Abstract
After acute myocardial infarction, the presence of no-reflow (or microvascular obstruction: MVO) has been associated with adverse left ventricular (LV) remodeling and worse clinical outcome. This study examined the effects of mechanical ischemic postconditioning on early and late MVO size in acute ST-elevation myocardial infarction (STEMI) patients. Fifty patients undergoing primary coronary angioplasty for a first STEMI with TIMI grade flow 0–1 and no collaterals were randomized to ischemic postconditioning (PC) (n = 25) or control (n = 25) groups. Ischemic PC consisted in the application of four consecutive cycles of a 1-min balloon occlusion, each followed by a 1-min deflation at the onset of reperfusion. Early (3 min post-contrast) and late (10 min post-contrast) MVO size were assessed by contrast-enhanced cardiac-MRI within 96 h after reperfusion. PC was associated with smaller early and late MVO size (3.9 ± 4.8 in PC versus 7.8 ± 6.6 % of LV in controls for early MVO, P = 0.02; and 1.8 ± 3.1 in PC versus 4.1 ± 3.9 % of LV in controls for late MVO; P = 0.01). This significant reduction was persistent after adjustment for thrombus aspiration, which neither had any significant effect on infarct size, nor on early or late MVO (P = NS for all). Attenuation of MVO was associated to infarct size reduction. Mechanical postconditioning significantly reduces MVO in patients with acute STEMI treated with primary angioplasty.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Therapeutic management of acute ST-elevated myocardial infarction (STEMI) patients in the cardiac catheterization laboratory for primary percutaneous coronary intervention (PPCI) has considerably evolved in the last 10 years [26]. Both recommended medications given to patients and angioplasty procedures are constantly evolving as illustrated by the recent changes in guidelines [36]. If these changes are globally associated with a significant reduction of cardiovascular adverse events, 60–70 % of patients with optimal angiographic reperfusion still display no-reflow [also termed microvascular obstruction (MVO)] lesions, as detected by cardiac magnetic resonance studies after reperfusion [23]. The presence of MVO lesions is associated with larger infarct size, increased left ventricular remodeling and adverse CV outcome [23, 24, 44].
Ischemic postconditioning is a phenomenon whereby repeated cycles of brief episodes of ischemia and reperfusion applied immediately after re-opening of the culprit coronary artery significantly reduce infarct size in experimental preparations [8, 45, 46]. Few reports suggest that ischemic postconditioning may limit MVO in in vivo animal models [46]. In patients undergoing ST-elevation acute myocardial infarction, angioplasty postconditioning [19] has been shown to limit the final infarct size (IS) and improve functional recovery significantly [6, 19, 34, 35, 40]. However, the postconditioning (PC) effect on MVO in a clinical setting is unknown [7]. Further, it has been questioned whether postconditioning by repeated balloon inflations at the site of the culprit thrombotic occlusion might favor micro-emboli and enhance MVO [33].
The primary objective of this study was therefore to assess the effect of angioplasty PC on early and late MVO assessed by contrast-enhanced cardiac magnetic resonance (ce-CMR) in a population of patients with acute STEMI.
Methods
Study population
The “Postconditioning No-Reflow” study was a multi-center, prospective, randomized, open-label, controlled study. The study was performed according to the Declaration of Helsinki (revised version of Somerset West, Republic of South Africa, 1996) and according to the European Guidelines of Good Clinical practice (version 11, July 1990) and French laws. The study protocol was approved by the Ethics Committee of our institution (IRB 123406519). All subjects gave written informed consent before inclusion (Clinical trial.gov: NCT01208727). Previous data from this same patient population showing a significant effect of postconditioning on myocardial edema have been reported in a previous publication [41].
Men and women, 18 years or older, who presented within 12 h after the onset of chest pain, who had ST-segment elevation of more than 0.1 mV in two contiguous leads, and referred for primary percutaneous coronary intervention (PPCI) were considered for inclusion into our study. Patients were eligible for the study whether they were undergoing PPCI or rescue PCI. Occlusion of the culprit coronary artery [Thrombolysis in Myocardial Infarction (TIMI) flow grade 0–1] at the time of admission was also a criterion for inclusion.
Patients with acute myocardial infarction complications at the time of presentation (cardiac arrest, ventricular fibrillation, cardiogenic shock, stent thrombosis) were excluded from the study. Also patients with previous myocardial infarction, angina within 48 h before infarction, and contraindication to ce-CMR were not included in the study. Patients with occlusion of the left circumflex coronary artery were included only in case of left circulation dominance. Patients with occlusion of the left main or with evidence of coronary collaterals (Rentrop grade ≥ 1) to the region at risk on initial coronary angiography (at the time of admission) were not included.
Angiography and PPCI
Left ventricular and coronary angiography were performed with the use of standard techniques, just before revascularization. The size of the area at risk was estimated for each patient by measuring the circumferential extent of abnormally contracting segments (ACS), according to the method of Feild et al. [3, 16] as performed in previous randomized trials [35, 40]. Briefly, the length of the end-diastolic ventricular endocardial perimeter (circumference) and the length of the abnormally contracting segments of the end-diastolic perimeter were determined by computerized planimetry (Image J® 1.38× software). Abnormally contracting segments were expressed (percentage) as ACS = (abnormally contracting length of end-diastolic circumference/total end-diastolic circumference) × 100. Measurement of ACS was done in a blinded manner by an experienced investigator.
Experimental protocol
Patients were randomly allocated to either the control or the PC group. Just before revascularization, randomization was performed with the use of a computer-generated randomization sequence.
Revascularization was performed with the use of direct stenting. Thrombus aspiration was performed according to the attending interventional cardiologist’s judgment. In the control group, no additional intervention was performed during the first 8 min of reperfusion. In the PC group, within 1 min of reflow after direct stenting, the angioplasty balloon was re-inflated four times for 1 min, with low-pressure inflations (4–6 atmospheres), each separated by 1 min of reflow. Re-inflation was performed at a site proximal to the index lesion, in order to reduce distal embolization. After the eighth minute of reperfusion, the PCI procedure was completed according to the physician’s judgment with respect to patient status.
All patients received aspirin (≥250 mg), clopidogrel (600 mg) and enoxaparine (0.1 IU/kg) after confirmation of ST-segment elevation on ECG. The standard treatment after primary PCI included aspirin, clopidogrel, β-blockers, lipid-lowering agents and angiotensin-converting enzyme inhibitors.
Microvascular obstruction and infarct size assessment
CMR acquisition
All CMR studies were performed on a 1.5T MAGNETOM Avanto TIM system (Siemens, Erlangen), using vectocardiogram monitoring and a phased-array cardiac receiver coil. We aimed at performing CMR studies 48–72 h after admission. Localizers and LV functional assessment were performed using steady-state, free-precession images. In the short-axis orientation, the LV was completely encompassed by contiguous slices.
Early MVO was determined using a breath-hold 3D inversion recovery gradient-echo pulse sequence (TR, 1.0 ms; TE, 3.4 ms; flip angle, 20°; typical spatial resolution, 1.4 × 1.4 × 5, 2 mm gap between each slice) covering the whole ventricle performed 3 min after intravenous administration of 0.2 mmol/kg gadolinium-based contrast agents (Dotarem®, Guerbet France). Late MVO and delayed enhanced images covering the entire LV were acquired 10 min after contrast administration. Inversion times were individually adjusted to optimize nulling of apparently normal myocardium (typical values, 270–300 ms).
Image analysis
Off-line image analyses were performed by two experienced observers completely blinded to clinical status and treatment allocation group. All images were transferred to a dedicated OsiriX workstation (OsiriX Foundation, Geneva, Switzerland) for analysis and measurement. Left ventricular volumes, ejection fraction, mass measurements and segmental thickening were performed from the cine images with dedicated software (Argus, Siemens Medical Solutions, Malvern, PA). MVO was measured on delayed enhanced images by manual delineation of the hypoenhanced areas within the hyperintense infarcted myocardium. Infarcted myocardium was measured by manual delineation of the hyperintense myocardium on delayed enhanced images at 10 min. The extent of myocardial early and late MVO as well as infarcted myocardium was expressed in % of LV mass according to the following formula: [∑ (hypoenhanced or hyperenhanced area (cm2)) × slice thickness (cm) × myocardial specific density (1.05 g/cm3) × 100]/LV mass. Also, the percentage of MVO within the final infarct size ratio was calculated according to the following formula: MVO extent within infarct = (late MVO mass/infarct size mass) × 100.
Statistical analysis
Calculation of sample size was performed according to the previous studies. Considering an expected reduction of 30 % in the late MVO extent (comparable to the edema and infarct size reduction in our previous studies) with a statistical power of 80 % and a probability of type I error of 0.05 with a 2-sided test, we calculated a total sample size of 50 patients. To compensate for patient dropout, a total of 62 patients was planned to be recruited.
Results are expressed as mean ± SD or median and interquartile range, depending on normal distribution as assessed by the Shapiro–Wilk test. Comparisons between groups were performed using unpaired t test or Wilcoxon rank sum test for continuous variables and χ 2 or Fisher’s exact test for categorical variables. To compare the relationship between early, late MVO and the infarct size, univariate and multivariate linear regression analyses were performed. We performed analyses of covariance to compare the treatment effect on early and late MVO after adjustment on the size of the area at risk. To adjust for potential confounders of the effect of PC on MVO, we created a multivariate linear regression model with early or late MVO as dependent variables and age, ischemia time, size of the area at risk, postconditioning and thrombus aspiration as independent variables. Results were considered statistically significant at a P value of 0.05. Statistical analyses were done using STATA version SE 11.2 (StataCorp, TX, USA).
Results
Study population
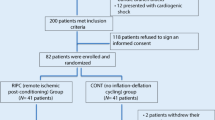
Between June 2008 and October 2010, 76 patients diagnosed with STEMI met the inclusion criteria and were considered eligible for the study. The study patient population is described in Fig. 1.
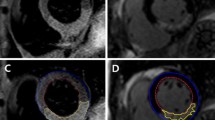
At clinical admission in the catheterization laboratory there were no significant differences in the clinical characteristics between PC patients and controls (Table 1). Figure 2 illustrates early and late enhancement CMR images of a representative patient from the study population.
Early and late contrast-enhanced CMR left ventricular study in a patient with anterior acute myocardial infarction. On the short-axis images (a and b) the hypoenhanced area within the hyperintense infarcted myocardium was defined as the microvascular obstruction (MVO) area and measured at 3 min (early MVO) (Panel A) and 10 min (late MVO) (b) after contrast administration. Corresponding 4-chamber view late-enhancement study showing the late MVO at the apex (arrows) (c)
Postconditioning effect on infarct size and MVO
CMR studies were performed 93 ± 57 h after admission and there was no significant difference with respect to this timing between the two study groups (P = 0.97). There were no significant differences in LV volumes, ejection fraction and LV mass between both groups at baseline. The final infarct size measured according to the LGE extent was significantly reduced in PC patients compared to controls (13 ± 7 versus 21 ± 14 g/m2, respectively, P = 0.01). This mean 38 % difference was maintained after adjustment for the angiographic area at risk.
MVO was present in 19 (78 %) PC patients compared to 21 (84 %) controls (P = 0.61). As reported in Table 2, early and late MVO were both significantly reduced in the PC group compared to controls. There was a significant 50 % reduction of early MVO extent (% of LV mass) and a significant 56 % reduction of late MVO extent (% of LV mass) in the PC group. As shown in Fig. 3, this difference was maintained after adjustment for the angiographic area at risk. There was a significant correlation between the extent of early and late MVO and the final infarct size in both groups (R = 0.81 and R = 0.77, respectively; P < 0.001) and the extent of late MVO within the final infarct was significantly reduced by 50 % by postconditioning (P = 0.007).
Effect of postconditioning on early and late microvascular obstruction. The relative mass of early (a) and late (b) microvascular obstruction (MVO) were significantly reduced in the postconditioned group (Postcond) compared with the control group. The relative mass of early (c) and late (d) MVO was expressed as a function of the angiographic area at risk (ACS). There was a significant correlation between the early and late MVO extent and the area at risk in the Control group, whereas the correlation was not significant in the Postcond group. The line in the box-plot represents the median, and box boundaries the 25th and 75th percentiles. The whiskers represent the upper and lower boundaries defining outliers (median ± 1.5 interquartile range)
In both groups, we found a significant correlation between infarct size and the extent of late MVO as shown in Fig. 4. A similar significant relationship was found between early MVO and infarct size (R 2 = 0.58, P < 0.0001 in the control group and R 2 = 0.42, P = 0.001 in the PC group) showing that infarct extent was a major determinant of MVO.
Mutivariable regression of MVO on postconditioning and thrombus aspiration and other confounders
As shown in Table 3, after adjustment for the angiographic area at risk, thrombus aspiration (TA) did not have a significant effect on infarct size, early MVO size or late MVO size.
In the multivariate linear regression of late MVO size (in % of LV) adjusting for the size of AAR, PC, TA, ischemia time and age, the reduction effect of PC remained significant [β = −2.34 95 % IC (−4.4; −0.3); P = 0.02].
After adjustment for the size of AAR, PC, TA, ischemia time and age, TA had no significant effect [β = 0.38; 95 % IC (−1.6; 2.4); P = 0.39] on late MVO size.
Discussion
This randomized study showed that angioplasty postconditioning applied at reperfusion in patients with acute STEMI affords a significant reduction of early and late MVO extent. This protective effect against MVO is associated to infarct size reduction.
In experimental models, the reduction effect of PC on the final infarct size has been shown to be mediated through various mechanisms [46]. PC has been shown to block the opening of the mitochondrial transition pore [2, 11], thus reducing the bioenergetic catastrophe as well as the activation of the final apoptotic pathways triggered by the abrupt reflow of the previously ischemic myocardial tissue. It has also been shown to reduce significantly all the cytotoxic phenomena that occur at reperfusion onset as well as the secondary inflammatory response to the ischemic myocardial injury [38, 45]. All these different factors participate in the reperfusion injury process that increases the final infarct size.
In the present study, we found a 38 % reduction of infarct size by postconditioning, very similar to that found in previous proof-of-concept trials [8, 35, 40]. MVO as assessed by ce-CMR is an indicator of the myocardial ischemia–reperfusion injury [1]. It is a dynamic imaging marker that may vary within the first 48 h of reperfusion [21, 28]. The pathophysiology of the MVO is poorly understood [10, 15, 31]. Following prolonged ischemia reperfusion, absence of flow in a given territory may be due to intravascular and/or extravascular factors. Endothelium destruction or swelling, vasospasm, plugging of leukocytes or red blood cells and micro-thrombi may contribute to the microcirculation perfusion deficit. Extrinsic compression by myocardial edema and hemorrhage can also cause micro-vessel closure and contribute to MVO. Each of these factors may be more or less prominent according to several conditions including duration of ischemia, coronary artery territory, hemodynamic status, reperfusion therapy modality, as well as confounders like the patient’s characteristics or comorbidities (e.g., diabetes, age) or concurrent pharmacological treatments.
Yet, two major characteristics of MVO remain: first, it exists even when coronary occlusion is mechanical and not thrombotic (e.g., experimental models) [15]. Second, the MVO area is always located within the infarcted area. The latter point implies that any intervention that can reduce infarct size should, at least partly, attenuate MVO. In our study, we observed a significant reduction of early and late MVO by postconditioning. None of the pharmacological treatment currently available in STEMI patients has been shown able to reduce MVO extent. Surprisingly, we noticed that early and late MVO were significantly reduced by 50 and 56 %, respectively, while infarct size reduction averaged 38 %. This suggests an additional beneficial effect of postconditioning on MVO although, differences were not significantly different. When plotting MVO size against infarct size, we observed a significant correlation between both variables, for control as well as for postconditioned hearts, confirming that infarct size is a major determinant of the extent of MVO or vice versa. This study was not sufficiently powered to show a direct protection of postconditioning on coronary microvasculature. This effect was shown in the initial experimental report by Zhao et al. [46] who demonstrated that ischemic postconditioning could reduce infarct size but also improve maximal vasodilator response of the reperfused myocardium to acetylcholine, an index of endothelial function.
Also, in healthy humans, Loukogeorgakis et al. have reported that repeated brief episodes of regional ischemia could improve flow-mediated dilatation of the brachial artery after a 20-min arm ischemia. These results showed that ischemic postconditioning protected the forearm arterial endothelium after reversible ischemic injury [25]. While this type of demonstration was made for non-coronary circulation, our results seem to show that ischemic postconditioning might protect coronary vasculature [12].
The present demonstration of the protective effect of postconditioning on MVO in STEMI patients was made despite two confounding factors that are not usually addressed in experimental preparations: thrombus aspiration and distal micro-embolization. Thrombus aspiration has rapidly become current practice during PPCI in acute STEMI patients and recent reports show that it is used in up to 62 % of patients [20]. It is part of the most recent recommendations for the therapeutic management of acute STEMI patients in the catheterization laboratory [36]. Several reports have shown beneficial effects of thrombus aspiration on the final culprit coronary artery blood flow, the angiographic myocardial blush grade, ST-segment resolution, infarct size as well as MVO [17, 30, 39, 47]. Reports propose that those beneficial effects may be responsible for a significant reduction of the adverse clinical events incidence in STEMI patients [13, 22, 42]. In our study, in disagreement with the above-mentioned studies, thrombus aspiration neither reduced infarct size nor attenuated MVO in the control group (data not shown). This is, however, in accordance with other trials showing no effect of thrombus aspiration on various parameters of reperfusion or myocardial injury [18, 37], and clinical outcomes [22, 42]. We questioned whether thrombus aspiration had influenced the protective effect of postconditioning. In theory, thrombus aspiration might alter the efficiency of postconditioning since it slightly modifies the conditions of reflow. Experimental reports have clearly indicated that any delay in the application of postconditioning might prevent the infarct size reduction [14]. In clinical practice, the use of a thrombus aspiration device requires several minutes and thereby delays the application of the postconditioning protocol which might allow some reperfusion injury to develop. We, however, found that thrombus aspiration did not decrease the beneficial effect of postconditioning on infarct size and on MVO, suggesting that a few minutes delay for the application of the brief cycles of ischemia and reperfusion does not prevent postconditioning’s protection in STEMI patients.
Distal embolization of athero-thrombotic debris consecutive to the dilatation and stenting maneuvers of the culprit coronary artery lesion could be an important component of MVO [9, 33]. To avoid inadvertent micro-embolization during the postconditioning procedure, we have always carefully applied the repeated angioplasty balloon inflations and deflations, at low pressure and upstream of the culprit lesion [35, 40, 41]. Conversely, Freixa et al. [4] who suggested a detrimental effect of postconditioning on MVO and IS, performed the postconditioning protocol at the site of the index lesion where thrombotic mass is maximal. This situation may enhance thrombus dislodgement and therefore distal embolization, which in turn aggravates MVO and the final myocardial damage [32, 33]. Recent experimental and clinical evidence suggest that some antiplatelet agents, including clopidogrel, might modify infarct size, hence possibly MVO [29]. This, however, likely did not influence our results since the loading doses, type of medication and administration timing of antiplatelet treatments were equivalent between the postconditioning and control groups.
Overall, our study demonstrates that ischemic postconditioning reduces MVO in STEMI patients. It indicates that this protective effect is associated to the expected infarct size reduction. Although experimental data show that postconditioning has a protective effect on the coronary microvasculature, our results are not sufficiently powered to show this significant specific effect of postconditioning in human patients.
Limitations
The study sample size is small which could participate in the significance of the present findings. Also, the limited sample size does not allow us to account for all potential confounding variables that could impact results in the multivariable analysis. However, in a highly selected and well-defined population, our results are consistent with our previous findings and with the growing evidence of ischemic postconditioning trials showing a significant benefit on infarct size as reported by Heusch et al. [8].
The mean time-point when CMR studies were performed was 93 ± 57 h after admission. Although the goal was set to perform CMR studies between 48 and 72 h after reperfusion, because of local organization limitations we performed studies at a later time-point. There were no significant differences between study groups. This could influence our findings, knowing the microvascular obstruction is a highly dynamic phenomenon that seems to reach its peak after 48 h of reperfusion in experimental models [28].
Recent publications have shown that intra-myocardial hemorrhage was an important part of microvascular obstruction as defined by CMR [5, 27]. In our study, it may be difficult to distinguish between true microvascular obstruction and myocardial hemorrhage. However, we believe that both entities represent different manifestations of a common underlying pathophysiological mechanism.
In the literature, microvascular obstruction by CMR has been assessed by several methods: first-pass perfusion imaging, early “late” enhancement and delayed enhancement at 10 min after gadolinium injection [24, 43, 44]. We did not assess first-pass perfusion imaging in this study that would have allowed us to study signal intensity curves in the myocardium within the first minute. This might have added further information with regard to dynamic myocardial tissue perfusion. Instead we chose to assess early and late MVO as performed in a previous study by Nijveldt et al. [24]. In this same study, Nijveldt et al. showed that the most robust prognosis marker was MVO as defined by late enhancement.
References
Albert TS, Kim RJ, Judd RM (2006) Assessment of no-reflow regions using cardiac MRI. Basic Res Cardiol 101:383–390. doi:10.1007/s00395-006-0617-0
Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M (2005) Postconditioning inhibits mitochondrial permeability transition. Circulation 111:194–197. doi:10.1161/01.CIR.0000151290.04952.3B
Feild BJ, Russell ROJ, Dowling JT, Rackley CE (1972) Regional left ventricular performance in the year following myocardial infarction. Circulation 46:679–689
Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X, De Caralt TM, Betriu A, Masotti M (2012) Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J 33:103–112. doi:10.1093/eurheartj/ehr297
Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van de Werf F, Bogaert J (2009) Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J 30:1440–1449. doi:10.1093/eurheartj/ehp093
Garcia S, Henry TD, Wang YL, Chavez IJ, Pedersen WR, Lesser JR, Shroff GR, Moore L, Traverse JH (2011) Long-term follow-up of patients undergoing postconditioning during ST-elevation myocardial infarction. J Cardiovasc Transl Res 4:92–98. doi:10.1007/s12265-010-9252-0
Heusch G (2004) Postconditioning: old wine in a new bottle? J Am Coll Cardiol 44:1111–1112. doi:10.1016/j.jacc.2004.06.013
Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, Erbel R (2009) Coronary microembolization: from bedside to bench and back to bedside. Circulation 120:1822–1836. doi:10.1161/CIRCULATIONAHA.109.888784
Heusch G, Schulz R (2011) Preservation of peripheral vasodilation as a surrogate of cardioprotection? The mechanistic role of ATP-dependent potassium channels and the mitochondrial permeability transition pore. Eur Heart J 32:1184–1186. doi:10.1093/eurheartj/ehq511
Heusch G, Musiolik J, Gedik N, Skyschally A (2011) Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109:1302–1308. doi:10.1161/CIRCRESAHA.111.255604
Heusch G, Kleinbongard P, Skyschally A, Levkau B, Schulz R, Erbel R (2012) The coronary circulation in cardioprotection: more than just one confounder. Cardiovasc Res 94:237–245. doi:10.1093/cvr/cvr271
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175. doi:10.1016/S0140-6736(12)60916-7
Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, Maeng M, Kristensen J, Thuesen L, Krusell LR, Kristensen SD, Andersen HR, Lassen JF, Rasmussen K, Rehling M, Nielsen TT, Botker HE (2006) Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation 114:40–47. doi:10.1161/CIRCULATIONAHA.105.595660
Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J (2004) Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res 62:74–85. doi:1016/j.cardiores.2004.01.006
Kloner RA, Ganote CE, Jennings RB (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54:1496–1508. doi:10.1172/JCI107898
Lapeyre ACr, St Gibson W, Bashore TM, Gibbons RJ (2003) Quantitative regional wall motion analysis with early contrast ventriculography for the assessment of myocardium at risk in acute myocardial infarction. Am Heart J 145:1051–1057. doi:10.1016/S0002-8703(03)00112-1
Liistro F, Grotti S, Angioli P, Falsini G, Ducci K, Baldassarre S, Sabini A, Brandini R, Capati E, Bolognese L (2009) Impact of thrombus aspiration on myocardial tissue reperfusion and left ventricular functional recovery and remodeling after primary angioplasty. Circ Cardiovasc Interv 2:376–383. doi:10.1161/CIRCINTERVENTIONS.109.852665
Lipiecki J, Monzy S, Durel N, Cachin F, Chabrot P, Muliez A, Morand D, Maublant J, Ponsonnaille J (2009) Effect of thrombus aspiration on infarct size and left ventricular function in high-risk patients with acute myocardial infarction treated by percutaneous coronary intervention. Results of a prospective controlled pilot study. Am Heart J 157:583.e1–583.e7. doi:10.1016/j.ahj.2008.11.017
Lonborg J, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Treiman M, Jensen JS, Engstrom T (2010) Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv 3:34–41. doi:10.1161/CIRCINTERVENTIONS.109.905521
Mangiacapra F, Wijns W, De Luca G, Muller O, Trana C, Ntalianis A, Heyndrickx G, Vanderheyden M, Bartunek J, De Bruyne B, Barbato E (2010) Thrombus aspiration in primary percutaneous coronary intervention in high-risk patients with ST-elevation myocardial infarction: a real-world registry. Catheter Cardiovasc Interv 76:70–76. doi:10.1002/ccd.22465
Mewton N, Rapacchi S, Augeul L, Ferrera R, Loufouat J, Boussel L, Micolich A, Rioufol G, Revel D, Ovize M, Croisille P (2011) Determination of the myocardial area at risk with pre- versus post-reperfusion imaging techniques in the pig model. Basic Res Cardiol. doi:10.1007/s00395-011-0214-8
Mongeon FP, Belisle P, Joseph L, Eisenberg MJ, Rinfret S (2010) Adjunctive thrombectomy for acute myocardial infarction: a bayesian meta-analysis. Circ Cardiovasc Interv 3:6–16. doi:10.1161/CIRCINTERVENTIONS.109.904037
Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, Algra PR, Twisk JW, van Rossum AC (2008) Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 52:181–189. doi:10.1016/j.jacc.2008.04.006
Nijveldt R, Hofman MB, Hirsch A, Beek AM, Umans VA, Algra PR, Piek JJ, van Rossum AC (2009) Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study. Radiology 250:363–370. doi:10.1148/radiol.2502080739
Okorie MI, Bhavsar DD, Ridout D, Charakida M, Deanfield JE, Loukogeorgakis SP, MacAllister RJ (2011) Postconditioning protects against human endothelial ischaemia-reperfusion injury via subtype-specific KATP channel activation and is mimicked by inhibition of the mitochondrial permeability transition pore. Eur Heart J 32:1266–1274. doi:10.1093/eurheartj/ehr041
Puymirat E, Simon T, Steg PG, Schiele F, Gueret P, Blanchard D, Khalife K, Goldstein P, Cattan S, Vaur L, Cambou JP, Ferrieres J, Danchin N (2012) Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA 308:998–1006. doi:10.1001/2012.jama.11348
Robbers LF, Eerenberg ES, Teunissen PF, Jansen MF, Hollander MR, Horrevoets AJ, Knaapen P, Nijveldt R, Heymans MW, Levi MM, van Rossum AC, Niessen HW, Marcu CB, Beek AM, van Royen N (2013) Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J 34:2346–2353. doi:10.1093/eurheartj/eht100
Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA (1998) Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 98:1006–1014. doi:10.1161/01.CIR.98.10.1006
Roubille F, Lairez O, Mewton N, Rioufol G, Ranc S, Sanchez I, Cung TT, Elbaz M, Piot C, Ovize M (2012) Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol 107:275. doi:10.1007/s00395-012-0275-3
Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, Francone M, Di Roma A, Benedetti G, Conti G, Fedele F (2009) Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol 53:309–315. doi:10.1016/j.jacc.2008.10.017
Schwartz BG, Kloner RA (2012) Coronary no reflow. J Mol Cell Cardiol 52:873–882. doi:10.1016/j.yjmcc.2011.06.009
Skyschally A, Leineweber K, Gres P, Haude M, Erbel R, Heusch G (2006) Coronary microembolization. Basic Res Cardiol 101:373–382. doi:10.1007/s00395-006-0616-1
Skyschally A, Walter B, Heusch G (2012) Coronary microembolization during early reperfusion: infarct extension, but protection by ischaemic postconditioning. Eur Heart J. doi:10.1093/eurheartj/ehs434
Sorensson P, Saleh N, Bouvier F, Bohm F, Settergren M, Caidahl K, Tornvall P, Arheden H, Ryden L, Pernow J (2010) Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart 96:1710–1715. doi:10.1136/hrt.2010.199430
Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M (2005) Postconditioning the human heart. Circulation 112:2143–2148. doi:10.1161/CIRCULATIONAHA.105.558122
Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, Van’t Hof A, Widimsky P, Zahger D, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Hasdai D, Astin F, Astrom-Olsson K, Budaj A, Clemmensen P, Collet JP, Fox KA, Fuat A, Gustiene O, Hamm CW, Kala P, Lancellotti P, Maggioni AP, Merkely B, Neumann FJ, Piepoli MF, Van de Werf F, Verheugt F, Wallentin L (2012) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 33:2569–2619. doi:10.1093/eurheartj/ehs215
Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, Dambrink JH, Ochala A, Carlton TW, Cristea E, Wolff SD, Brener SJ, Chowdhary S, El-Omar M, Neunteufl T, Metzger DC, Karwoski T, Dizon JM, Mehran R, Gibson CM (2012) Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA 307:1817–1826. doi:10.1001/jama.2012.421
Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ (2005) Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+overload. Am J Physiol Heart Circ Physiol 288:H1900–H1908. doi:10.1152/ajpheart.01244.2003
Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F (2008) Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 358:557–567. doi:10.1056/NEJMoa0706416
Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, Andre-Fouet X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M (2008) Long-term benefit of postconditioning. Circulation 117:1037–1044. doi:10.1161/CIRCULATIONAHA.107.729780
Thuny F, Lairez O, Roubille F, Mewton N, Rioufol G, Sportouch C, Sanchez I, Bergerot C, Thibault H, Cung TT, Finet G, Argaud L, Revel D, Derumeaux G, Bonnefoy-Cudraz E, Elbaz M, Piot C, Ovize M, Croisille P (2012) Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 59:2175–2181. doi:10.1016/j.jacc.2012.03.026
Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F (2008) Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 371:1915–1920. doi:10.1016/S0140-6736(08)60833-8
Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA (1998) Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97:765–772. doi:10.1161/01.CIR.97.8.765
Wu KC, Kim RJ, Bluemke DA, Rochitte CE, Zerhouni EA, Becker LC, Lima JA (1998) Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol 32:1756–1764. doi:10.1016/S0735-1097(98)00429-X
Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV (2004) Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol 44:1103–1110. doi:10.1016/j.jacc.2004.05.060
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H579–H588. doi:10.1152/ajpheart.01064.2002
Zia MI, Ghugre NR, Connelly KA, Joshi SB, Strauss BH, Cohen EA, Wright GA, Dick AJ (2012) Thrombus aspiration during primary percutaneous coronary intervention is associated with reduced myocardial edema, hemorrhage, microvascular obstruction and left ventricular remodeling. J Cardiovasc Magn Reson 14:19. doi:10.1186/1532-429X-14-19
Acknowledgments
The authors wish to express their sincere acknowledgements to all the research coordinators, nurses and catheterization laboratory staff in the different centers for their help on the screening, enrollment and data collection of participants. This project was supported by a research grant from the Hospices Civils de Lyon (Appel d’Offre HCL Actions Incitatives 2007). Dr. Hélène Thibault was supported by a joint research grant from the French Federation of Cardiology and the French Society of Cardiology (Dotations de Recherche Communes) for this project.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
To this article an Editorial is available at doi:10.1007/s00395-013-0380-y.
Rights and permissions
About this article
Cite this article
Mewton, N., Thibault, H., Roubille, F. et al. Postconditioning attenuates no-reflow in STEMI patients. Basic Res Cardiol 108, 383 (2013). https://doi.org/10.1007/s00395-013-0383-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0383-8