeptide domain. This 303 Dalton increase in molecular weight is detected by mass spectrometry, but cannot be resolved by SDS-PAGE [116]. This S-glutathiolated 72 kD MMP-2 is proteolytically active, and cleaves both artificial and endogenous substrates (i.e., OmniMMP fluorescent MMP substrate and troponin I, respectively) [60, 116].
MMP-2 activity can be further modulated by its phosphorylation (Fig. 1) [60, 95, 96]. Native MMP-2 expressed in human cells is phosphorylated on at least five residues. As these residues are on the surface of the enzyme and some are near the catalytic cleft, they are predicted to affect substrate docking and/or access to the cleft. Although the protein kinases regulating MMP-2 ability in vivo are as of yet unknown, protein kinase C can phosphorylate it in vitro [96]. Dephosphorylation with alkaline phosphatase increases human recombinant MMP-2 activity in vitro [96], or endogenous MMP-2 in rat heart homogenates [95]. Treatment of isolated rat hearts subjected to I/R injury with okadaic acid, a serine/threonine phosphatase inhibitor, at a concentration which inhibits protein phosphatase 2a, but not phosphatase 1 activity, maintained MMP-2 in a more phosphorylated state, and decreased the I/R-induced impairment of contractile function and loss of troponin I [95]. This is consistent with in vitro results showing that the native phosphorylation state of MMP-2 is required for the peroxynitrite-induced increase in activity [60]. The phosphorylation status of the MMP-2NTT-50 and MMP-2NTT-76 isoforms is yet unknown. Important regulatory proteins controlling MMP activity include the tissue inhibitors of metalloproteinases (TIMPs) (reviewed in [85]). Mammals have four TIMPs (TIMP-1 through TIMP-4) that typically interact with MMPs with 1-to-1 stoichiometry, binding to the MMP active site and thereby inhibiting proteolytic activity. With the exception of TIMP-1, all TIMPs appear to be capable of inhibiting all MMPs. TIMP-1, -2 and -3 are expressed across most mammalian tissues, with TIMP-4 having a more restricted distribution, including the heart. We found that TIMP-4 is localized to the thin myofilaments of cardiomyocytes, and is released from hearts subjected to I/R injury, allowing for a further increase of MMP-2 activity during reperfusion [99]. Of note, TIMPs themselves can be inactivated by peroxynitrite, as was shown for TIMP-1 [40] and TIMP-4 [33].
Synthetic MMP inhibitors
The finding of an association between MMPs and tumor cell invasiveness [16, 97] and inflammatory disease states such as osteoarthritis [15] led to a great effort to develop and bring into clinical practice small molecule MMP inhibitors for these conditions [reviewed by 39].
The earliest pan-MMP inhibitors to reach clinical trials in the early 1990’s were hydroxamate based (e.g. marimastat and ilomastat/GM6001). These drugs bind the catalytic Zn2+, resulting in inhibitory action against several MMPs, including related metalloproteinases such as ADAMs and TACE. Furthermore, at this time the large number of existent MMPs was not apparent, and which of the known MMPs to target was unclear. Nothing was known then about intracellular MMP activity and targets, nor MMP-2 isoforms or post-translational modifications, including the ability of RONS to directly activate MMP-2.
Despite success in preclinical studies treating animal models of disease, the clinical trials of the time were unsuccessful. Across multiple trials, many patients suffered from unexpected side effects (musculoskeletal syndrome–described as joint pain, stiffness, edema and reduced mobility) severe enough that some MMP-inhibitor treated patients requested to be removed from the study. Consequently, the dosages were often lowered to the point where they were less than what was used in preclinical studies. The non-specificity of MMP inhibitors was believed to account for these unexpected and trial limiting side effects. So-called “third generation” MMP inhibitors were designed to spare certain other metalloproteinases and MMPs from inhibition, typically MMP-1, whose inhibition was hypothesized (but never conclusively established) to be a cause of the musculoskeletal syndrome. Despite the lack of success in clinical trials, the research and development of novel MMP inhibitors with increased specificity is on-going [34].
All the tested MMP inhibitors were designed based on the smaller size and structure of proteolytically activated MMPs, as peroxynitrite-activated, intracellular MMPs were then unknown. From the perspective of rational drug design, the differences between extracellular and intracellular MMPs invoke the ‘apples to oranges’ comparison. Finally, also unrecognized was the fact that MMP-2 also has other post-translational modifications, such as phosphorylation, with consequences on its three dimensional structure and proteolytic activity [60, 96]. These factors together likely resulted in the failure and unexpected off-target actions of these poorly targeted MMP inhibitors. Although the idea to target MMPs was fundamentally a good one, it was at least 20 years ahead of its time.
One positive aspect of the extensive research attempts to develop MMP inhibitors is that there is a large selection of inhibitors available for experimental use. These range from Zn2+ chelators such as 1,10-phenanthroline, broad-spectrum MMP inhibitors such as GM6001 (ilomastat), and inhibitors with selective activity against only some MMPs, such as ONO-4817, with an IC50 for MMP-1 approximately 2000× higher than for MMP-2 [121].
Some members of the tetracycline class of antibiotics inhibit MMPs at sub-microbial doses, with doxycycline being the most potent of these [48]. This inhibitory action has been attributed to chelation of the catalytic Zn2+ or of protease-associated Ca2+, resulting in conformational changes to the protease [44, 102, 103]. Interestingly, doxycycline inhibits MMP-1 to a much lesser extent than other MMPs [102]. Due to its well-characterized safety profile and efficacy, a sub-antimicrobial formulation of doxycycline is the only MMP inhibitor approved for clinical practice for the treatment of periodontal disease [22] and rosacea [14]. It is no doubt for these reasons that doxycycline is the most commonly used pharmaceutical treatment in preclinical and clinical studies, as reviewed below.
Ischemic heart disease
Ischemic heart disease is the result of a reduction in blood flow to heart muscle (ischemia), commonly induced by atherosclerotic plaques in the coronary arteries, thrombosis, or coronary vasoconstriction. Acute ischemia, if prolonged, results in a myocardial infarct (MI). Cardiomyocytes are especially sensitive to oxygen-deprivation due to their extensive dependence on oxidative phosphorylation for the production of ATP to drive contractile function. The result of an MI is a myocardial lesion, containing necrotic tissue, and contractile dysfunction.
Over the past several decades, short-term survival rates for victims of MI have improved dramatically, in substantial part due to the recognition of the importance of the rapid restoration of blood flow to the infarcted tissue, now recognized as a critical clinical step in patients presenting with MI [125]. However, the restoration of blood flow and the consequent reoxygenation paradoxically cause additional tissue damage (reperfusion injury), and short-term death rates after MI are still approaching 10 %, with a chance of long-term cardiac failure of 25 % [64, 125]. The underlying causes of reperfusion injury remain unclear but several important factors have been identified [125]. One pronounced and very short-term consequence of reoxygenation is oxidative stress, mediated by a burst of RONS that occurs within seconds to minutes upon reperfusion [18, 124]. In addition to the direct activation of MMP-2, RONS have been shown to be a potential mediator of other factors implicated in I/R injury, such as intracellular and mitochondrial Ca2+ overload and opening of the mitochondrial permeability transition pore [125].
Despite great biomedical and clinical interest, there are still no pharmacological treatments capable of decreasing reperfusion injury. This has been the subject of a great deal of research since reperfusion is a procedure that normally occurs after hospital admission and, therefore, there is much more scope for direct pharmaceutical intervention than there is for preventing the original MI, which would require identification of susceptible individuals followed by chronic administration of drugs, coupled with lifestyle changes.
Following MI, the myocardium undergoes a series of compensatory structural changes, collectively termed “myocardial remodeling”, ultimately contributing to heart failure [54]. Left ventricle (LV) remodeling results in an enlargement and dilation of the LV, along with wall thinning. Long-term post-MI ventricular remodeling continues to be a significant cause of heart failure in these patients, resulting in significant mortality. These structural changes have, at their base, both intra- and extracellular roots. The relationship between extracellular MMP activity, the extracellular matrix, and myocardial function has been extensively studied (reviewed in [107]), and has driven an interest in using MMP inhibitors as a treatment to prevent the progression of MI to heart failure. However, the unique biological actions of intracellular MMP-2, especially its activation by oxidative stress, suggest that its inhibition could also reduce some of the damage induced by reperfusion.
Novel targets and roles of MMP-2 in ischemic heart injury
There is now substantial evidence that MMP-2 plays a critical role as an intracellular mediator of cardiac I/R injury, also prior to the occurrence of remodeling. MMP-2 contributes to the acute, reversible mechanical dysfunction that occurs immediately following reperfusion (stunning injury) [27]. MMP-2 activity is decreased with pre- and postconditioning [10, 32, 72], which provide cardioprotection against I/R injury [53]. However, it has yet to be shown if decreased MMP-2 plays a causative role in the cardioprotection phenomena observed with pre- and postconditioning. MMP-2 is located in or co-localized with several subcellular organelles of cardiac myocytes and other cells (Fig. 2), including the sarcomere [5, 29, 118], cytoskeleton [109], nuclei [69], caveolae [28], mitochondria [57, 77, 118], and the mitochondria-associated membrane [57].
The best, thus far, characterized intramyocyte targets of MMP-2 in I/R injury are specific sarcomeric proteins (Fig. 2). Troponin I (TnI), a regulator of actin–myosin interaction, is readily proteolyzed by MMP-2. The presence of TnI and its fragments in the circulation is widely recognized as a biomarker for cardiac injury. In isolated rat hearts subjected to I/R injury, cardiac TnI levels are decreased and MMP inhibitors prevented TnI loss and decreased the severity of the post-I/R contractile dysfunction that is characteristic of myocardial stunning [118]. Titin, the molecular spring of the sarcomere which determines both systolic and diastolic function, is also proteolyzed by MMP-2 in I/R injury; this could be prevented by MMP inhibition or by genetic ablation of MMP-2 [3]. In addition, myosin light chain-1, which is degraded in cardiac I/R injury [112], and α-actinin, which plays an important role in maintaining sarcomere structure and contractile force conductance, can also be proteolyzed by MMP-2 [98, 109]. MMP-2 localizes to these proteins in both thin and thick myofilaments, as well as to the Z-line region of titin. In addition, TIMP-4 is associated with the myofilaments and is lost during reperfusion [99]. The full spectrum of intracellular MMP-2 targets relevant to ischemic heart disease will undoubtedly grow with further study. Indeed a review of unbiased, degradomics-based data of putative and confirmed intracellular targets for multiple MMPs suggests that 99 out of 120 of these are targets of MMP-2 [23].
Hearts from transgenic mice expressing heart-specific, constitutively active MMP-2 (by single point mutation), exhibit functional impairments, including a significantly decreased LV ejection fraction, as well as histological abnormalities including myocyte hypertrophy, sarcomeric and mitochondrial disorganization, and myofilament lysis [12]. Their hearts were more sensitive to I/R injury, resulting in greater impairment of contractile function associated with impaired mitochondrial function and larger infarct size [128]. MMP-2 was also found to be physically associated with intracellular TnI, and TnI levels were also reduced. Hearts from mice expressing cardiac-specific MMP-2NTT-76 which, as described above localizes to mitochondria, develop spontaneous systolic heart failure associated with cardiac hypertrophy and inflammation. Like MMP-2 transgenics, they exhibit increased sensitivity to I/R injury. However, MMP-2NTT-76 hearts show little evidence of myofilament lysis, consistent with different isoforms of intracellular MMP-2 having different effects [77, 78].
This very rapid remodeling of the susceptible intracellular sarcomeric and cytoskeletal protein targets (the “intracellular matrix”) by MMP-2 results in defects in both systolic and diastolic contractile function. In addition we predict that the cleavage fragments of these proteins have their own biological activities, which include “non-self” recognition of fragments, activation of the immune response and inflammation, which are all part of the transition to failing myocardium. This contrasts with the traditional view of MMP involvement in extracellular matrix remodeling which generated great interest in the use MMP inhibitors to prevent adverse remodeling and thus the longer-term progression to heart failure. An important point of comparison is to consider the role of MMP-2 in myocardial stunning versus that which may cause irreversible injury to muscle cells (necrosis, apoptosis) as a result of myocardial infarct. We will, therefore, interpret both the preclinical and the clinical literature on ischemic heart injury from the perspective that MMP-2 could contribute to both these types of injury by proteolysis of both intracellular and extracellular protein targets.
Preclinical studies of MMP-2 in ischemic heart disease
For the purpose of this review, we identified 37 preclinical studies which used animal models to determine the effect of MMP inhibition on cardiac ischemic injury. Two main methodologies were used: (a) induction of MI in an anesthetized animal (in vivo) and (b) induction of global myocardial ischemia in isolated perfused hearts (ex vivo).
For in vivo studies, MI was typically induced by surgical ligation of the left anterior descending coronary artery in an anesthetized animal. This was most commonly practiced without a subsequent reperfusion phase (i.e. coronary artery occlusion was initiated, the chest was closed and the animal was left to recover). The involvement of MMPs was evaluated in MMP-2 knockout (KO) mice (Table 1), or pharmacologically by the use of MMP inhibitors (Table 2). For ex vivo studies (Table 3), the heart is rapidly removed from an anesthetized animal and promptly perfused with a glucose-containing, oxygenated buffer which supports physiological contractile function. Typically, a Langendorff heart preparation is used, where the perfusate is applied in a retrograde fashion into the aorta, resulting in closure of the aortic valves and perfusion of the heart via the coronary circulation. Global ischemia is induced by complete blockage of flow of the perfusate, followed by reperfusion triggered by the resumption of perfusate flow.
To facilitate comparison between studies, we classified results from each study as to whether the tested drugs decreased infarct size, improved cardiac contractile performance, decreased mortality, or decreased LV remodeling. In every study the measurements in question were worsened by ischemic injury relative to uninjured controls. Contractile performance was measured in terms of LV pressure and/or heart rate (ex vivo), or, typically, hemodynamic measures via echocardiography (in vivo). Mortality observed was the result of acute injury suffered during, or, in the immediate aftermath of in vivo MI, mostly occurring during the days immediately following MI induction (although some studies followed animals for up to 2 months post-MI, no additional mortality was reported). LV remodeling was determined via measures of LV geometry (such as hypertrophy and wall thinning). This is only an incomplete measure of remodeling, since it does not incorporate the molecular events (such as intracellular and extracellular matrix protein remodeling) through which MMPs are expected to act. However, LV remodeling can proceed even when extracellular matrix components are protected from degradation by MMP inhibition or genetic ablation of MMP-2 [79].
The most commonly used species were rats and mice, together accounting for more than three-quarters of studies analyzed. Other animals used were pigs, rabbits, and Syrian hamsters. All animals used were young, healthy, adults, with the exception of one study which fed one group of rats a cholesterol-rich diet prior to ex vivo I/R [45]. Strikingly, the great majority of studies were conducted in male animals (30 studies). Of the remainder, the sex used was either not specified or both sexes were analyzed together. It has been reported that male mice suffer much greater mortality than females following in vivo MI relative to females [55]. This is consistent with what has been reported for humans, with increased resistance of the female heart to I/R injury, both in terms of acute injury and remodeling post-MI [89]. This is, therefore, a potentially important factor that has been overlooked. It is also worthy to note that none of the studies reported any adverse effects of MMP inhibition. However, no studies specifically looked for evidence of the musculoskeletal syndrome which plagued past clinical trials of MMP inhibitors.
Given the relatively recent appreciation of the importance of intracellular MMP-2 biology, many preclinical studies on the efficacy of MMP inhibitors were designed to test the action of MMPs on LV remodeling by focusing on changes to the extracellular matrix. The majority of these studies consisted of the induction of MI, and examining the effects of this over time intervals of days to months (Table 2).
In vivo MI–MMP-2 knockout mice
Given the current lack of pharmacological MMP inhibitors that are specific towards MMP-2, genetic ablation of MMP-2 represents one method of disentangling the effects of different MMPs (Table 1). Two studies reported the response of MMP-2 KOs to in vivo MI [52, 79]—both reported decreased mortality rates in the week following MI, but only one found protective effects in terms of hemodynamic performance or remodeling [52]. Interestingly, even where genetic ablation of MMP-2 did not prevent LV enlargement, it did prevent the degradation of ECM structural components (laminin and fibronectin) observed in control hearts [79].
In vivo MI–MMP inhibitors
The most commonly assessed endpoint when MMP inhibitor drugs were used to treat animals subjected to in vivo MI was myocardial remodeling, typically in terms of LV geometry (Table 2). Most studies (14 out of 16) that assessed remodeling found evidence of a protective effect of MMP inhibition. This was observed in all studies (5) that started treatment prior to infarct induction, and most (9 out of 11) that started treatment at or after MI induction. Positive effects were observed with the broad-spectrum MMP inhibitor doxycycline as well as with a TIMP-4 viral transgene, expected to inhibit most MMPs, but also with the third generation MMP inhibitors PD166793 (selective for MMP-2, -3 and -13 over MMP-1, -7 and -9), PGE-530742 (selective for MMP-2, -3, -8, -9, -13 and -14 over MMP-1 and -7; re-named to PG-116800, as used in the PREMIER clinical trial described below), CP-471474 (selective for MMP-2, -3, -9 and -13 over MMP-1) and ONO-4817 (selective for MMP-2, -8, -9, -12 and -13 over MMP-1 and -7). One study used what was claimed to be a specific inhibitor of MMP-2 (TISAM), which did not show a protective effect on remodeling [79]. No evidence for the specificity or efficacy of this inhibitor was presented and we were unable to identify any in the published literature. Thus, as a whole, these studies are consistent with MMP-2 having a detrimental role in post-MI ECM remodeling.
LV remodeling is most significant in that it can ultimately progress to heart failure. Since overt heart failure in rats undergoing coronary artery occlusion and MI does not become apparent until 3–4 months post-MI [90], shorter-term studies such as those described here must rely on predictors such as hemodynamic performance (e.g. ejection fraction). It is striking that of the 14 in vivo MI studies reporting hemodynamic data, only 7 found evidence of a protective effect of MMP inhibition. These were distributed across studies initiating treatment both prior to- and post-MI, and among treatment with broad spectrum or third generation MMP inhibitors, as well as among both short- or longer-term treatment periods. The same broad distribution of experimental conditions is present among studies not showing a protective effect. MMP inhibition decreased infarct size and mortality in a minority of the studies in which they were reported (2 out of 11 and 1 out of 7 studies, respectively).
The majority of studies were done using rats and mice. However, a small number of studies used swine, which, as a large animal model, may be more clinically representative. These studies used the MMP-1-sparing inhibitors PD166793 [82] or PGE-530742/PG-116800 [7, 123]. To replicate the likely clinical scenario of post-MI treatment, PD166793 was given starting 5 days post-MI and was found to reduce infarct size at 2 and 8 weeks post-MI. PGE-530742 was started 3 days prior- or 3 days post- MI. Measures of LV geometry suggested a decrease in LV remodeling for both treatments, but infarct size was only decreased in PD166793-treated animals. Measures of myocardial performance were not reported for PD166793, but LV contractile performance was improved in animals treated with 1 mg/kg PGE-530742 starting 3 days prior to MI [7]. Interestingly, treatment with 10 mg/kg did not show this protective effect [123]. However, treatment with this dosage starting 3 days post-MI was protective [123].
In vivo–I/R
Prompt reperfusion is currently understood to be an important therapeutic response to MI, meaning that reperfusion injury is a common clinical phenomenon. These preclinical studies (Table 2) induced MI by coronary artery ligation for a short duration (30–60 min) followed by re-opening the blocked artery to allow for reperfusion (2–48 h; in one study animals were retained for 21 days). MMP inhibitors were applied either prior to surgery or starting during the reperfusion phase. These studies primarily used infarct size as an end point, and, in contrast to the in vivo MI studies described above, which largely focused on MMP-1 sparing inhibitors, the drugs used in these studies were the broad-spectrum MMP inhibitors GM6001 and doxycycline. These studies reported a consistent protective effect of MMP inhibition on infarct size. This was observed with both pre-treatment with inhibitors and when treatment was initiated at the time of surgery. Only one study reported effects on contractile performance and remodeling, finding a protective effect of TIMP-3 gene delivery on both, after a 1-h occlusion followed by a 21-day treatment period [122].
Ex vivo I/R studies
These studies (Table 3) were primarily designed to investigate the intracellular actions of MMP inhibition on I/R-induced myocardial stunning injury, with LV contractile function measured via an inserted balloon. MMP inhibitors were added to the perfusate starting either prior to ischemia or at the start of reperfusion. Protective effects on cardiac performance were consistently observed, with the broad-spectrum MMP inhibitor 1,10-phenanthroline (4 studies), doxycycline (9 studies), the third generation inhibitors ONO-4817 and PD166793 (1 study each), as well as for a small number of novel inhibitors (2 studies) and a neutralizing antibody against MMP-2 (1 study). In addition, infarct size was decreased by the broad-spectrum MMP inhibitor GM6001 in hearts from rats that had been fed either regular chow or a high-cholesterol diet [45]. Notably, this was the only study we identified that attempted to account for the prevalence of other pathological conditions, such as atherosclerosis, in the target patient population.
The initiation of treatment prior to induction of ischemia means that this would be most applicable to predicted I/R events, such as that which occur during cardiopulmonary bypass, explanted heart preservation or coronary angioplasty procedures. Two studies (one on rat hearts, the other on rabbit hearts) examined the effect of initiating MMP inhibition at the onset of reperfusion only (using doxycycline and GM6001, for the first 2 and 15 min of reperfusion only, respectively). Strikingly, both found that infarct size was decreased by this very short-duration treatment [9, 32]. However, only one of these studies measured LV function and showed that it was not improved by a 2 min treatment with doxycycline [32].
Neonatal asphyxia and cardiac hypoxia-reoxygenation injury
Asphyxia is a common cause of neonatal mortality and morbidity of which ischemic injury to the myocardium plays a significant role in overall outcome, and the injury to the heart can extend from stunning to infarction. A recent study determined the dose-dependent effects of doxycycline in hypoxia–reoxygenation injury to the heart [70]. In this clinically translatable model of injury, where piglets were subjected to 2 h of hypoxia followed by 4 h of reoxygenation, doxycycline was administered intravenously (at 3, 10 or 30 mg/kg) 5 min into the reoxygenation period. Post-resuscitation administration of doxycycline improved cardiac and stroke volume indices, systemic arterial pressure, and systemic oxygen delivery and consumption. This was associated with decreased MMP-2 activation, enhanced myocardial TnI and reduced plasma TnI levels, and reduced myocardial lactate and lipid hydroperoxide levels. Dose-dependent protective effects of doxycycline were seen at 10 and 30, but not 3 mg/kg [70].
Preclinical studies–summary
It is clear that treatment with MMP inhibitors prior to induction of MI has a protective effect on LV remodeling. However, other measurements of clinical relevance, such as myocardial performance, mortality or infarct size were affected to a lesser extent. On the other hand, infarct size was consistently reduced by MMP inhibition when a short period of in vivo ischemia was followed by reperfusion, and this was true even when treatment was not initiated until the onset of the reperfusion stage. Furthermore, myocardial stunning induced by ex vivo I/R injury was consistently ameliorated by MMP inhibition.
Because the drugs used are not specific to any one MMP, it is not currently possible to ascribe protective effects only to the inhibition of MMP-2. Genetic ablation of MMP-2 did result in some protective effects in mice subjected to MI, notably of acute mortality. However, LV remodeling was not impacted as consistently as was the case for pharmaceutical MMP inhibition. Similar studies have been performed using other MMP KO mice. Post-MI LV remodeling was unaffected in MMP-7 KO mice [73], but decreased in MMP-9 KO mice [35, 74] and MMP-14 heterozygote KO mice (MMP-14 homozygous KO mice are non-viable) [65, 126]. However, a disadvantage of genetic KO mice is that they have been subjected (and thus adapted) to the effects of the genetic manipulation since birth (or before, in the case of homozygous mothers), increasing the possibility of confounding variables. For example, MMP-2 KO mice exhibit smaller birth weights and delayed growth [59] as well as reduced embryonic survival rates (Mosig RA, Schulz R, Kassiri Z, Martignetti J, submitted for publication) relative to wild-type mice. The development of inducible KOs would allay this concern.
Clinical studies of MMP-2 in ischemic heart disease
MMP-2 as a clinical biomarker of ischemic heart disease
Given the association of secreted MMPs with myocardial remodeling there has been considerable interest in developing MMPs as clinical biomarkers for ischemic heart injury and heart failure [36]. Nilsson et al. [87] investigated the diagnostic value of plasma MMP-2 levels with final infarct size and ventricular dysfunction in ST-segment elevation acute MI (STEMI) patients. Fifty-eight patients receiving primary percutaneous coronary intervention (PCI) had their blood sampled immediately prior and 12–48 h afterwards. Infarct size, LV dysfunction and remodeling were measured by cardiac magnetic resonance imaging 5 days and 4 months after their infarct. Plasma MMP-2 at both 0 and 12 h showed a consistent and significant correlation with infarct size and LV dysfunction at both time points after their infarct, and this also correlated well with serum TnI levels. Interestingly, neither MMP-8, MMP-9 nor myeloperoxidase activity was consistently correlated with the outcome measures. Thus, monitoring plasma MMP-2 activity as early as possible in STEMI patients may be helpful to assess the extent of myocardial damage and guide the stratification of clinical management to reduce the consequences of ischemic heart injury. Matasunga et al. [80] studied the activity of plasma MMP-2 14 days after STEMI, finding that acute STEMI patients with higher MMP-2 activity had a greater increase in LV end diastolic and systolic volume indices 6 months after STEMI.
Squire et al. [108] measured MMP-2 and -9 by ELISA in plasma samples from acute STEMI patients, sampling the blood within 6–12 h of the onset of symptoms and after the delivery of thrombolytic therapy, and then every 24 h for the first 5 days post-MI. Echocardiographic parameters were measured upon admission and 6 weeks later. These authors found a significant negative correlation between peak MMP-2 levels and LV volumes, whereas there was a positive correlation between peak MMP-9 and LV volumes. Interestingly, higher plasma levels of MMP-2 were observed after inferior compared to anterior acute MI, suggesting that there may be a greater induction of MMP-2 activity in surviving myocardium than is possible when the infarct is anterior, the latter resulting in larger infarcts.
Changes in MMP-2 from I/R as a result of cardiac surgery
We examined coronary artery bypass graft patients (CABG) undergoing elective cardiopulmonary bypass (CPB) surgery for evidence of MMP-2 activation in the heart [71]. Fifteen patients with stable angina were examined. During CPB the stilled heart undergoes mild ischemic injury as a result of cardioplegia and then is subjected to reperfusion injury once the repair is complete and the aortic cross-clamp is removed, reestablishing normal blood flow through the coronary circulation. MMP-2 and MMP-9 activities (measured by gelatin zymography) were markedly elevated in right atrial biopsies obtained within 10 min of aortic cross-clamp release, compared to samples obtained immediately after the start of CPB, but before cardioplegia. There was a significant inverse correlation between atrial MMP-9 and MMP-2 activities in the reperfused myocardium and LV stroke work index 3 h post-surgery, at the time point when cardiac function was most severely depressed during the first 24 h post-surgery. A positive correlation between atrial biopsy MMP-2 or MMP-9 activities and the aortic cross-clamp duration, but not the duration of CPB, was also observed. Thus, MMPs are activated in the human heart within 10 min of ischemia, and the timing of their maximum activation correlates with the nadir of contractile function 3 h after reperfusion. Interestingly, in similar patients, a transient increase in MMP-9 activity was observed in myocardial interstitial fluid during the onset of CPB and was enhanced after reperfusion [106].
This prompted us to perform the first randomized, double-blinded, placebo-controlled trial to evaluate whether sub-antimicrobial dosing of doxycycline could, therefore, reduce myocardial stunning injury in the first 24 h in patients undergoing primary elective CABG surgery with CPB [100]. Forty-two patients were randomized to receive either a sub-antimicrobial dosage of doxycycline (20 mg, twice a day orally) or matching placebo pill, beginning at least 2 days prior to surgery, on the day of surgery, and for the first 3 post-operative days. Right atrial biopsies were collected as above to measure MMP-2 activity and TnI levels. Blood was collected for measuring plasma TnI and the inflammatory markers IL-6 and C-reactive protein. Atrial biopsy MMP-2 activity was lower upon reperfusion in the doxycycline group, and the increase in MMP-2 in the placebo group due to reperfusion did not occur in the doxycycline group. However, TnI levels were not significantly altered in atrial biopsies or blood samples upon reperfusion, suggesting that cardiac injury was minimal. Thus, the severity of the temporary and reversible cardiac dysfunction seen in these patients was modest. Doxycycline did not affect ventricular stroke work index following reperfusion, nor the CPB-CABG induced increase in plasma MMP-9 activity, IL-6 or C-reactive protein levels. However, it is possible that MMP-2 and TnI in right atrial biopsies may not reflect those seen in the LV. Together with the lack of changes in TnI levels in both tissue and plasma, it may not be surprising that doxycycline did not show any protective effect, at least on a parameter which is more indicative of systolic function, given that left ventricular stroke work index does not estimate diastolic dysfunction which occurs post-CPB, and which may be more reflective of myocardial performance [38]. Other limitations of this study include: (a) the small sample size, (b) the dose of doxycycline may have been too low, or the use of a loading dose should have been considered, and (c) the impact of doxycycline use during cardiac surgery on longer-term outcomes following CPB-CABG should also be considered. Importantly, doxycycline was well tolerated and not associated with any adverse events.
Acute coronary syndrome
MIDAS (MMP Inhibition with sub-antimicrobial doses of Doxycycline to prevent Acute coronary Syndromes). The MIDAS trial determined the effect of sub-antimicrobial doxycycline (20 mg twice daily) in angina patients [17]. This prospective, double-blinded, placebo-controlled trial started patients on either placebo or doxycycline within 2 weeks of the onset of symptoms and followed them for a total of 6 months. Doxycycline reduced the plasma level of MMP-9 and other inflammatory markers, including C-reactive protein (the primary biochemical outcome) but showed no benefit in the composite clinical endpoint comprised of sudden death, fatal or non-fatal myocardial infarct, or troponin-positive unstable angina. However, this trial was likely underpowered, with only 2 out of 50 participants meeting any of the clinical endpoints. However, as C-reactive protein is a strong predictor of cardiovascular disease morbidity and mortality [113], this trial shows an important beneficial effect of doxycycline over a relatively short study period.
Myocardial infarct
Moving from myocardial stunning and angina we now consider two clinical trials of MMP inhibitors to prevent detrimental ventricular remodeling following myocardial infarct. This remodeling results in excessive LV dilatation which increases the risk of further complications including congestive heart failure, aneurysm formation, and cardiac rupture. Both of the studies follow successful preclinical studies in which short-term use of third generation proprietary MMP inhibitors [62] or doxycycline [49, 117], administered around the time of experimental infarction, reduced infarct size and preserved left ventricular structure.
PREMIER (PREvention of Myocardial Infarction Early Remodeling). This prospective, double-blind, placebo-controlled, multi-center study investigated the use of an MMP-1-sparing MMP inhibitor, PG116800 (identified as PGE-530742 in the studies cited in Table 2), in STEMI patients who showed an immediate and severe reduction in LV ejection fraction of <40 % [56]. The drug or placebo treatment began within 48 h of MI and continued for 3 months. PG116800 treatment resulted in no benefit in any clinical outcomes or left ventricular remodeling. Beyond the already discussed problems that this drug was designed as a pan-specific MMP inhibitor, without knowledge of which MMPs to inhibit or not to inhibit, nor of intracellular MMP-2 biology, this trial had a major limitation severely limiting its interpretation [104]. In consideration of possible undesired side effects seen in previous trials (i.e. musculoskeletal syndrome), the dose used was one quarter of that used in preclinical studies in swine, and this was lowered even further in some patients in the midst of the study. Other limitations of this study were that MMP inhibition was likely started too late (mean 54 h post-MI) to counteract infarct expansion, a major determinant of the remodeling process, and was likely too prolonged, potentially resulting in adverse effects on LV remodeling.
TIPTOP (Tetracycline (Doxycycline) In Patients with large acute myocardial infarction TO Prevent left ventricular remodeling). In this single site, open-label, randomized, phase II trial, 110 STEMI patients with LV ejection fraction of <40 % were given 100 mg doxycycline or placebo immediately after primary percutaneous coronary intervention, and then every 12 h for 7 days, in addition to standard therapy [25]. Following them for 6 months the authors found that doxycycline patients had a significantly reduced increase in LV end-diastolic volume index (0.4 vs 13.4 %), infarct size (5.5 vs 18.8 %) and infarct severity as determined by echocardiography or single photon emission computed tomography. Despite the fact that the trial was not powered for clinical endpoints, they also reported a statistically significant 50 % reduction in the composite of death, myocardial infarct, congestive heart failure and stroke, with the majority of the effect stemming from the difference between the incidence of fatal and non-fatal heart failure. Although well designed and executed, limitations of the study include that it was designed as an open label study and not all baseline characteristics were evenly matched (notably, more women and diabetics in the control versus the doxycycline groups). It is important to note that the study evaluated the use of an antimicrobial dose of doxycycline. Interestingly, a post hoc analysis found that doxycycline improved myocardial function and decreased remodeling in MI patients with a pre-intervention occluded infarct-related artery (with a thrombolysis in myocardial infarction (TIMI) flow grade of ≤1), but had no effect in MI patients with an open infarct-related artery with a TIMI flow grade of 2–3 [24].
Without doubt this promising study, which shows the effectiveness of a short-term course of doxycycline, an inexpensive drug with a well-understood and excellent safety profile, needs to be proven in a large, multi-center, placebo-controlled trial. A key consideration in such a trial is the dose to be used, as the widespread use of broad spectrum antibiotics can contribute to the development of antibacterial resistance. Whether sub-antimicrobial dosing would be effective in such studies is unknown. We recommend that this point be given very special consideration in the design of future trials of doxycycline in the treatment of ischemic heart disease.
Implications
Of four published clinical trials testing the efficacy of MMP inhibitors for ischemic heart disease, one had design issues which prevented a meaningful interpretation (PREMIER), one showed efficacy of sub-antimicrobial doxycycline medium-term treatment for angina in terms of biochemical endpoints but had insufficient power to assess its clinical endpoints (MIDAS), one demonstrated efficacy of short-term treatment with doxycycline in preventing adverse clinical outcomes in patients who had suffered MI (TIPTOP), and another failed to show therapeutic benefits for short-term treatment with sub-antimicrobial doxycycline in patients undergoing CPB-CABG.
Generalizing across these multiple clinical scenarios of myocardial ischemia, doxycycline exerted beneficial effects with conventional, antimicrobial doses over the short-term (1 week), whereas a sub-antimicrobial dose was only effective over the medium-term (6 months), and not in the short-term. Given the predicted lack of serious side effects, we, therefore, recommend that future clinical trials adopt one of the two former dosage regimes. The potential for side effects from other MMP inhibitors would seem to contraindicate the use of these drugs in clinical trials until these issues are resolved, especially since there is currently little evidence of increased effectiveness of these drugs over doxycycline.
In the context of trials testing doxycycline for ischemic heart disease, a retrospective epidemiological study from the UK must be mentioned. This study examined the hypothesis that bacterial infection contributes to coronary artery disease leading to MI. The records of more than 16,000 primary practice patients were examined to determine if there was a relationship between antecedent use of any antibiotic drug and the risk of a first-time acute MI. They found that patients who took tetracyclines, but not any other class of antibiotics, showed a lower incidence of MI [46, 81]. Given that several tetracyclines but not any other classes of antibiotics are capable of inhibiting MMP activity, it is tempting to speculate that sub-antimicrobial dosing of doxycycline could be used to reduce the risk of MI in susceptible patient populations.
When comparing the above clinical trials with preclinical studies investigating the effects of MMP inhibition (Tables 1, 2, 3), one is struck by the absence of preclinical studies that recapitulate clinical scenarios. Numerous studies investigated the effects of MMP inhibition on animals subject to short- to medium-term MI, but most of the examined studies did not include typical clinical events such as myocardial reperfusion via thrombolytic therapy or primary percutaneous coronary intervention (Table 2). There are substantial differences in outcome between MI with and without subsequent reperfusion, with reperfusion decreasing infarct expansion and mortality rate and improving some hemodynamic measures of LV function [76]. Four studies did follow-up short periods of MI (30–45 min) with reperfusion and treatment with doxycycline or GM6001, and all found that treatment decreased infarct size [9, 11, 49, 94]. Although promising, none of these studies followed the animals for more than 2 days post-MI, or reported measurements of myocardial performance. However, protective effects on myocardial performance, infarct size and remodeling after a 21 day reperfusion period following 1 h MI were shown for administration of TIMP-3 via gene delivery [122]. We recommend that such an experiment should be performed using clinically viable agents such as doxycycline.
Disconnects between preclinical and clinical studies have been noted before, both in the context of earlier clinical trials of MMP inhibitors for cancer or inflammatory diseases [30, 91], as well as more generally for treatments of cardiac I/R injury [125]. In that context (and in addition to differences between species in the response to myocardial ischemia [53]) there are a number of likely characteristics of patient populations that are rarely re-capitulated in preclinical studies [54, 125]:
-
(a)
Likely suffering from the effects of aging and the presence of other comorbidities, notably metabolic syndrome, atherosclerosis and diabetes.
-
(b)
Likely consumption of additional medications.
-
(c)
A duration of ischemia (3–12 h) that is not commonly used in preclinical studies.
-
(d)
Relevant outcomes are long-term (such as the incidence of subsequent heart failure and mortality over months to years).
-
(e)
Inflammatory origins of infarct (e.g., atherosclerosis).
-
(f)
Variable timing of reperfusion relative to infarct duration.
-
(g)
Infarct regions of a smaller relative size.
Resolution of these issues via improvements to the animal models used can, admittedly, be challenging from logistical and financial (and possibly ethical) points of view. However, given the expense and potential medical risks undergone by patients during clinical trials, it is important that attempts are made to increase their odds of success. There are also issues that are relatively simple to address, such as the rarity of female animals in preclinical studies (Tables 1, 2, 3). The bias in biomedical research towards male animals has been previously noted [8], but it seems to be particularly egregious among the studies examined here.
An alternative approach suggested by Yellon and Hausenloy [125] is to design clinical trials that better reflect the findings and limitations of existent preclinical studies. For example, one could only include patients who have suffered MI without any subsequent pharmacological or surgical reperfusion. An example of a preclinical study of direct clinical relevance is that of neonatal hypoxic injury [70], described above. This is especially well suited for clinical trials since few extrapolations need to be made in the translation to human treatment.
Conclusions
MMPs, and MMP-2 in particular, remains a very promising target for the management of ischemic heart disease. Issues we have discussed here include preclinical studies that should be more representative of clinical scenarios encountered in human patients, and, of great importance, include both sexes. More attention needs to be paid to the proper design of future clinical trials, in particular to experimental power and dosage. Doxycycline, with a well-understood safety profile, should be a subject of future clinical trials utilizing either short-term antimicrobial or long-term, sub-antimicrobial doses. The design of improved, safer, and more specific MMP inhibitors needs to continue. A key issue is that no drug has yet been shown or specifically designed to inhibit intracellular MMP-2 or its isoforms. These future inhibitors will need a thorough evaluation in well-designed preclinical studies.
Notes
In gelatin zymography, proteins are separated on a SDS-PAGE gel co-polymerized with gelatin. The SDS is subsequently removed by incubation of the gel in a non-denaturing detergent, re-activating gelatinolytic MMPs which then degrade gelatin in their vicinity. Coomassie blue staining reveals zones of gelatinolytic activity as clear areas against a stained background. Gelatin zymography allows for unambiguous differentiation of MMP-2 from other MMPs, without the requirement for immunological detection. Furthermore, this method can also detect changes to MMP-2 activity due to post-translational modifications [60].
References
Agrawal A, Romero-Perez D, Jacobsen JA, Villarreal FJ, Cohen SM (2008) Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem 3:812–820. doi:10.1002/cmdc.200700290
Alfonso-Jaume MA, Bergman MR, Mahimkar R, Cheng S, Jin ZQ, Karliner JS, Lovett DH (2006) Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB. Am J Physiol Heart Circ Physiol 291:H1838–H1846. doi:10.1152/ajpheart.00026.2006
Ali MA, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R (2010) Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation 122:2039–2047. doi:10.1161/circulationaha.109.930222
Ali MAM, Fan X, Schulz R (2011) Cardiac sarcomeric proteins: novel intracellular targets of matrix metalloproteinase-2 in heart disease. Trends Cardiovasc Med 21:112–118. doi:10.1016/j.tcm.2012.03.008
Ali MAM, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R (2010) Titin is a target of matrix metalloproteinase-2. Circulation 122:2039–2047. doi:10.1161/circulationaha.109.930222
Ali MAM, Chow AK, Kandasamy AD, Fan X, West LJ, Crawford BD, Simmen T, Schulz R (2012) Mechanisms of cytosolic targeting of matrix metalloproteinase-2. J Cell Physiol 227:3397–3404. doi:10.1002/jcp.24040
Apple KA, Yarbrough WM, Mukherjee R, Deschamps AM, Escobar PG, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Stroud RE, O’Neill TP, Spinale FG (2006) Selective targeting of matrix metalloproteinase inhibition in post-infarction myocardial remodeling. J Cardiovasc Pharmacol 47:228–235. doi:10.1097/01.fjc.0000200989.23987.b8
Beery AK, Zucker I (2011) Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35:565–572. doi:10.1016/j.neubiorev.2010.07.002
Bell R, Kunuthur S, Hendry C, Bruce-Hickman D, Davidson S, Yellon D (2013) Matrix metalloproteinase inhibition protects CyPD knockout mice independently of RISK/mPTP signalling: a parallel pathway to protection. Basic Res Cardiol 108:331. doi:10.1007/s00395-013-0331-7
Bencsik P, Kupai K, Giricz Z, Görbe A, Pipis J, Murlasits Z, Kocsis GF, Varga-Orvos Z, Puskás LG, Csonka C, Csont T, Ferdinandy P (2010) Role of iNOS and peroxynitrite–matrix metalloproteinase-2 signaling in myocardial late preconditioning in rats. Am J Physiol Heart Circ Physiol 299:H512–H518. doi:10.1152/ajpheart.00052.2010
Bencsik P, Paloczi J, Kocsis GF, Pipis J, Belecz I, Varga ZV, Csonka C, Gorbe A, Csont T, Ferdinandy P (2014) Moderate inhibition of myocardial matrix metalloproteinase-2 by ilomastat is cardioprotective. Pharmacol Res 80:36–42. doi:10.1016/j.phrs.2013.12.007
Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu B-Q, Nguyen A, Dahi S, Karliner JS, Lovett DH (2007) Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol 292:H1847–H1860. doi:10.1152/ajpheart.00434.2006
Bergman MR, Cheng S, Honbo N, Piacentini L, Karliner JS, Lovett DH (2003) A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem J 369:485–496. doi:10.1042/bj20020707
Berman B, Perez OA, Zell D (2007) Update on rosacea and anti-inflammatory-dose doxycycline. Drugs Today (Barc) 43:27–34. doi:10.1358/dot.2007.43.1.1025697
Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, VanWart H, Poole AR (1997) Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 99:1534–1545. doi:10.1172/jci119316
Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 85:683–693. doi:10.1016/s0092-8674(00)81235-0
Brown DL, Desai KK, Vakili BA, Nouneh C, Lee H-M, Golub LM (2004) Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol 24:733–738. doi:10.1161/01.ATV.0000121571.78696.dc
Bulteau A-L, Lundberg KC, Ikeda-Saito M, Isaya G, Szweda LI (2005) Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc Natl Acad Sci USA 102:5987–5991. doi:10.1073/pnas.0501519102
Cadete VJJ, Sawicka J, Jaswal JS, Lopaschuk GD, Schulz R, Szczesna-Cordary D, Sawicki G (2012) Ischemia/reperfusion-induced myosin light chain 1 phosphorylation increases its degradation by matrix metalloproteinase 2. FEBS J 279:2444–2454. doi:10.1111/j.1742-4658.2012.08622.x
Cadete VJJ, Sawicka J, Bekar LK, Sawicki G (2013) Combined subthreshold dose inhibition of myosin light chain phosphorylation and MMP-2 activity provides cardioprotection from ischaemic/reperfusion injury in isolated rat heart. Br J Pharmacol 170:380–390. doi:10.1111/bph.12289
Camp TM, Tyagi SC, Aru GM, Hayden MR, Mehta JL (2004) Doxycycline ameliorates ischemic and border-zone remodeling and endothelial dysfunction after myocardial infarction in rats. J Heart Lung Transplant 23:729–736. doi:10.1016/j.healun.2003.06.005
Caton J, Ryan ME (2011) Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol Res 63:114–120. doi:10.1016/j.phrs.2010.12.003
Cauwe B, Opdenakker G (2010) Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol 45:351–423. doi:10.3109/10409238.2010.501783
Cerisano G, Buonamici P, Valenti R, Moschi G, Taddeucci E, Giurlani L, Migliorini A, Vergara R, Parodi G, Sciagrà R, Romito R, Colonna P, Antoniucci D (2014) Effects of a timely therapy with doxycycline on the left ventricular remodeling according to the pre-procedural TIMI flow grade in patients with ST-elevation acute myocardial infarction. Basic Res Cardiol 109:412. doi:10.1007/s00395-014-0412-2
Cerisano G, Buonamici P, Valenti R, Sciagrà R, Raspanti S, Santini A, Carrabba N, Dovellini EV, Romito R, Pupi A, Colonna P, Antoniucci D (2014) Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: the TIPTOP trial. Eur Heart J 35:184–191. doi:10.1093/eurheartj/eht420
Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T (2003) Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253:269–285. doi:10.1023/a:1026028303196
Cheung P-Y, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R (2000) Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101:1833–1839. doi:10.1161/01.CIR.101.15.1833
Chow AK, Cena J, El-Yazbi AF, Crawford BD, Holt A, Cho WJ, Daniel EE, Schulz R (2007) Caveolin-1 inhibits matrix metalloproteinase-2 activity in the heart. J Mol Cell Cardiol 42:896–901. doi:10.1016/j.yjmcc.2007.01.008
Coker ML, Doscher MA, Thomas CV, Galis ZS, Spinale FG (1999) Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am J Physiol Heart Circ Physiol 277:H777–H787
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science 295:2387–2392. doi:10.1126/science.1067100
Descamps FJ, Martens E, Opdenakker G (2002) Analysis of gelatinases in complex biological fluids and tissue extracts. Lab Invest 82:1607–1608. doi:10.1097/01.LAB.0000038556.54069.73
Donato M, D’Annunzio V, Buchholz B, Miksztowicz V, Carrion CL, Valdez LB, Zaobornyj T, Schreier L, Wikinski R, Boveris A, Berg G, Gelpi RJ (2010) Role of matrix metalloproteinase-2 in the cardioprotective effect of ischaemic postconditioning. Exp Physiol 95:274–281. doi:10.1113/expphysiol.2009.049874
Donnini S, Monti M, Roncone R, Morbidelli L, Rocchigiani M, Oliviero S, Casella L, Giachetti A, Schulz R, Ziche M (2008) Peroxynitrite inactivates human-tissue inhibitor of metalloproteinase-4. FEBS Lett 582:1135–1140. doi:10.1016/j.febslet.2008.02.080
Dorman G, Kocsis-Szommer K, Spadoni C, Ferdinandy P (2007) MMP inhibitors in cardiac diseases: an update. Recent Pat Cardiovasc Drug Discov 2:186–194. doi:10.2174/157489007782418964
Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT (2000) Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106:55–62. doi:10.1172/jci8768
Fan X, Schulz R (2012) Matrix metalloproteinase-2: an emerging biomarker for reperfusion injury following percutaneous coronary intervention. Heart 98:1–2. doi:10.1136/heartjnl-2011-300886
Fert-Bober J, Leon H, Sawicka J, Basran RS, Devon RM, Schulz R, Sawicki G (2008) Inhibiting matrix metalloproteinase-2 reduces protein release into coronary effluent from isolated rat hearts during ischemia-reperfusion. Basic Res Cardiol 103:431–443. doi:10.1007/s00395-008-0727-y
Finegan BA (2009) What about the other two-thirds of the cardiac cycle? Can J Anaesth 56:348–351. doi:10.1007/s12630-009-9072-3
Fingleton B (2007) Matrix metalloproteinases as valid clinical targets. Curr Pharm Des 13:333–346. doi:10.2174/138161207779313551
Frears ER, Zhang Z, Blake DR, O’Connell JP, Winyard PG (1996) Inactivation of tissue inhibitor of metalloproteinase-1 by peroxynitrite. FEBS Lett 381:21–24. doi:10.1016/0014-5793(96)00065-8
Galis ZS, Sukhova GK, Lark MW, Libby P (1994) Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94:2493–2503. doi:10.1172/jci117619
Gao L, Chen L, Lu Z, Gao H, Wu L, Chen Y, Zhang C, Jiang Y, Jing Q, Zhang YY, Yang HT (2014) Activation of α1B-adrenoceptors contributes to intermittent hypobaric hypoxia-improved post-ischemic myocardial performance via inhibiting MMP-2 activation. Am J Physiol Heart Circ Physiol 306:H1569–H1581. doi:10.1152/ajpheart.00772.2013
Garcia RA, Go KV, Villarreal FJ (2007) Effects of timed administration of doxycycline or methylprednisolone on post-myocardial infarction inflammation and left ventricular remodeling in the rat heart. Mol Cell Biochem 300:159–169. doi:10.1007/s11010-006-9379-0
García RA, Pantazatos DP, Gessner CR, Go KV, Woods VL, Villarreal FJ (2005) Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Mol Pharmacol 67:1128–1136. doi:10.1124/mol.104.006346
Giricz Z, Lalu MM, Csonka C, Bencsik P, Schulz R, Ferdinandy P (2006) Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: role of matrix metalloproteinase-2 inhibition. J Pharmacol Exp Ther 316:154–161. doi:10.1124/jpet.105.091140
Glenn L (1999) Antibiotic use and risk of myocardial infarction. JAMA 282(21):1997
Goldberg GI, Wilhelm SM, Kronberger A, Bauer EA, Grant GA, Eisen AZ (1986) Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem 261:6600–6605
Golub LM, Lee H-M, Ryan ME, Giannobile WV, Payne J, Sorsa T (1998) Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 12:12–26. doi:10.1177/08959374980120010501
Griffin MO, Jinno M, Miles LA, Villarreal FJ (2005) Reduction of myocardial infarct size by doxycycline: a role for plasmin inhibition. Mol Cell Biochem 270:1–11. doi:10.1007/s11010-005-2540-3
Gross J, Lapiere CM (1962) Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA 48:1014–1022
Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA (2002) S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297:1186–1190. doi:10.1126/science.1073634
Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A (2003) Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol 285:H1229–H1235. doi:10.1152/ajpheart.00207.2003
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175. doi:10.1016/S0140-6736(12)60916-7
Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943. doi:10.1016/S0140-6736(14)60107-0
Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JPM, Shipley M, Angellilo A, Levi M, Nue O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JFM, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJAP, Carmeliet P (1999) Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5:1135–1142. doi:10.1038/13459
Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD (2006) Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol 48:15–20. doi:10.1016/j.jacc.2006.02.055
Hughes BG, Fan X, Cho WJ, Schulz R (2014) MMP-2 is localized to the mitochondria-associated membrane of the heart. Am J Physiol Heart Circ Physiol 306:H764–H770. doi:10.1152/ajpheart.00909.2013
Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG (2005) Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol 288:H149–H158. doi:10.1152/ajpheart.00370.2004
Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S (1997) Unaltered secretion of β-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 272:22389–22392. doi:10.1074/jbc.272.36.22389
Jacob-Ferreira AL, Kondo MY, Baral PK, James MNG, Holt A, Fan X, Schulz R (2013) Phosphorylation status of 72 kDa MMP-2 determines its structure and activity in response to peroxynitrite. PLoS One 8:e71794. doi:10.1371/journal.pone.0071794
Jayasankar V, Woo YJ, Bish LT, Pirolli TJ, Berry MF, Burdick J, Bhalla RC, Sharma RV, Gardner TJ, Sweeney HL (2004) Inhibition of matrix metalloproteinase activity by TIMP-1 gene transfer effectively treats ischemic cardiomyopathy. Circulation 110:II-180–II-186. doi:10.1161/01.cir.0000138946.29375.49
Kaludercic N, Lindsey ML, Tavazzi B, Lazzarino G, Paolocci N (2008) Inhibiting metalloproteases with PD 166793 in heart failure: impact on cardiac remodeling and beyond. Cardiovasc Drug Rev 26:24–37. doi:10.1111/j.1527-3466.2007.00034.x
Kandasamy AD, Chow AK, Ali MAM, Schulz R (2010) Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 85:413–423. doi:10.1093/cvr/cvp268
Keeley EC, Boura JA, Grines CL (2003) Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 361:13–20. doi:10.1016/S0140-6736(03)12113-7
Koenig GC, Rowe RG, Day SM, Sabeh F, Atkinson JJ, Cooke KR, Weiss SJ (2012) MT1-MMP-dependent remodeling of cardiac extracellular matrix structure and function following myocardial infarction. Am J Pathol 180:1863–1878. doi:10.1016/j.ajpath.2012.01.022
Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kyto V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R (2010) Mice with tissue inhibitor of metalloproteinases 4 (TIMP4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285:24487–24493. doi:10.1074/jbc.M110.136820
Krishnamurthy P, Peterson JT, Subramanian V, Singh M, Singh K (2009) Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol Cell Biochem 322:53–62. doi:10.1007/s11010-008-9939-6
Kryzhanovskii SA, Ionova EO, Stolyaruk VN, Tsorin IB, Vititnova MB (2013) Effect of metalloproteinase inhibitor on early postinfarction remodeling in the most acute phase of myocardial infarction. Bull Exp Biol Med 156:19–24. doi:10.1007/s10517-013-2267-1
Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R (2004) Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J 18:690–692. doi:10.1096/fj.02-1202fje
Labossiere JR, Pelletier JS, Ali MA, Thiesen A, Schulz R, Bigam DL, Cheung PY (2013) Postresuscitation administration of doxycycline preserves cardiac contractile function in hypoxia-reoxygenation injury of newborn piglets. Crit Care Med 42:e260–e269. doi:10.1097/CCM.0000000000000135
Lalu MM, Pasini E, Schulze CJ, Ferrari-Vivaldi M, Ferrari-Vivaldi G, Bachetti T, Schulz R (2005) Ischaemia–reperfusion injury activates matrix metalloproteinases in the human heart. Eur Heart J 26:27–35. doi:10.1093/eurheartj/ehi007
Lalu MM, Csonka C, Giricz Z, Csont T, Schulz R, Ferdinandy P (2002) Preconditioning decreases ischemia/reperfusion-induced release and activation of matrix metalloproteinase-2. Biochem Biophys Res Commun 296:937–941. doi:10.1016/S0006-291X(02)02019-3
Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG (2006) Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 113:2919–2928. doi:10.1161/circulationaha.106.612960
Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG (2005) Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290:H232–H239. doi:10.1152/ajpheart.00457.2005
Lindsey ML, Gannon J, Aikawa M, Schoen FJ, Rabkin E, Lopresti-Morrow L, Crawford J, Black S, Libby P, Mitchell PG, Lee RT (2002) Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation 105:753–758. doi:10.1161/hc0602.103674
Lindsey ML, Zamilpa R (2012) Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther 30:31–41. doi:10.1111/j.1755-5922.2010.00207.x
Lovett DH, Mahimkar R, Raffai RL, Cape L, Maklashina E, Cecchini G, Karliner JS (2012) A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS One 7:e34177. doi:10.1371/journal.pone.0034177
Lovett DH, Mahimkar R, Raffai RL, Cape L, Zhu B-Q, Jin Z-Q, Baker AJ, Karliner JS (2013) N-terminal truncated intracellular matrix metalloproteinase-2 induces cardiomyocyte hypertrophy, inflammation and systolic heart failure. PLoS One 8:e68154. doi:10.1371/journal.pone.0068154
Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y (2005) Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest 115:599–609. doi:10.1172/jci22304
Matsunaga T, Abe N, Kameda K, Hagii J, Fujita N, Onodera H, Kamata T, Ishizaka H, Hanada H, Osanai T, Okumura K (2005) Circulating level of gelatinase activity predicts ventricular remodeling in patients with acute myocardial infarction. Int J Cardiol 105:203–208. doi:10.1016/j.ijcard.2005.01.011
Meier CR, Derby LE, Jick SS, Vasilakis C, Jick H (1999) Antibiotics and risk of subsequent first-time acute myocardial infarction. JAMA 281:427–431. doi:10.1001/jama.281.5.427
Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG (2003) Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 107:618–625. doi:10.1161/01.CIR.0000046449.36178.00
Müller AL, Freed D, Dhalla NS (2013) Activation of proteases and changes in Na+-K+-ATPase subunits in hearts subjected to ischemia-reperfusion. J Appl Physiol 114:351–360. doi:10.1152/japplphysiol.01239.2012
Murphy G, Nagase H (2008) Progress in matrix metalloproteinase research. Mol Aspects Med 29:290–308. doi:10.1016/j.mam.2008.05.002
Murphy G (2011) Tissue inhibitors of metalloproteinases. Genome Biol 12:233. doi:10.1186/gb-2011-12-11-233
Nelson KK, Melendez JA (2004) Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 37:768–784. doi:10.1016/j.freeradbiomed.2004.06.008
Nilsson L, Hallen J, Atar D, Jonasson L, Swahn E (2012) Early measurements of plasma matrix metalloproteinase-2 predict infarct size and ventricular dysfunction in ST-elevation myocardial infarction. Heart 98:31–36. doi:10.1136/heartjnl-2011-300079
Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H (2001) Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 276:29596–29602. doi:10.1074/jbc.M102417200
Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I (2009) Gender differences in cardiac ischemic injury and protection—experimental aspects. Exp Biol Med 234:1011–1019. doi:10.3181/0812-mr-362
Peterson JT, Li H, Dillon L, Bryant JW (2000) Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res 46:307–315. doi:10.1016/s0008-6363(00)00029-8
Peterson JT (2004) Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev 9:63–79. doi:10.1023/B:HREV.0000011395.11179.af
Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT (1999) Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation 99:3063–3070. doi:10.1161/01.cir.99.23.3063
Romero-Perez D, Agrawal A, Jacobsen J, Yan Y, Thomas R, Cohen S, Villarreal F (2009) Effects of novel semiselective matrix metalloproteinase inhibitors on ex vivo cardiac structure-function. J Cardiovasc Pharmacol 53:452–461. doi:10.1097/FJC.0b013e3181a6aa83
Romero-Perez D, Fricovsky E, Yamasaki KG, Griffin M, Barraza-Hidalgo M, Dillmann W, Villarreal F (2008) Cardiac uptake of minocycline and mechanisms for in vivo cardioprotection. J Am Coll Cardiol 52:1086–1094. doi:10.1016/j.jacc.2008.06.028
Sariahmetoglu M, Skrzypiec-Spring M, Youssef N, Jacob-Ferreira ALB, Sawicka J, Holmes C, Sawicki G, Schulz R (2012) Phosphorylation status of matrix metalloproteinase 2 in myocardial ischaemia–reperfusion injury. Heart 98:656–662. doi:10.1136/heartjnl-2011-301250
Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, Holmes C, Berthiaume LG, Holt A, Sawicki G, Schulz R (2007) Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J 21:2486–2495. doi:10.1096/fj.06-7938com
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M (1994) A matrix metalloproteinase expressed on the surface of invasive tumor-cells. Nature 370:61–65. doi:10.1038/370061a0
Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R (2005) Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury. Circulation 112:544–552. doi:10.1161/circulationaha.104.531616
Schulze CJ, Wang W, Suarez-Pinzon WL, Sawicka J, Sawicki G, Schulz R (2003) Imbalance between tissue inhibitor of metalloproteinase-4 and matrix metalloproteinases during acute myoctardial ischemia-reperfusion injury. Circulation 107:2487–2492. doi:10.1161/01.cir.0000065603.09430.58
Schulze CJ, Castro MM, Kandasamy AD, Cena J, Bryden C, Wang SH, Koshal A, Tsuyuki RT, Finegan BA, Schulz R (2013) Doxycycline reduces cardiac matrix metalloproteinase-2 activity but does not ameliorate myocardial dysfunction during reperfusion in coronary artery bypass patients undergoing cardiopulmonary bypass. Crit Care Med 41:2512–2520. doi:10.1097/CCM.0b013e318292373c
Singh RB, Hryshko L, Freed D, Dhalla NS (2012) Activation of proteolytic enzymes and depression of the sarcolemmal Na+-K+-ATPase in ischemia–reperfused heart may be mediated through oxidative stress. Can J Physiol Pharmacol 90:249–260. doi:10.1139/y11-128
Smith GN Jr, Mickler EA, Hasty KA, Brandt KD (1999) Specificity of inhibition of matrix metalloproteinase activity by doxycycline: relationship to structure of the enzyme. Arthritis Rheum 42:1140–1146. doi:10.1002/1529-0131(199906)42:6<1140:AID-ANR10>3.0.CO;2-7
Smith GN Jr, Brandt KD, Hasty KA (1996) Activation of recombinant human neutrophil procollagenase in the presence of doxycycline results in fragmentation of the enzyme and loss of enzyme activity. Arthritis Rheum 39:235–244. doi:10.1002/art.1780390209
Spinale F, Wilbur N (2009) Matrix metalloproteinase therapy in heart failure. Curr Treat Options Cardiovasc Med 11:339–346. doi:10.1007/s11936-009-0034-4
Spinale FG (2002) Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res 90:520–530. doi:10.1161/01.res.0000013290.12884.a3
Spinale FG, Koval CN, Deschamps AM, Stroud RE, Ikonomidis JS (2008) Dynamic changes in matrix metalloproteinase activity within the human myocardial interstitium during myocardial arrest and reperfusion. Circulation 118:S16–S23. doi:10.1161/circulationaha.108.786640
Spinale FG (2007) Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87:1285–1342. doi:10.1152/physrev.00012.2007
Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM (2004) Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail 10:328–333. doi:10.1016/j.cardfail.2003.11.003
Sung MM, Schulz CG, Wang W, Sawicki G, Bautista-López NL, Schulz R (2007) Matrix metalloproteinase-2 degrades the cytoskeletal protein [alpha]-actinin in peroxynitrite mediated myocardial injury. J Mol Cell Cardiol 43:429–436. doi:10.1016/j.yjmcc.2007.07.055
Takai S, Jin D, Inagaki S, Yamamoto D, Tanaka K, Miyazaki M (2007) Significance of matrix metalloproteinase-9 in cardiac dysfunction during the very acute phase after myocardial infarction in hamsters. Eur J Pharmacol 572:57–60. doi:10.1016/j.ejphar.2007.07.001
Tessone A, Feinberg MS, Barbash IM, Reich R, Holbova R, Richmann M, Mardor Y, Leor J (2005) Effect of matrix metalloproteinase inhibition by doxycycline on myocardial healing and remodeling after myocardial infarction. Cardiovasc Drugs Ther 19:383–390. doi:10.1007/s10557-005-5201-6
Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ (1998) Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa-force relation. Circ Res 82:261–271. doi:10.1161/01.res.82.2.261
van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer IE, Pasterkamp G, Prins MW, Roest M (2013) Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One 8:e62080. doi:10.1371/journal.pone.0062080
Van Wart HE, Birkedal-Hansen H (1990) The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA 87:5578–5582. doi:10.1073/pnas.87.14.5578
Vanhoutte D, Schellings M, Pinto Y, Heymans S (2006) Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res 69:604–613. doi:10.1016/j.cardiores.2005.10.002
Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, León H, van Mulligen T, Schulz R (2009) Activation and modulation of 72 kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol 77:826–834. doi:10.1016/j.bcp.2008.11.004
Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J (2003) Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation 108:1487–1492. doi:10.1161/01.cir.0000089090.05757.34
Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JRB, Sawicki G, Schulz R (2002) Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 106:1543–1549. doi:10.1161/01.cir.0000028818.33488.7b
Will H, Atkinson SJ, Butler GS, Smith B, Murphy G (1996) The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation: regulation by TIMP-2 and TIMP-3. J Biol Chem 271:17119–17123. doi:10.1074/jbc.271.29.17119
Woolley DE, Glanville RW, Crossley MJ, Evanson JM (1975) Purification of rheumatoid synovial collagenase and its action on soluble and insoluble collagen. Eur J Biochem 54:611–622. doi:10.1111/j.1432-1033.1975.tb04173.x
Yamada A, Uegaki A, Nakamura T, Ogawa K (2000) ONO-4817, an orally active matrix metalloproteinase inhibitor, prevents lipopolysaccharide-induced proteoglycan release from the joint cartilage in guinea pigs. Inflamm Res 49:144–146. doi:10.1007/s000110050573
Yan P, Chen KJ, Wu J, Sun L, Sung HW, Weisel RD, Xie J, Li RK (2014) The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhance Timp3 gene transfection and improve cardiac function. Biomaterials 35:1063–1073. doi:10.1016/j.biomaterials.2013.10.043
Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O’Neill TP, Spinale FG (2003) Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation 108:1753–1759. doi:10.1161/01.cir.0000091087.78630.79
Yasmin W, Strynadka KD, Schulz R (1997) Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res 33:422–432. doi:10.1016/s0008-6363(96)00254-4
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135. doi:10.1056/NEJMra071667
Zavadzkas JA, Mukherjee R, Rivers WT, Patel RK, Meyer EC, Black LE, McKinney RA, Oelsen JM, Stroud RE, Spinale FG (2011) Direct regulation of membrane type 1 matrix metalloproteinase following myocardial infarction causes changes in survival, cardiac function, and remodeling. Am J Physiol Heart Circ Physiol 301:H1656–H1666. doi:10.1152/ajpheart.00141.2011
Zavadzkas JA, Stroud RE, Bouges S, Mukherjee R, Jones JR, Patel RK, McDermott PJ, Spinale FG (2014) Targeted overexpression of tissue inhibitor of matrix metalloproteinase-4 modifies post–myocardial infarction remodeling in mice. Circ Res 114:1435–1445. doi:10.1161/circresaha.114.303634
Zhou H-Z, Ma X, Gray MO, Zhu B, Nguyen AP, Baker AJ, Simonis U, Cecchini G, Lovett DH, Karliner JS (2007) Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem Biophys Res Commun 358:189–195. doi:10.1016/j.bbrc.2007.04.094
Acknowledgments
The Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Heart and Stroke Foundation of Canada funded research in the author’s laboratory.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
We dedicate this review in the memory of Daniel (Danny) Schulz, January 13, 1989–April 30, 2014, youngest son of Petra and Richard Schulz. A loving and talented young man who touched so many people’s hearts.
Rights and permissions
About this article
Cite this article
Hughes, B.G., Schulz, R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol 109, 424 (2014). https://doi.org/10.1007/s00395-014-0424-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0424-y