Abstract
The implantable cardioverter-defibrillator significantly improves survival in patients with malignant ventricular arrhythmias but does not target the underlying pathological substrate responsible for arrhythmic events. A significant proportion of defibrillator recipients experience multiple ventricular tachycardia/fibrillation episodes over a short period of time, termed electrical storm (ES). The current therapeutic strategy for ES is complex and unsatisfactory because simultaneous administration of several medications and additional invasive procedures are often required to control ES. Moreover, this treatment does not favorably influence the long-term outcome. Clearly, improved ES therapies are necessary and desirable, but a lack of understanding of the pathophysiological mechanisms underlying ES has hindered the development of more effective, rationally based therapeutic approaches. This paper reviews emerging experimental and clinical findings that provide insights into the pathophysiology of ES and discusses mechanism-based innovative therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The implantable cardioverter-defibrillator (ICD; see Table 1 for an overview of abbreviations) is the first line of treatment for malignant ventricular tachyarrhythmias and there has been an exponential increase in its use for prevention of sudden cardiac death (SCD) [33]. However, the ICD does not target the pathological substrate(s) responsible for arrhythmic events. Consequently, a significant proportion of ICD recipients experience multiple ventricular tachycardia (VT) and/or ventricular fibrillation (VF) episodes in a syndrome called “electrical storm” (ES). ES is said to be present when three or more separate VT/VF episodes occur within 24 h, although alternative definitions have been proposed [30]. ES is an increasingly common life-threatening emergency that requires hospitalization and intensive treatment.
Electrical storm can occur in a variety of settings, including acute ischemia, structural heart diseases (non-ischemic and ischemic cardiomyopathies), and inherited channelopathies. The clinical presentation of arrhythmias is also diverse, ranging from repetitive hemodynamically destabilizing polymorphic VT/VF episodes requiring multiple ICD-shocks to stable monomorphic VTs treated by antitachycardia-pacing therapy. An organized approach for evaluating and managing ES, based on the underlying cardiac disease and the electrocardiographic morphology of the arrhythmia, has been proposed (see reviews by Eifling et al. [27]; Huang et al. [43]). Pharmacological and/or non-pharmacological interventions (e.g., β-blockers, amiodarone, anesthesia, left stellate ganglion blockade, overdrive pacing, and/or catheter ablation) can successfully terminate ES in most patients. Nevertheless, this form of ES treatment is challenging because of the simultaneous administration of several medications and the frequent requirement of additional invasive procedures.
Electrical storm has serious prognostic implications, especially in the setting of structural heart disease. Even when ES is suppressed acutely, patients remain at high risk of cardiac death. Analyses of recent large clinical trials indicate that patients with depressed left-ventricular (LV) function who experience ES have a high risk of death within a few months [28, 98]. Most of these deaths are due to cardiac, non-sudden mechanisms, particularly progressive heart failure (HF). Clearly, improved ES therapies are required, but a lack of understanding of the pathophysiology and the molecular basis of ES has limited the development of innovative management strategies.
Aberrant intracellular Ca2+-handling, ionic imbalances associated with acute ischemia, neurohumoral changes, and genetic predisposition have been identified as major contributors to the susceptibility to, and initiation of, malignant VT/VF, although the immediate event triggering tachyarrhythmias at a specific time in an otherwise stable condition often remains unclear [93, 107]. A few recent studies have specifically addressed recurrent ventricular arrhythmias in novel experimental animal models to investigate underlying mechanisms [82, 109]. In combination with known arrhythmogenic mechanisms that could be involved in the pathophysiology of ES, these may enable us to identify factors that are central in triggering repetitive malignant VT/VF, thereby pointing to novel potential therapeutic approaches. This paper reviews emerging experimental and clinical findings that provide insights into pathophysiology and potential mechanisms of ES and explores mechanism-based innovative therapeutic strategies. We discuss (1) electrophysiological characteristics of ES in the clinical setting, (2) cellular and molecular basis of ES revealed by experimental studies, and (3) potential new targets for treating and preventing ES.
Key basic concepts
Cardiac action potential and intracellular Ca2+-handling
The basic mechanisms of cardiac cellular electrophysiology have been extensively studied for several decades (see [11, 78, 93, 107] for reviews on this subject) and are therefore only briefly summarized here.
A complex ensemble of transmembrane ionic currents and transporters shapes the cardiac action potential (AP). Depolarization of the membrane potential of a ventricular myocyte from its resting level close to the K+ reversal potential (≈−85 mV) activates voltage-gated Na+ current (I Na; Fig. 1a) responsible for the AP upstroke (phase 0; Fig. 1a), subsequently activating voltage-gated L-type Ca2+ current (I Ca,L). The Ca2+ entering the cell via I Ca,L triggers a much larger release of Ca2+ from the intracellular sarcoplasmic reticulum (SR) stores through the ryanodine receptor channels (RyR2 = cardiac isoform), giving rise to the systolic Ca2+-transient (Fig. 1b). Binding of cytosolic Ca2+ to the contractile machinery initiates contraction of the ventricular myocyte. Relaxation occurs when the Ca2+-transient declines via Ca2+ extrusion through the electrogenic Na+−Ca2+ exchanger (NCX1 = cardiac isoform) and the sarcolemmal Ca2+-ATPase, as well as due to resequestration of Ca2+ into the SR by the SR Ca2+-ATPase (SERCA2a = cardiac isoform). The AP upstroke is followed by a brief period of rapid repolarization (phase 1) resulting from activation of the transient-outward K+ current (I to), which is particularly prominent in epicardial regions. The subsequent AP plateau (phase 2) results from a balance between depolarizing currents, particularly those through NCX1, I Ca,L and the late component of I Na (INa,late), and repolarizing K+ currents, including the rapid and slow delayed-rectifier K+ currents (I Kr and I Ks, respectively) and the Na+-K+-ATPase current (I NaK). Further activation of I Kr and I Ks and the inward-rectifier K+ current (I K1) are responsible for final repolarization (phase 3).
a Prototype of the cardiac action potential (AP) and its underlying ionic currents. Time-point at which a current has its maximal effect on the AP approximately corresponds to the location of its label. b Schematic representation of a ventricular myocyte indicating the major ionic currents, Ca2+-handling proteins, as well as βAR and CaMKII-dependent signaling pathways. Elements from ‘Servier Medical Art’ were used in the design of this figure. Abbreviations are defined in Table 1
The cardiac AP and intracellular Ca2+-handling can be fine-tuned by a wide variety of signaling cascades affecting transcription, translation, and/or post-translational modification. The β-adrenoceptor (βAR) and Ca2+/calmodulin (CaM)-dependent kinase II (CaMKII) signaling pathways play critical roles in post-translational modifications mediating the neurohumoral regulation of cardiac electrophysiology and cardiac output [34]. Sympathetic stimulation results in increased cylic AMP levels, which activate protein kinase A (PKA), phosphorylating serine, and threonine residues of a large number of proteins, including the L-type Ca2+ channel, RyR2, and the SERCA2a-inhibitor phospholamban (PLB; Fig. 1b), which contribute to the inotropic response to sympathetic stimulation by augmenting the cellular Ca2+-transient [39]. In addition, PKA-dependent phosphorylation of I Kr, I Ks, and I NaK (via the accessory subunit phospholemman) tends to shorten APD, offsetting the APD-prolonging effect of increased I Ca,L [37, 39] (Fig. 1b).
CaMKII is activated by binding of a Ca2+/CaM complex. Prolonged activation results in CaMKII autophosphorylation at Thr286, leading to an increased affinity for Ca2+/CaM as well as Ca2+/CaM-independent CaMKII activity [102]. These mechanisms make CaMKII sensitive to repetitive Ca2+/CaM signals, with activity that is strongly dependent on heart rate. In addition, CaMKII can be activated by oxidation of the methionine residues Met281/Met282 [13, 102].
The cyclic AMP and CaMKII signaling pathways are strongly interconnected. Sympathetic stimulation activates CaMKII by increasing Ca2+ influx through I Ca,L [4], through PKA-dependent activation of inhibitor-1 of type-1 protein phosphatase (PP1) [120] (thereby reducing PP1-dephosphorylation/inactivation of CaMKII [34]), as well as through exchange protein activated by cyclic AMP [34]. PKA and CaMKII phosphorylate the same key Ca2+-handling proteins (Fig. 1b), albeit usually at different sites. Recent research identified Ser516, Ser571, and Thr594 as CaMKII-dependent phosphorylation sites of the cardiac Na+ channel [12]. Other validated phosphorylation sites with important physiological consequences include Ser16 (PKA) and Thr17 (CaMKII) on PLB and Ser2808 (PKA/CaMKII) and Ser2814 (CaMKII) on RyR2 (Ser2809 and Ser2815 in some species). Several phosphorylation sites on the α1C and β2 subunits of the L-type Ca2+ channel have also been proposed, including α1C Ser1512 and Ser1570 (CaMKII), α1C Ser1928 (PKA), β2 Ser479/Ser480 (PKA), and β2 Thr498 (CaMKII), but a recent study found that genetic manipulations of the β2-subunit sites do not affect regulation of the L-type Ca2+ channel by βAR stimulation [14].
Mechanisms of arrhythmogenesis
In a wide variety of pathological conditions, alterations in Ca2+-handling or electrophysiological properties of the cardiac myocyte can give rise to electrical signals originating outside of the sinoatrial node, which may act as a trigger for the development of cardiac arrhythmias. The classical mechanisms causing arrhythmias are abnormal automaticity, triggered activity induced by afterdepolarizations, and reentry [92].
Automaticity
Automaticity causes spontaneous generation of APs that do not require induction by previous beats. Normal automaticity occurs at diastolic membrane potentials typical for the cell/tissue, whereas abnormal automaticity is characterized by depolarized membrane potentials [92]. Working myocardium is not normally automatic. However, under disease conditions, resting membrane potential depolarization to more positive values may cause abnormal automaticity. For example, in the acute phase of myocardial infarction, injury currents flowing across the border zones of the infarct depolarize neighboring myocytes, which may then exhibit spontaneous activity [46]. Electrically coupled myofibroblasts can promote depolarization-induced spontaneous activity in cultured cardiomyocytes [69]. Pacemaker activity also occurs in isolated ventricular myocytes when resting membrane potential is destabilized by genetic inhibition of the I K1 channel [67].
Physiological pacemaker activity in sinoatrial node cells is produced by multiple time- and voltage-dependent ionic currents [57]. Upregulation or re-expression of such currents normally absent or less abundant in adult cardiomyocytes may contribute to arrhythmias due to abnormal automaticity under pathological conditions [42, 52]. In addition, recent work has highlighted a role for the so-called “Ca2+ clock” in pacemaking mediated by SR Ca2+-release through RyR2 and NCX1-induced membrane depolarization [31, 57], making it difficult to distinguish automaticity from triggered activity due to delayed afterdepolarizations (see below), particularly in vivo where a preceding beat is always present.
Early afterdepolarizations
Early afterdepolarizations (EADs) are secondary depolarizations occurring before full repolarization of the cardiac AP, which typically occur in a setting of excessive AP prolongation (Fig. 2a). Loss of repolarizing K+ currents or augmentation of depolarizing currents causes APD prolongation, allowing recovery of L-type Ca2+ channels from inactivation, creating the EAD upstroke. Recent evidence points toward an important role for Ca2+-handling abnormalities in EAD formation. Ca2+-dependent inactivation is the dominant type of I Ca,L inactivation under normal physiological conditions [8]. Loss of normal Ca2+-dependent inactivation prolongs APD and causes EADs [2]. CaMKII can phosphorylate Na+ channels, increasing I Na,late and causing APD-prolongation/EAD-generation [12]. Moreover, CaMKII-dependent phosphorylation of I Ca,L slows inactivation and accelerates recovery from inactivation, further enhancing the likelihood of EADs [8]. Accordingly, CaMKII inhibition has been shown to prevent oxidative stress-induced EADs [122]. Finally, a specific type of EAD occurring during late-phase 3 of the AP, which is dependent on SR Ca2+-release and therefore mechanistically related to delayed afterdepolarizations (DADs, see below), has been described under conditions of intense βAR stimulation [115, 129]. In contrast to more classical EAD-forms which are most common during bradycardia or following a sinus pause, these late-phase 3 EADs are tachycardia-dependent [49, 92, 115].
Schematic representations of early (a) and delayed (b) afterdepolarizations and their dominant underlying mechanisms. Abbreviations are defined in Table 1
Delayed afterdepolarizations
Delayed afterdepolarizations typically occur during conditions of elevated cellular Ca2+ loading, like fast heart rates, and/or βAR stimulation. When SR Ca2+-content exceeds a certain limit or when the sensitivity of RyR2 is increased, Ca2+ can be released from the SR through RyR2 in the absence of the normal I Ca,L trigger (Fig. 2b). This Ca2+ activates a transient-inward current mostly carried by NCX1, which depolarizes the membrane potential. If the DAD is of sufficient amplitude, I Na is activated and a triggered AP occurs. In addition to promoting triggered APs, diastolic SR Ca2+ release events are also associated with a prolongation of the following AP due to a reduction in SR Ca2+ load and subsequent reduction in Ca2+-dependent I Ca,L inactivation [48]. This interspersed prolongation contributes to increased spatial and temporal dispersion of repolarization, which are established proarrhythmic factors.
CaMKII plays a critical role in RyR2 dysfunction, abnormal SR Ca2+-handling and DADs. PKA- and CaMKII-dependent phosphorylation of PLB enhances Ca2+ uptake through SERCA2a disinhibition, increasing SR Ca2+ load and the likelihood of DADs [91]. In addition, CaMKII-dependent phosphorylation of RyR2 at Ser2814 increases its Ca2+-sensitivity, further promoting SR Ca2+-release events [91]. Studies in mice with the phosphomimetic substitution Ser2814Asp have shown that phosphorylation of this CaMKII site is sufficient for the development of ventricular tachyarrhythmias upon catecholaminergic provocation or following transverse aortic constriction [112]. Accordingly, CaMKII-inhibition has been shown to normalize Ca2+-handling and prevent DAD occurrence in various cell types and pathologies [60, 112, 114]. Purkinje cells are more susceptible to spontaneous SR Ca2+-release than ventricular myocytes, suggesting an important role in RyR2-mediated arrhythmogenesis [50].
Reentry
Reentry is a central mechanism for sustained ventricular arrhythmias. It requires a vulnerable substrate, which can be caused by disease-related remodeling, predisposing genetic background, etc., and a trigger, usually in the form of afterdepolarization-induced premature APs. Reentry can occur around a fixed anatomical obstacle or in a substrate in which functional properties permit initiation and maintenance of the reentrant circuit [131]. Different conceptual models of functional reentry have been proposed, with leading circle reentry around a functionally refractory core and spiral wave reentry with an excitable but unexcited core being the most common [20]. In the leading-circle model, reentry is critically determined by wavelength, the distance an impulse travels in one effective refractory period. The refractory period is largely determined by APD, whereas conduction velocity largely depends on I Na availability, expression and localization of gap-junction proteins, and composition of the extracellular matrix (notably fibrosis). Reduced availability of I Na or gap-junction proteins, or interruption of longitudinal muscle-bundle continuity by intervening fibrosis, reduces effective depolarization of neighboring tissue, producing slow conduction that provides more time for the surrounding tissue to regain excitability, thereby increasing the likelihood of reentry.
ES pathophysiology
Clinical conditions predisposing to ES
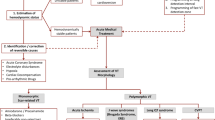
Electrical storm has been described in association with a variety of clinical conditions including rare genetic disorders [Brugada syndrome (BrS), early repolarization syndrome (ERS), long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT)] and more common acquired conditions (cardiac hypertrophy, HF). In the ICD era, ES has become a more common clinical finding, occurring at some point in up to 20 % of patients with an ICD for secondary prevention [30, 43]. The clinical conditions acutely contributing to ES initiation remain incompletely understood. In the SHIELD trial, a precipitating cause could be identified in 13 % of the ES cases and mostly consisted of worsening HF and electrolyte disturbances [41]. In addition, ischemic events, renal insufficiency, and prior arrhythmic events have been considered to be major risk factors for ES [43]. A significant correlation between the type of arrhythmia requiring ICD implantation and the type of arrhythmia responsible for ES has also been identified [113]. However, in one study, no independent predictors of ES were identified using univariate or multivariable analyses [41]. As such, it is likely that ES results from a complex interplay between preexisting pathological conditions creating a vulnerable substrate and acute initiating factors in a patient-specific manner. Figure 3 highlights the major electrophysiological consequences and the predominant arrhythmogenic mechanisms in the pathologies predisposing to ES, which are reviewed below with the aim of identifying factors shared among the heterogeneous clinical conditions predisposing to ES.
Overview of clinical conditions that can lead to recurrent ventricular arrhythmias and electrical storm, their main pathophysiological characteristics, arrhythmogenic mechanisms as well as common initiating factors. There appears to be a central role for abnormal Ca2+-handling in a wide range of clinical conditions leading to electrical storm
Brugada syndrome and early repolarization syndrome
Brugada syndrome is an inherited SCD arrhythmia syndrome, characterized by ST-segment elevation in the right precordial leads, complete or incomplete right bundle-branch block, and high incidence of polymorphic VT/VF. Mutations affecting I Na have been linked to BrS, with other cases involving genes encoding I Ca,L and I to [71]. ERS is an emerging primary arrhythmia syndrome [6] characterized by J wave and ST-segment elevation in the inferolateral leads, and is associated with an increased risk of idiopathic VF [35]. Proposed mechanisms of arrhythmogenesis are similar in BrS and ERS and involve accelerated repolarization due to the underlying ion-channel abnormalities in regions with prominent I to, leading to a regional loss of AP dome and marked APD abbreviation, resulting in a transmural voltage gradient, ST-segment elevation, phase 2 reentry and VT/VF [6, 9]. An alternative explanation relates the I Na dysfunction in BrS to delayed activation of the right-ventricular outflow tract due to conduction abnormalities [119].
Brugada syndrome and ERS seem to share a number of predisposing factors for ventricular tachyarrhythmias including hypokalemia, a high vagal tone, bradycardia, and fever [36, 71, 119]. ES consisting of multiple VF episodes (VF storm) occurs in 18 % of patients with symptomatic BrS [83] and in 13–45 % of patients with ERS [36, 76, 117]. Ohgo et al. [83] recently reported that no predictive clinical, laboratory, ECG, or electrophysiological characteristics could discriminate BrS patients with and without ES, whereas Nam et al. [75, 76] showed that ERS patients display transient augmentation of global J waves before the development of ES.
VF storm associated with BrS is successfully treated acutely with intravenous infusion of the βAR agonist isoprenaline and prevented with isoprenaline, denopamine or cilostazol (a type-3 phosphodiesterase inhibitor increasing I Ca,L) [71, 83, 119]. These results have been explained by epicardial AP dome restoration due to increased I Ca,L or I Na, resulting in reduced transmural repolarization heterogeneity preventing reentry. A similar effect may also be achieved by decreasing I to with quinidine [63]. Isoprenaline and quinidine are also effective in suppressing and preventing VF storm in ERS patients [10, 36, 76]. In contrast to isoprenaline, endogenous catecholamine release after repeated ICD shocks does not prevent ES in BrS patients. The associated α-adrenergic stimulation, which leads to ST-segment elevation in BrS [70], is suspected to counteract the beneficial effects of βAR stimulation in this situation [65]. These studies suggest reentry as the predominant arrhythmogenic mechanism in BrS and ERS. However, it remains unclear which factors contribute to the recurrent nature of arrhythmias in BrS and ERS patients with ES.
Congenital long QT syndrome
The congenital LQTS is an inherited disorder characterized by prolongation of the QT interval on the ECG and susceptibility to ventricular arrhythmias and SCD with thirteen disease-causing genes described to date [70]. Ventricular arrhythmias in LQTS are thought to arise through a combination of EAD-mediated triggered beats due to excessive repolarization prolongation and large spatial gradients in repolarization, creating functional block and reentry, giving rise to torsade de pointes (TdP) arrhythmias.
Antiadrenergic (sympatholytic) therapies including β-blockers and left cardiac sympathetic denervation effectively reduce the risk for TdP and VF. An ICD is currently recommended when antiadrenergic therapy was unsuccessful [94]. Shimizu et al. [99] identified the clinical characteristics of LQTS patients with repetitive TdP episodes. Female probands with LQT2 genotype and remarkable QTc prolongation were at risk of repetitive TdP even during β-blocker therapy. The Ca2+-channel blocker verapamil (injection followed by continuous infusion) effectively suppressed recurrence in all cases. During contraceptive medication with high progesterone to estrogen ratios, there appears to be a lower incidence of arrhythmic events than when contraceptive medication is not used in female LQTS patients [73]. These findings, together with experimental findings in transgenic LQT2 rabbits showing that progesterone prevented isoprenaline-induced Ca2+-oscillations and EADs [81], suggest that acute alterations in intracellular Ca2+-handling associated with changes in sex hormone levels or autonomic nervous system tone importantly contribute to repetitive occurrence of arrhythmias in the setting of LQTS. The use of ICDs in LQTS is often problematic. LQTS patients frequently have multiple episodes of TdP that spontaneously terminate, such that programming the device with a VT zone and a relatively short detection time leads to an overestimation of appropriate ICD shocks. Sympathetic hyperactivity after ICD discharge can trigger additional tachyarrhythmias and initiate ES. Reprogramming avoids unnecessary shocks and may reduce the incidence of ES [96]. Recently, the MADIT investigators demonstrated reduction in inappropriate therapy and mortality through ICD programming [73]. In the European LQTS ICD Registry, 43 % of patients with appropriate ICD therapies had recurrent episodes fulfilling the definition of ES [96]. In addition, it has been shown that left cardiac sympathetic denervation is highly effective in preventing recurrent arrhythmia events in LQTS patients who experience ES with an ICD [95].
Taken together, these findings suggest major roles for sympathetic nervous system and Ca2+-handling abnormalities in the development of ES in LQTS patients. There is evidence that genetic variations of adrenergic receptors [97] and NOS1AP [21, 106] and variants in 3′ untranslated region of KCNQ1 [3] are associated with increased incidence of cardiac events. A genetic predisposition that affects sympathetic nervous activity or Ca2+ sensitivity may therefore also be involved in the development of ES in some LQTS patients.
Catecholaminergic polymorphic ventricular tachycardia
Catecholaminergic polymorphic ventricular tachycardia is an inherited SCD arrhythmia syndrome characterized by bidirectional or polymorphic VT elicited by increased sympathetic tone in a structurally normal heart without electrocardiographic manifestation under resting conditions. Mutations in the genes encoding RyR2 or the SR Ca2+-buffer calsequestrin increase sensitivity of the channel to luminal and/or cytosolic Ca2+ levels, thereby decreasing the threshold for RyR2 opening. The latter causes inappropriate diastolic SR Ca2+-leak with enhanced incidence of spontaneous SR Ca2+-release events, thereby predisposing to DADs and triggered activity. Increased sympathetic tone further amplifies these abnormalities.
The majority of CPVT patients are well controlled by β-blocker therapy, but arrhythmic event rates remain significant in some individuals. Left cardiac sympathetic denervation and the class 1c antiarrhythmic agents flecainide and propafenone are alternative treatment options. In addition to inhibition of I Na, limiting cardiac excitability, flecainide and propafenone have recently been shown to also directly inhibit RyR2, thereby reducing the occurrence of arrhythmogenic Ca2+ waves and DADs in CPVT mice [45]. RyR2 inhibition may contribute to their antiarrhythmic efficacy in CPVT since other class I antiarrhythmics did not prevent arrhythmias despite similar I Na inhibition [45, 88, 111]. The Ca2+-channel blockers verapamil and diltiazem also reduce arrhythmic event rates, but the underlying mechanism(s) by which they suppress CPVT remain unclear [88, 111], although suppression of catecholamine release through inhibition of neuronal-type of Ca2+ channels is likely to contribute [25]. The use of ICDs in CPVT patients is discouraged because of its proarrhythmic potential, since both appropriate and inappropriate ICD shocks can trigger catecholamine release, resulting in ES with multiple shocks and ultimately death [111]. There is a case report of a patient with CPVT caused by a calsequestrin mutation showing suppression of shock-induced ES by flecainide [75]. The electrophysiological characteristics and genetic backgrounds of CPVT patients with ES have not been clarified yet.
Hypertrophy and heart failure
Patients with cardiac hypertrophy and HF are at a high risk for SCD from VT/VF. VT/VF episodes tend to cluster in HF [61] and HF is a risk factor for ES [38]. On the other hand, analyses of large clinical trials have shown that ES is associated with increased mortality, predominantly due to progressive HF [28, 98]. These findings indicate an interaction between HF and ES. A recent preclinical study using a rabbit model of ES (see the section below) showed that ES events themselves worsen HF by activating the cellular signaling involved in the progression of HF [109].
Clustered VT/VF and ES associated with HF may involve both reentry and focal (triggered) activity. The reentry mechanism is supported (1) by the presence of fibrosis in HF patients, independent of its ischemic or non-ischemic etiology, (2) by the clinical evidence that ES recurrence is suppressed by the class III antiarrhythmic drug azimilide, which prolongs repolarization and increases wavelength [41], and (3) by the efficacy of catheter ablation [15], which modifies the arrhythmogenic substrate. Repeated focal activity is another candidate ES mechanism. Three-dimensional mapping studies in humans and animals with HF showed that inducible and spontaneous ventricular tachyarrhythmias are frequently due to a focal mechanism [19, 86, 87]. Abnormal intracellular Ca2+-homeostasis in HF (discussed in detail below) naturally suggests Ca2+-triggered arrhythmias facilitated by elevated sympathetic tone as a major driver of ES.
The greatest reduction in SCD in patients with HF resulted from the use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, lipid-lowering agents and aldosterone-receptor inhibitors [93, 131]. These drugs most likely exert their antiarrhythmic potential indirectly by inhibiting or delaying adverse functional and structural remodeling in the diseased heart and by affecting “upstream events” that trigger electrophysiological instability [93]. Beneficial effects of such HF therapy in post-ES management have been underscored [43]. Cardiac resynchronization therapy with biventricular pacing can also reverse ventricular remodeling in HF by attenuating dyssynchrony, resulting in improved cardiac function and reduced mortality. A retrospective study showed a much lower incidence of ES in patients who had biventricular pacing with defibrillator versus ICD-only patients [80]. Interestingly, the percentage of patients who had isolated VT/VF episodes did not differ between the two groups. These findings suggest that HF improvement may contribute to ES suppression.
Factors contributing to VT/VF recurrence
The initiating factors for ES are generally unknown, although psychological stress, acute myocardial ischemia, electrolyte imbalances and HF progression have been implicated [43]. It remains mysterious why ES develops in certain individuals, whereas others with similar pathological conditions have isolated VT/VF episodes.
It should be noted that the definition of ES as three or more separate episodes of VT/VF per 24 h is somewhat arbitrary [30] and may conceal mechanistic differences between a ‘train’ of recurrent VT/VF episodes occurring in quick succession and episodes occurring less frequently. In the former case, it can be expected that the preceding VT/VF episode and/or defibrillation directly contribute to the recurrence of VT/VF. Acute global ischemia during VT/VF [76], shock-induced sympathetic activation [16] and/or shock-induced myocardial damage and dysfunction [16] are potential candidates. It is likely that these factors may interact synergistically to create predisposing conditions for the reinitiation of VF. For example, it has been shown that sympathetic stimulation can cause vasoconstriction and amplify myocardial ischemia in the setting of decreased coronary reserve [40]. This could promote arrhythmogenesis, which in turn would shorten diastolic duration, aggravate ischemia and promote further ICD-discharges, starting a vicious cycle of ES. Moreover, VT/VF episodes themselves result in increased intracellular Ca2+-levels due to the rapid ventricular rate, repeated activation of I Ca,L and short diastolic intervals, limiting Ca2+-extrusion via NCX, which could contribute to the reinduction of VF via afterdepolarizations (discussed in detail below).
Recurrent VT/VF episodes occurring over larger time intervals may be due to other time-dependent factors. The incidence of SCD exhibits diurnal variation, occurring predominantly early after rising in the morning and in the evening [74]. Similarly, diurnal variations in certain risk factors, notably autonomic tone, may contribute to ES. Interestingly, Jeyaraj et al. [47] recently demonstrated that ion-channel expression and QT interval duration also show diurnal variation, contributing to enhanced susceptibility to ventricular arrhythmias. Based on the aforementioned importance of increased sympathetic tone and aberrant Ca2+-homeostasis-favoring afterdepolarizations for ES in a variety of pathological conditions (LQTs, CPVT, and HF as shown in Fig. 3), we speculate that time-dependent alterations in the sensitivity of target molecules to intracellular Ca2+-levels may also contribute to the recurrent nature of ventricular tachyarrhythmias, although this remains to be tested in future studies.
Experimental studies of ES mechanisms
The unpredictable nature of ES and the invasive character of many experiments preclude a detailed characterization of the cellular and molecular mechanisms contributing to the recurrence of VT/VF in humans. Recently, two experimental models have been developed, which have provided important first steps to elucidate these mechanisms.
Repolarization alterations contributing to ES
Chen et al. [18, 82] have investigated the occurrence and mechanism of spontaneous VF following defibrillation shocks in a rabbit model of tachypacing-induced HF. In this model, rabbits underwent ventricular pacing (250, 300, and 350 beats per minute for 3 days, 3 days, and 3 weeks, respectively), resulting in pacing-induced HF with increased end-diastolic and end-systolic LV dimensions, reduced fractional shortening, and increased ventricular fibrosis. The failing hearts retrogradely perfused on a Langendorff setup showed increased average APD and increased spatial dispersion of APD. Following burst pacing-induced VF and subsequent defibrillation, 4 out of 12 HF rabbit hearts (and 3 out of 9 in a follow-up study [18]) but no sham-operated controls developed spontaneous recurrent VF. We have recently developed an experimental ES model based on a rabbit model of complete atrioventricular block (CAVB) to study the basic mechanisms contributing to ES in vivo [109]. In this model, CAVB rabbits underwent ICD implantation and were followed for ~80 days. 53 % of rabbits developed ES consisting of frequent VF episodes (≥3 VF episodes per 24 h) and the average number of VF episodes/rabbit was >100.
APD and QT prolongation are observed in both experimental models of recurrent ventricular arrhythmias [82, 109] as well as in several clinical conditions predisposing to ES (HF, LQTS), suggesting that reduced repolarization reserve is an important arrhythmogenic risk factor. Electrical remodeling, characterized by changes in ion channel and transporter function and expression, underlies this APD prolongation and likely predisposes to VT/VF through the development of EADs and/or spatial APD gradients [68], although the molecular basis of APD prolongation is diverse [110]. However, APD prolongation does not appear to be a necessary requirement for ES in all conditions, since patients with CPVT, BrS, or ERS can also develop ES with normal or reduced APD.
Interestingly, Chua et al. [18] have recently provided evidence that small-conductance Ca2+-dependent K+ (SK) channels contribute to recurrent VT/VF in the tachypacing-induced HF model. The proposed mechanism involves pronounced APD shortening in the beats following defibrillation due to VT/VF-dependent increases in Ca2+-levels and activation of SK channels. The abbreviated APD after defibrillation occurred despite the overall APD prolongation at rest and resulted in late-phase 3 EAD-mediated VF reinduction [18]. These findings suggest that ion-channel alterations and changes in cellular Ca2+-handling (discussed below) are strongly interconnected and may interact to produce ES. Additional points of interaction include the increased INCX in combination with reduced IK1 observed in HF models, which would produce larger membrane depolarizations for a given SR Ca2+-release, potentially facilitating DAD-triggered arrhythmias [77]. In addition, CaMKII-dependent phosphorylation of Nav1.5 is linked to enhanced I Na,late in human HF [56] and combined with CaMKII-dependent phosphorylation of I Ca,L may contribute to the development of EADs.
Linking ES to abnormal intracellular Ca2+-handling
Changes in intracellular Ca2+-handling alter contractile function and promote potentially arrhythmogenic afterdepolarizations. There is substantial evidence that CaMKII activation has an important role in promoting arrhythmias in the setting of cardiac hypertrophy and HF [102, 103, 121, 127]. CaMKII inhibition improved Ca2+-handling abnormalities in failing cardiomyocytes [1, 100] and suppressed afterdepolarization-triggered arrhythmias in a variety of animal models [5, 51, 89, 121].
Excessive βAR stimulation can activate CaMKII by increased intracellular Ca2+-concentrations and cyclic AMP-dependent and -independent mechanisms (Fig. 1), although the transcriptional regulation of CaMKII remains unclear. This suggests that the sympathetic activation that characterizes ES would potentiate CaMKII activation and promote afterdepolarizations (Fig. 2). Rabbits with CAVB for 2 weeks show cardiac hypertrophy, acquired QT prolongation and spontaneous TdP arrhythmias [89, 110]. Their cardiomyocytes are hypercontractile and show APD prolongation with a high incidence of EADs [89]. Enhanced SR Ca2+-uptake due to APD prolongation and PLB-hyperphosphorylation at Ser16 (PKA phosphorylation site) and Thr17 (CaMKII site) increase SR Ca2+-content, leading to increased systolic Ca2+-transients, enhanced contractile function, and CaMKII activation. Isoprenaline facilitates EADs and induces DADs in CAVB myocytes. These afterdepolarizations are prevented by interventions that suppress SR Ca2+-loading, SR Ca2+-release, NCX function, or activation of CaMKII.
Electrical storm manifested as VF storm was observed in approximately half of CAVB rabbits [109]. ES-rabbits showed LV function deterioration along with striking CaMKII activation and enhanced expression of protein-phosphatases PP1 and 2a (PP2a) compared to non-ES CAVB rabbits. These alterations were associated with hyperphosphorylation of the L-type Ca2+ channel α-subunit CaV1.2 and RyR2 at both Ser2809 and Ser2815 and dephosphorylation of PLB at both Ser16 and Thr17, a phosphorylation pattern similar to that reported previously in humans and animals with HF [108]. Repeated VF/defibrillation with ICD shocks over 1 h in baseline rabbits reproduced ES-associated changes in CaMKII activation and PLB phosphorylation, but repeated ICD-shocks alone without VF induction did not. CaM inhibition with W7 suppressed VT/VF episodes and rescued LV dysfunction in association with a reduction in CaMKII-activation. Caspase-3 subunits as well as ANF and BNP mRNA levels were increased in ES-rabbits, changes that were not suppressed by W7 treatment. These findings suggest that CaMKII hyperactivity is importantly involved in recurrent ventricular arrhythmias with progressive contractile failure and support the notion that CaMKII activation and Ca2+-handling abnormalities resulting from ES events might be responsible for negative outcomes.
Figure 4, based on the data from our rabbit ES model, illustrates a putative model for the pathophysiology of ES in the setting of acquired diseases with LV dysfunction. Electrical remodeling associated with structural heart diseases (cardiac hypertrophy and HF) leads to APD prolongation, which favors EADs by lengthening the AP plateau phase with increased opportunities for L-type Ca2+ channel reactivation. Although not present in all pathological conditions predisposing to ES, APD prolongation may increase Ca2+-loading and activate CaMKII. Subsequent hyperphosphorylation of L-type Ca2+ channels and RyR2 would promote EADs, DADs, and mechanical dysfunction. CaMKII also activates apoptotic and hypertrophic signaling pathways that lead to structural remodeling [102], further contributing to a substrate predisposing to VF. Repeated VF/defibrillation cycles may, by themselves, cause CaMKII activation and PLB dephosphorylation. Such a sequence of events would produce a positive feed-back loop, leading to an increase of VF occurrence and deterioration of contractile dysfunction. Our data, showing that CaMKII inhibition with W7 in ES-rabbits suppressed VT/VF and rescued LV dysfunction without significantly altering apoptosis or hypertrophy, suggest that Ca2+-handling protein phosphorylation abnormalities rather than progressive structural remodeling importantly contribute to ES in this model. Of note, the proposed model cannot apply to conditions with normal APD (e.g., BrS, CPVT) or abbreviated APD (ERS), suggesting that other mechanisms may contribute to the development of a vulnerable substrate for ES in these entities.
Proposed pathophysiology of electrical storm (ES) based on a rabbit ES model. Predisposing conditions, result in increased APD and cellular Ca2+ loading, thereby activating CaMKII and, through unknown mechanisms, reducing protein phosphatase type-1 (PP1) activity, which contributes to the altered phosphorylation state of several Ca2+-handling proteins, predisposing to afterdepolarization-mediated arrhythmogenesis. VF/defibrillation cycles further increase cellular Ca2+ loading. The positive feedback loop induces CaMKII hyperactivity and prominent alterations of protein-phosphorylation, leading to increased VF occurrence. Since CaMKII favors apoptosis and stimulates hypertrophic transcriptional programs, structural remodeling progression may also be involved in ES pathogenesis (dashed lines). Further details in the text. Modified from Tsuji [108]
The precise role of PLB dephosphorylation in association with ES and repeated VF/defibrillation has not yet been determined (Fig. 4). Zhang et al. [126] have recently demonstrated by crossing CaMKII transgenic mice with PLB knockout mice that increasing SR Ca2+-load by reducing PLB-dependent SERCA inhibition in the face of elevated CaMKII and RyR2 phosphorylation leads to enhanced SR Ca2+-leak and mitochondrial Ca2+-elevation, which exacerbated cell death, HF and mortality. On the other hand, SERCA2a gene therapy targeted to improve SR Ca2+-load and mechanical dysfunction has been shown to possess antiarrhythmic effects in rats with HF following myocardial infarction [62]. SERCA2a gene therapy stabilized SR Ca2+-load, reduced RyR2 phosphorylation and decreased SR Ca2+-leak, reducing cellular triggered activity in vitro, and spontaneous and catecholamine-induced ventricular arrhythmias in vivo in failing hearts, in contrast to PLB knockout. Interestingly, restoring SERCA2a protein levels and activity in the failing heart also resulted in attenuation of electrical remodeling as well as reduced susceptibility to programmed electrical stimulation-induced arrhythmias [22, 62].
Role of Ca2+ in recurrence of arrhythmias following defibrillation
Recurrence of ventricular arrhythmias following defibrillation shocks is often seen in ICD patients as well as in experimental models of ES. Mechanisms of post-shock arrhythmias have been investigated in several studies. Changes in intracellular Ca2+ and APD after defibrillation appear to be important contributors to VF reinitiation. Ogawa et al. [82] have shown that in the rabbit tachypacing-induced HF model, reinitiation of VF following defibrillation occurs in regions where intracellular Ca2+ is elevated and there is a disproportionate shortening of APD relative to the duration of the Ca2+-transient. In addition, a study in rat hearts showed that VF-induced Ca2+-overload causes failed electrical defibrillation and post-shock reinitiation of VF [125]. Successful defibrillation shocks led to a sudden reduction in intracellular Ca2+, whereas incomplete reduction of Ca2+-overload after defibrillation shocks was followed by spontaneous Ca2+-oscillations and subsequent reinitiation of VF [125]. A voltage- and Ca2+-optical mapping study demonstrated that the heterogeneous distribution of intracellular Ca2+ during the post-shock isoelectric window determines the defibrillation outcome [44]. The first post-shock activation always occurred from a region of low Ca2+ surrounded by high Ca2+ (Ca2+ sinkhole) after unsuccessful defibrillation. Recently, it has been found in normal rabbit hearts that spontaneous Ca2+-elevation can emerge in the ventricles after prolonged rapid ventricular pacing or after successful defibrillation [64]. This phenomenon was observed after defibrillation in 80 % of VF episodes due to isoprenaline infusion. Despite similar amplitudes and slopes of spontaneous Ca2+-elevations between epicardial and endocardial surfaces, DAD-related triggered activities originated from endocardial surfaces with Purkinje-like potentials. This could be partly due to the smaller I K1 in the Purkinje system contributing to a higher Ca2+-voltage coupling gain, promoting triggered activity. Indeed, pharmacological suppression of I K1 enhanced Ca2+-voltage coupling gain and enabled the epicardium to also generate triggered activities [64]. In addition, the approximately one-dimensional structure of Purkinje fibers limits the electronic sink that needs to be overcome to generate triggered APs, thereby requiring synchronization of afterdepolarizations among fewer cells [123].
Taken together, there is substantial experimental evidence implicating multiple Ca2+-handling abnormalities and subsequent electrophysiological disturbances in recurrent ventricular arrhythmogenesis and ES, providing a range of potential therapeutic approaches for ES. Although the ex vivo nature of many investigations into post-shock VF recurrence has helped to determine the direct role of defibrillation and Ca2+, it should be noted that post-shock recurrence of VF in patients is likely modulated by many extracardiac factors, including increased sympathetic tone, ischemia and electrolyte imbalances [43].
Therapeutic approaches for ES
Currently available drug therapy for ES is empiric, complex, and only of moderate efficacy. The cornerstone of current therapy is sympathetic blockade by β-blockers or left stellate ganglion denervation, suppressing the adrenergic drive that can stimulate recurrent ventricular arrhythmias [43]. Sedation and anesthesia with propofol may also be effective. Conventional antiarrhythmic agents provide little benefit, although class III antiarrhythmic agents with antiadrenergic properties like sotalol and amiodarone are moderately effective [43]. The L-type Ca2+-channel blocker verapamil has been shown to suppress repetitive TdP in LQTS patients [99] and appears to suppress ES in some patients with CPVT [29, 111] or idiopathic Purkinje-related VF [79]. Once ES has occurred, the therapeutic focus is typically shifted toward treatment of changes in the underlying substrate, notably worsening HF, particularly in patients with structural heart disease. According to the results of larger-scale clinical trials indicating that the first few months of ES are critical [28, 98], the optimization of HF therapy is strongly recommended [43]. β-Blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, lipid-lowering agents, and spironolactone reduce cellular Ca2+-load and reactive oxygen species production. Recent clinical studies have demonstrated that lack of therapy with angiotensin-converting enzyme inhibitors and/or β-blockers is a predictor of ES [7, 101] and that statin use is associated with a reduction in mortality, mostly attributable to a reduction in arrhythmic death in ICD-patients with non-ischemic dilated cardiomyopathy [32]. Several HF regimens with proven mortality-protection benefits improve defective Ca2+-handling in experimental HF. Metoprolol reverses hyperphosphorylation of RyR2, restores the stoichiometry of the RyR2 macromolecular complex, and normalizes single-channel function [90]. Low-dose propranolol corrects the defective interaction of FKBP12.6 with RyR2, restoring proper RyR2 conformation and preventing SR Ca2+-leak through RyR2 [26]. The angiotensin-receptor blocker valsartan restores SR function along with attenuation of RyR2 hyperphosphorylation and a decrease in abnormal SR Ca2+-leak [84]. In the failing rat heart, the statin rosuvastatin has been shown to prevent LV remodeling and dysfunction at least partly by normalization of SERCA2a expression, PLB phosphorylation, and SERCA2a function [124]. Because many of these therapeutic approaches directly or indirectly affect intracellular Ca2+-handling, their effects may be attributed at least in part to reduced CaMKII activity.
Agents that could provide lead compounds for novel therapeutic opportunities are illustrated in Fig. 5 (orange boxes). The data in the rabbit model of ES, as well as features of the clinical conditions, point to CaMKII as a potentially important molecular contributor to ES and its consequences. CaMKII inhibition has also been demonstrated to suppress or prevent ventricular arrhythmias in a variety of animal models of hypertrophy/HF and ischemic heart disease and inherited arrhythmia syndromes [91]. In addition, CaMKII inhibition also has a significant beneficial effect on the development of HF [34, 102] and in atrial fibrillation [17, 59, 114]. Catecholamine-induced sustained ventricular arrhythmias are completely prevented by CaMKII inhibition with KN93 infusion in RyR2 R4496C+/− mice with CPVT [60]. Rat ventricular myocytes overexpressing a Cav1.2 mutant responsible for Timothy syndrome (referred to as LQTS 8) exhibit increased CaMKII activity and a proarrhythmic phenotype including APD prolongation, increased I Ca,L facilitation and afterdepolarizations [105]. Intracellular dialysis of a CaMKII-inhibitory peptide reverses I Ca,L facilitation to normal levels, normalizes APD and prevents afterdepolarizations. Infusion of the CaMKII-blocker KN93 suppresses EADs and the CaM antagonist W7 prevents TdP in rabbit models of drug-induced LQTS [5, 66]. These data strongly suggest that CaMKII inihibition might be a promising strategy to treat ES. However, cardiac-specific CaMKII inhibitors preferentially targeting CaMKIIδ, the predominant cardiac CaMKII isoform, will first have to be developed since global CaMKII inhibition may cause impairment of memory and fertility [24].
Recent experimental studies demonstrated that Ca2+-overload coupled with shortened APD promotes late-phase 3 EAD and triggered activity, leading to VF. I KATP channel activation along with cellular Ca2+-overload due to rapid pacing is associated with the development of late-phase 3 EAD and VF [104]. A Ca2+-chelator BAPTA-AM prevents Ca2+-accumulation, resulting in suppression of EAD and arrhythmia induction. Selective blockers of cardiac sarcolemmal I KATP channels HMR 1883/1098 and HMR 1402 may therefore also be beneficial for treating ischemia-related ES. As described above, HF increases the sensitivity of Ca2+-activated SK channels to Ca2+ [18], resulting in APD shortening at high stimulation frequencies. The selective SK-channel blocker apamin attenuates post-shock APD abbreviation and prevents recurrent spontaneous VF in failing hearts [18], whereas the SK-channel inhibitor NS8593 terminates and prevents experimental atrial fibrillation [23]. These findings suggest that ion-channels responsible for APD shortening are potential therapeutic targets for preventing recurrent VF and ES.
New therapeutic approaches to prevent afterdepolarizations and triggered arrhythmias have recently emerged. RyR2 stabilizers JTV-519 and the more specific analog S107 reduce SR Ca2+-leak by increasing the binding affinity for FKBP12.6 to RyR2 [58, 118], whereas dantrolene, a therapeutic agent for malignant hyperthermia, reduces SR Ca2+-leak by correcting domain unzipping [53]. RyR2 stabilization with these agents prevents stress-induced arrhythmias in CPVT mouse models [58, 118] and improves contractile function in dogs with tachypacing-induced HF [54, 55]. Carvedilol [130] and ranolazine [85] have been demonstrated to possess direct antiarrhythmic effects by reducing RyR2 single-channel open probability. Inhibition of I Na,late with ranolazine also has the potential to treat and prevent ES. Ranolazine abolishes EADs due to angiotensin II-induced reactive oxygen species production, CaMKII activation, and enhancement of I Na,late in ventricular myocytes [128] and terminates multifocal VF induced by exposure to oxidative stress with H2O2 in perfused rat hearts [72]. It is conceivable that inhibition of I Na,late, which is upregulated in HF, may mitigate CaMKII-overactivity, because ranolazine reduces cellular Ca2+-load by preventing intracellular Na+-accumulation [116].
Conclusions
Electrical storm likely represents the most severe end-point of a wide variety of pathological conditions. Although abnormal Ca2+-handling appears to be an important common denominator with therapeutic potential in experimental models, patient-specific pathophysiological conditions will have to be taken into account when treating ES. Despite recent progress, we are just beginning to understand the pathophysiology and the precise cellular and molecular mechanisms of ES. An enormous amount of additional work is needed to clarify the multiple clinical and molecular aspects of ES and to develop novel mechanism-based therapeutic approaches.
References
Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM (2005) Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97:1314–1322. doi:10.1161/01.RES.0000194329.41863.89
Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT (2002) Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A 99:17185–17190. doi:10.1073/pnas.262372999
Amin AS, Giudicessi JR, Tijsen AJ, Spanjaart AM, Reckman YJ, Klemens CA, Tanck MW, Kapplinger JD, Hofman N, Sinner MF, Muller M, Wijnen WJ, Tan HL, Bezzina CR, Creemers EE, Wilde AA, Ackerman MJ, Pinto YM (2012) Variants in the 3′ untranslated region of the KCNQ1-encoded Kv7.1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur Heart J 33:714–723. doi:10.1093/eurheartj/ehr473
Anderson ME (2004) Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc Med 14:152–161. doi:10.1016/j.tcm.2004.02.005
Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ (1998) KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther 287:996–1006
Antzelevitch C (2012) Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J 76:1054–1065. doi:10.1253/circj.CJ-12-0284
Arya A, Haghjoo M, Dehghani MR, Fazelifar AF, Nikoo MH, Bagherzadeh A, Sadr-Ameli MA (2006) Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator. Am J Cardiol 97:389–392. doi:10.1016/j.amjcard.2005.08.058
Benitah JP, Alvarez JL, Gomez AM (2010) L-type Ca2+ current in ventricular cardiomyocytes. J Mol Cell Cardiol 48:26–36. doi:10.1016/j.yjmcc.2009.07.026
Benito B, Guasch E, Rivard L, Nattel S (2010) Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 56:1177–1186. doi:10.1016/j.jacc.2010.05.037
Bernard A, Genee O, Grimard C, Sacher F, Fauchier L, Babuty D (2009) Electrical storm reversible by isoproterenol infusion in a striking case of early repolarization. J Interv Card Electrophysiol 25:123–127. doi:10.1007/s10840-008-9348-5
Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49. doi:10.1146/annurev.physiol.70.113006.100455
Bers DM, Herren AW (2012) Na+ channel I-II loop mediates parallel genetic and phosphorylation-dependent gating changes. Circulation 126:2042–2046. doi:10.1161/CIRCULATIONAHA.112.140384
Boontje NM, Merkus D, Zaremba R, Versteilen A, de Waard MC, Mearini G, de Beer VJ, Carrier L, Walker LA, Niessen HW, Dobrev D, Stienen GJ, Duncker DJ, van der Velden J (2011) Enhanced myofilament responsiveness upon beta-adrenergic stimulation in post-infarct remodeled myocardium. J Mol Cell Cardiol 50:487–499. doi:10.1016/j.yjmcc.2010.12.002
Brandmayr J, Poomvanicha M, Domes K, Ding J, Blaich A, Wegener JW, Moosmang S, Hofmann F (2012) Deletion of the C-terminal phosphorylation sites in the cardiac beta-subunit does not affect the basic beta-adrenergic response of the heart and the Cav1.2 channel. J Biol Chem 287:22584–22592. doi:10.1074/jbc.M112.366484
Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, Riva S, Moltrasio M, Cireddu M, Veglia F, Della Bella P (2008) Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation 117:462–469. doi:10.1161/CIRCULATIONAHA.106.686534
Cevik C, Perez-Verdia A, Nugent K (2009) Implantable cardioverter defibrillators and their role in heart failure progression. Europace 11:710–715. doi:10.1093/europace/eup091
Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH (2009) Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119:1940–1951. doi:10.1172/JCI37059
Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T, Chen PS (2011) Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 108:971–979. doi:10.1161/CIRCRESAHA.110.238386
Chung MK, Pogwizd SM, Miller DP, Cain ME (1997) Three-dimensional mapping of the initiation of nonsustained ventricular tachycardia in the human heart. Circulation 95:2517–2527. doi:10.1161/01.cir.95.11.2517
Comtois P, Kneller J, Nattel S (2005) Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace 7(Suppl 2):10–20. doi:10.1016/j.eupc.2005.05.011
Crotti L, Monti MC, Insolia R, Peljto A, Goosen A, Brink PA, Greenberg DA, Schwartz PJ, George AL Jr (2009) NOS1AP is a genetic modifier of the long-QT syndrome. Circulation 120:1657–1663. doi:10.1161/CIRCULATIONAHA.109.879643
Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS (2009) Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol 2:686–694. doi:10.1161/CIRCEP.109.863118
Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS (2010) Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol 3:380–390. doi:10.1161/CIRCEP.110.957407
Dobrev D, Carlsson L, Nattel S (2012) Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov 11:275–291. doi:10.1038/nrd3682
Dobrev D, Milde AS, Andreas K, Ravens U (1999) The effects of verapamil and diltiazem on N-, P- and Q-type calcium channels mediating dopamine release in rat striatum. Br J Pharmacol 127:576–582. doi:10.1038/sj.bjp.0702574
Doi M, Yano M, Kobayashi S, Kohno M, Tokuhisa T, Okuda S, Suetsugu M, Hisamatsu Y, Ohkusa T, Kohno M, Matsuzaki M (2002) Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 105:1374–1379. doi:10.1161/hc1102.105270
Eifling M, Razavi M, Massumi A (2011) The evaluation and management of electrical storm. Tex Heart Inst J 38:111–121
Exner DV, Pinski SL, Wyse DG, Renfroe EG, Follmann D, Gold M, Beckman KJ, Coromilas J, Lancaster S, Hallstrom AP, Defibrillators AIAVI (2001) Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation 103:2066–2071. doi:10.1161/01.CIR.103.16.2066
Fagundes A, DE Magalhaes LP, Russo M, Xavier E (2010) Pharmacological treatment of electrical storm in cathecolaminergic polymorphic ventricular tachycardia. Pacing Clin Electrophysiol 33:e27–e31. doi:10.1111/j.1540-8159.2009.02586.x
Gao D, Sapp JL (2013) Electrical storm: definitions, clinical importance, and treatment. Curr Opin Cardiol 28:72–79. doi:10.1097/HCO.0b013e32835b59db
Gao Z, Rasmussen TP, Li Y, Kutschke W, Koval OM, Wu Y, Hall DD, Joiner ML, Wu X, Dominic Swaminathan P, Purohit A, Zimmerman KA, Weiss RM, Philipson K, Song LS, Hund TJ, Anderson ME (2012) Genetic inhibition of Na+-Ca2+ exchanger current disables fight or flight sinoatrial node activity without affecting resting heart rate. Circ Res doi:10.1161/CIRCRESAHA.111.300193
Goldberger JJ, Subacius H, Schaechter A, Howard A, Berger R, Shalaby A, Levine J, Kadish AH, Investigators D (2006) Effects of statin therapy on arrhythmic events and survival in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 48:1228–1233. doi:10.1016/j.jacc.2006.05.053
Goldberger Z, Lampert R (2006) Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA 295:809–818. doi:10.1001/jama.295.7.809
Grimm M, Brown JH (2010) Beta-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol 48:322–330. doi:10.1016/j.yjmcc.2009.10.016
Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquie JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O’Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J (2008) Sudden cardiac arrest associated with early repolarization. N Engl J Med 358:2016–2023. doi:10.1056/NEJMoa071968
Haissaguerre M, Sacher F, Nogami A, Komiya N, Bernard A, Probst V, Yli-Mayry S, Defaye P, Aizawa Y, Frank R, Mantovan R, Cappato R, Wolpert C, Leenhardt A, de Roy L, Heidbuchel H, Deisenhofer I, Arentz T, Pasquie JL, Weerasooriya R, Hocini M, Jais P, Derval N, Bordachar P, Clementy J (2009) Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol 53:612–619. doi:10.1016/j.jacc.2008.10.044
Harmati G, Banyasz T, Barandi L, Szentandrassy N, Horvath B, Szabo G, Szentmiklosi JA, Szenasi G, Nanasi PP, Magyar J (2011) Effects of beta-adrenoceptor stimulation on delayed rectifier K+ currents in canine ventricular cardiomyocytes. Br J Pharmacol 162:890–896. doi:10.1111/j.1476-5381.2010.01092.x
Haverkamp W (2006) Electrical storm: still a cryptogenic phenomenon? Eur Heart J 27:2921–2922. doi:10.1093/eurheartj/ehl396
Heijman J, Volders PG, Westra RL, Rudy Y (2011) Local control of β-adrenergic stimulation: effects on ventricular myocyte electrophysiology and Ca2+-transient. J Mol Cell Cardiol 50:863–871. doi:10.1016/j.yjmcc.2011.02.007
Heusch G, Deussen A (1983) The effects of cardiac sympathetic nerve stimulation on perfusion of stenotic coronary arteries in the dog. Circ Res 53:8–15
Hohnloser SH, Al-Khalidi HR, Pratt CM, Brum JM, Tatla DS, Tchou P, Dorian P, SHIELD investigators (2006) Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J 27:3027–3032. doi:10.1093/eurheartj/ehl276
Honjo H, Boyett MR, Niwa R, Inada S, Yamamoto M, Mitsui K, Horiuchi T, Shibata N, Kamiya K, Kodama I (2003) Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation 107:1937–1943. doi:10.1161/01.CIR.0000062645.38670.BD
Huang DT, Traub D (2008) Recurrent ventricular arrhythmia storms in the age of implantable cardioverter defibrillator therapy: a comprehensive review. Prog Cardiovasc Dis 51:229–236. doi:10.1016/j.pcad.2008.07.003
Hwang GS, Hayashi H, Tang L, Ogawa M, Hernandez H, Tan AY, Li H, Karagueuzian HS, Weiss JN, Lin SF, Chen PS (2006) Intracellular calcium and vulnerability to fibrillation and defibrillation in Langendorff-perfused rabbit ventricles. Circulation 114:2595–2603. doi:10.1161/CIRCULATIONAHA.106.630509
Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, Yin H, Knollmann BC (2011) Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 4:128–135. doi:10.1161/CIRCEP.110.959916
Janse MJ, Wit AL (1989) Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev 69:1049–1169
Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK (2012) Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 483:96–99. doi:10.1038/nature10852
Johnson DM, Heijman J, Bode EF, Greensmith DJ, van der Linde H, Abi-Gerges N, Eisner D, Trafford AW, Volders PG (2013) Diastolic spontaneous calcium release from the sarcoplasmic reticulum increases beat-to-beat variability of repolarization in canine ventricular myocytes after β-adrenergic stimulation. Circ Res 112:246–256. doi:10.1161/CIRCRESAHA.112.275735
Johnson DM, Heijman J, Pollard CE, Valentin JP, Crijns HJ, Abi-Gerges N, Volders PGA (2010) IKs restricts excessive beat-to-beat variability of repolarization during beta-adrenergic receptor stimulation. J Mol Cell Cardiol 48:122–130. doi:10.1016/j.yjmcc.2009.08.033
Kang G, Giovannone SF, Liu N, Liu FY, Zhang J, Priori SG, Fishman GI (2010) Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res 107:512–519. doi:10.1161/CIRCRESAHA.110.221481
Khoo MS, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, Grueter CE, Guan X, Oddis CV, Zhang R, Mendes L, Ni G, Madu EC, Yang J, Bass M, Gomez RJ, Wadzinski BE, Olson EN, Colbran RJ, Anderson ME (2006) Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation 114:1352–1359. doi:10.1161/CIRCULATIONAHA.106.644583
Kinoshita H, Kuwahara K, Takano M, Arai Y, Kuwabara Y, Yasuno S, Nakagawa Y, Nakanishi M, Harada M, Fujiwara M, Murakami M, Ueshima K, Nakao K (2009) T-type Ca2+ channel blockade prevents sudden death in mice with heart failure. Circulation 120:743–752. doi:10.1161/CIRCULATIONAHA.109.857011
Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N (2005) Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem 280:6580–6587. doi:10.1074/jbc.M408375200
Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, Xu X, Uchinoumi H, Okuda S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M (2009) Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol 53:1993–2005. doi:10.1016/j.jacc.2009.01.065
Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Yamamoto T, Matsuzaki M (2010) Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2R2474S/+ knock-in mouse model. Circ J 74:2579–2584. doi:10.1253/circj.CJ-10-0680
Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, Leymaster ND, Dun W, Wright PJ, Cardona N, Qian L, Mitchell CC, Boyden PA, Binkley PF, Li CL, Anderson ME, Mohler PJ, Hund TJ (2012) Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation 126:2084–2094. doi:10.1161/CIRCULATIONAHA.112.105320
Lakatta EG, DiFrancesco D (2009) What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47:157–170. doi:10.1016/j.yjmcc.2009.03.022
Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR (2008) Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118:2230–2245. doi:10.1172/JCI35346
Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH (2012) Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res 110:465–470. doi:10.1161/CIRCRESAHA.111.253229
Liu N, Ruan Y, Denegri M, Bachetti T, Li Y, Colombi B, Napolitano C, Coetzee WA, Priori SG (2011) Calmodulin kinase II inhibition prevents arrhythmias in RyR2 (R4496C+/−) mice with catecholaminergic polymorphic ventricular tachycardia. J Mol Cell Cardiol 50:214–222. doi:10.1016/j.yjmcc.2010.10.001
Lunati M, Gasparini M, Bocchiardo M, Curnis A, Landolina M, Carboni A, Luzzi G, Zanotto G, Ravazzi P, Magenta G, Denaro A, Distefano P, Grammatico A, InSync ICDIRI (2006) Clustering of ventricular tachyarrhythmias in heart failure patients implanted with a biventricular cardioverter defibrillator. J Cardiovasc Electrophysiol 17:1299–1306. doi:10.1111/j.1540-8167.2006.00618.x
Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, Hajjar RJ, Peters NS, Poole-Wilson PA, Macleod KT, Harding SE (2011) SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol 4:362–372. doi:10.1161/CIRCEP.110.961615
Marquez MF, Bonny A, Hernandez-Castillo E, De Sisti A, Gomez-Flores J, Nava S, Hidden-Lucet F, Iturralde P, Cardenas M, Tonet J (2012) Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: a case series and literature review. Heart Rhythm 9:1995–2000. doi:10.1016/j.hrthm.2012.08.027
Maruyama M, Joung B, Tang L, Shinohara T, On YK, Han S, Choi EK, Kim DH, Shen MJ, Weiss JN, Lin SF, Chen PS (2010) Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of purkinje fibers and triggered activity. Circ Res 106:399–408. doi:10.1161/CIRCRESAHA.109.211292
Maury P, Hocini M, Haissaguerre M (2005) Electrical storms in Brugada syndrome: review of pharmacologic and ablative therapeutic options. Indian Pacing Electrophysiol J 5:25–34
Mazur A, Roden DM, Anderson ME (1999) Systemic administration of calmodulin antagonist W-7 or protein kinase A inhibitor H-8 prevents torsade de pointes in rabbits. Circulation 100:2437–2442. doi:10.1161/01.cir.100.24.2437
Miake J, Marban E, Nuss HB (2002) Biological pacemaker created by gene transfer. Nature 419:132–133. doi:10.1038/419132b
Michael G, Xiao L, Qi XY, Dobrev D, Nattel S (2009) Remodelling of cardiac repolarization: how homeostatic responses can lead to arrhythmogenesis. Cardiovasc Res 81:491–499. doi:10.1093/cvr/cvn266
Miragoli M, Salvarani N, Rohr S (2007) Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 101:755–758. doi:10.1161/CIRCRESAHA.107.160549
Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S (1996) Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol 27:1061–1070. doi:10.1016/0735-1097(95)00613-3
Mizusawa Y, Wilde AA (2012) Brugada syndrome. Circ Arrhythm Electrophysiol 5:606–616. doi:10.1161/CIRCEP.111.964577
Morita N, Lee JH, Xie Y, Sovari A, Qu Z, Weiss JN, Karagueuzian HS (2011) Suppression of re-entrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol 57:366–375. doi:10.1016/j.jacc.2010.07.045
Moss AJ (2012) Sex hormones and ventricular tachyarrhythmias in LQTS: new insights regarding antiarrhythmic therapy. Heart Rhythm 9:833–834. doi:10.1016/j.hrthm.2012.01.014
Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH (1987) Circadian variation in the frequency of sudden cardiac death. Circulation 75:131–138. doi:10.1161/01.cir.75.1.131
Nam GB, Kim YH, Antzelevitch C (2008) Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med 358:2078–2079. doi:10.1056/NEJMc0708182
Nam GB, Ko KH, Kim J, Park KM, Rhee KS, Choi KJ, Kim YH, Antzelevitch C (2010) Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs Brugada syndrome. Eur Heart J 31:330–339. doi:10.1093/eurheartj/ehp423
Nattel S, Maguy A, Le Bouter S, Yeh YH (2007) Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87:425–456. doi:10.1152/physrev.00014.2006
Nerbonne JM, Kass RS (2005) Molecular physiology of cardiac repolarization. Physiol Rev 85:1205–1253. doi:10.1152/physrev.00002.2005
Nogami A (2011) Purkinje-related arrhythmias part ii: polymorphic ventricular tachycardia and ventricular fibrillation. Pacing Clin Electrophysiol 34:1034–1049. doi:10.1111/j.1540-8159.2011.03145.x
Nordbeck P, Seidl B, Fey B, Bauer WR, Ritter O (2010) Effect of cardiac resynchronization therapy on the incidence of electrical storm. Int J Cardiol 143:330–336. doi:10.1016/j.ijcard.2009.03.055
Odening KE, Choi BR, Liu GX, Hartmann K, Ziv O, Chaves L, Schofield L, Centracchio J, Zehender M, Peng X, Brunner M, Koren G (2012) Estradiol promotes sudden cardiac death in transgenic long QT type 2 rabbits while progesterone is protective. Heart Rhythm 9:823–832. doi:10.1016/j.hrthm.2012.01.009
Ogawa M, Morita N, Tang L, Karagueuzian HS, Weiss JN, Lin SF, Chen PS (2009) Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm 6:784–792. doi:10.1016/j.hrthm.2009.02.017
Ohgo T, Okamura H, Noda T, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S, Ohe T, Shimizu W (2007) Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm 4:695–700. doi:10.1016/j.hrthm.2007.02.014
Okuda S, Yano M, Doi M, Oda T, Tokuhisa T, Kohno M, Kobayashi S, Yamamoto T, Ohkusa T, Matsuzaki M (2004) Valsartan restores sarcoplasmic reticulum function with no appreciable effect on resting cardiac function in pacing-induced heart failure. Circulation 109:911–919. doi:10.1161/01.CIR.0000115526.92541.D2
Parikh A, Mantravadi R, Kozhevnikov D, Roche MA, Ye Y, Owen LJ, Puglisi JL, Abramson JJ, Salama G (2012) Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm 9:953–960. doi:10.1016/j.hrthm.2012.01.010
Pogwizd SM (1994) Focal mechanisms underlying ventricular tachycardia during prolonged ischemic cardiomyopathy. Circulation 90:1441–1458. doi:10.1161/01.cir.90.3.1441
Pogwizd SM (1995) Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation 92:1034–1048. doi:10.1161/01.cir.92.4.1034
Priori SG, Chen SR (2011) Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 108:871–883. doi:10.1161/CIRCRESAHA.110.226845
Qi X, Yeh YH, Chartier D, Xiao L, Tsuji Y, Brundel BJ, Kodama I, Nattel S (2009) The calcium/calmodulin/kinase system and arrhythmogenic afterdepolarizations in bradycardia-related acquired long-QT syndrome. Circ Arrhythm Electrophysiol 2:295–304. doi:10.1161/CIRCEP.108.815654
Reiken S, Gaburjakova M, Gaburjakova J, He Kl KL, Prieto A, Becker E, Yi Gh GH, Wang J, Burkhoff D, Marks AR (2001) beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104:2843–2848. doi:10.1161/hc4701.099578
Rokita AG, Anderson ME (2012) New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII). Circulation 126:2125–2139. doi:10.1161/CIRCULATIONAHA.112.124990
Rosen MR (1988) Mechanisms for arrhythmias. Am J Cardiol 61:2A–8A
Rubart M, Zipes DP (2005) Mechanisms of sudden cardiac death. J Clin Invest 115:2305–2315. doi:10.1172/JCI26381
Schwartz PJ, Crotti L, Insolia R (2012) Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol 5:868–877. doi:10.1161/CIRCEP.111.962019
Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, Zareba W, Robinson JL, Hall WJ, Brink PA, Toivonen L, Epstein AE, Li C, Hu D (2004) Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 109:1826–1833. doi:10.1161/01.CIR.0000125523.14403.1E
Schwartz PJ, Spazzolini C, Priori SG, Crotti L, Vicentini A, Landolina M, Gasparini M, Wilde AA, Knops RE, Denjoy I, Toivonen L, Monnig G, Al-Fayyadh M, Jordaens L, Borggrefe M, Holmgren C, Brugada P, De Roy L, Hohnloser SH, Brink PA (2010) Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation 122:1272–1282. doi:10.1161/CIRCULATIONAHA.110.950147
Schwartz PJ, Vanoli E, Crotti L, Spazzolini C, Ferrandi C, Goosen A, Hedley P, Heradien M, Bacchini S, Turco A, La Rovere MT, Bartoli A, George AL Jr, Brink PA (2008) Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol 51:920–929. doi:10.1016/j.jacc.2007.09.069
Sesselberg HW, Moss AJ, McNitt S, Zareba W, Daubert JP, Andrews ML, Hall WJ, McClinitic B, Huang DT, Group M-IR (2007) Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm 4:1395–1402. doi:10.1016/j.hrthm.2007.07.013
Shimizu W, Noda T, Satomi K, Suyama K, Kurita T, Aihara N, Horie M, Priori SG, Kamakura S (2005) Clinical characteristics and acute therapy for electrical storm of torsade de pointes in genotyped patients with congenital long QT syndrome. Circulation 112:II-493[abstract]
Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittkopper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS (2010) Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res 107:1150–1161. doi:10.1161/CIRCRESAHA.110.220418
Streitner F, Kuschyk J, Veltmann C, Mahl E, Dietrich C, Schimpf R, Doesch C, Streitner I, Wolpert C, Borggrefe M (2011) Predictors of electrical storm recurrences in patients with implantable cardioverter-defibrillators. Europace 13:668–674. doi:10.1093/europace/euq428
Swaminathan PD, Purohit A, Hund TJ, Anderson ME (2012) Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res 110:1661–1677. doi:10.1161/CIRCRESAHA.111.243956
Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME (2011) Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest 121:3277–3288. doi:10.1172/JCI57833
Tang L, Joung B, Ogawa M, Chen PS, Lin SF (2012) Intracellular calcium dynamics, shortened action potential duration, and late-phase 3 early afterdepolarization in Langendorff-perfused rabbit ventricles. J Cardiovasc Electrophysiol 23:1364–1371. doi:10.1111/j.1540-8167.2012.02400.x
Thiel WH, Chen B, Hund TJ, Koval OM, Purohit A, Song LS, Mohler PJ, Anderson ME (2008) Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation 118:2225–2234. doi:10.1161/CIRCULATIONAHA.108.788067
Tomas M, Napolitano C, De Giuli L, Bloise R, Subirana I, Malovini A, Bellazzi R, Arking DE, Marban E, Chakravarti A, Spooner PM, Priori SG (2010) Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol 55:2745–2752. doi:10.1016/j.jacc.2009.12.065
Tomaselli GF, Zipes DP (2004) What causes sudden death in heart failure? Circ Res 95:754–763. doi:10.1161/01.RES.0000145047.14691.db
Tsuji Y (2011) Electrical storm and calcium signaling: a review. J Electrocardiol 44:725–729. doi:10.1016/j.jelectrocard.2011.07.022
Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, Dobrev D, Nattel S, Kodama I, Kamiya K (2011) Ca2+-related signaling and protein phosphorylation abnormalities play central roles in a new experimental model of electrical storm. Circulation 123:2192–2203. doi:10.1161/CIRCULATIONAHA.110.016683
Tsuji Y, Opthof T, Yasui K, Inden Y, Takemura H, Niwa N, Lu Z, Lee JK, Honjo H, Kamiya K, Kodama I (2002) Ionic mechanisms of acquired QT prolongation and torsades de pointes in rabbits with chronic complete atrioventricular block. Circulation 106:2012–2018. doi:10.1161/01.cir.0000031160.86313.24
van der Werf C, Zwinderman AH, Wilde AA (2012) Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace 14:175–183. doi:10.1093/europace/eur277
van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH (2010) Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation 122:2669–2679. doi:10.1161/CIRCULATIONAHA.110.982298
Verma A, Kilicaslan F, Marrouche NF, Minor S, Khan M, Wazni O, Burkhardt JD, Belden WA, Cummings JE, Abdul-Karim A, Saliba W, Schweikert RA, Tchou PJ, Martin DO, Natale A (2004) Prevalence, predictors, and mortality significance of the causative arrhythmia in patients with electrical storm. J Cardiovasc Electrophysiol 15:1265–1270. doi:10.1046/j.1540-8167.2004.04352.x
Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D (2012) Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125:2059–2070. doi:10.1161/CIRCULATIONAHA.111.067306
Volders PGA, Kulcsar A, Vos MA, Sipido KR, Wellens HJJ, Lazzara R, Szabo B (1997) Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc Res 34:348–359. doi:10.1016/s0008-6363(96)00270-2
Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS (2011) Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res 108:555–565. doi:10.1161/CIRCRESAHA.110.221911
Watanabe H, Nogami A, Ohkubo K, Kawata H, Hayashi Y, Ishikawa T, Makiyama T, Nagao S, Yagihara N, Takehara N, Kawamura Y, Sato A, Okamura K, Hosaka Y, Sato M, Fukae S, Chinushi M, Oda H, Okabe M, Kimura A, Maemura K, Watanabe I, Kamakura S, Horie M, Aizawa Y, Shimizu W, Makita N (2012) Clinical characteristics and risk of arrhythmia recurrences in patients with idiopathic ventricular fibrillation associated with early repolarization. Int J Cardiol 159:238–240. doi:10.1016/j.ijcard.2012.05.091
Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR (2004) Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304:292–296. doi:10.1126/science.1094301
Wilde AA, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, Antzelevitch C (2010) The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol 49:543–553. doi:10.1016/j.yjmcc.2010.07.012
Wittkopper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsold B, Kirchhof P, Maier LS, Hasenfuss G, Dobrev D, Eschenhagen T, El-Armouche A (2010) Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest 120:617–626. doi:10.1172/JCI40545
Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME (2002) Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation 106:1288–1293. doi:10.1161/01.cir.0000027583.73268.e7
Xie LH, Chen F, Karagueuzian HS, Weiss JN (2009) Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104:79–86. doi:10.1161/CIRCRESAHA.108.183475
Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN (2010) So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J 99:1408–1415. doi:10.1016/j.bpj.2010.06.042
Yang Y, Mou Y, Hu SJ, Fu M (2009) Beneficial effect of rosuvastatin on cardiac dysfunction is associated with alterations in calcium-regulatory proteins. Eur J Heart Fail 11:6–13. doi:10.1093/eurjhf/hfn002
Zaugg CE, Wu ST, Barbosa V, Buser PT, Wikman-Coffelt J, Parmley WW, Lee RJ (1998) Ventricular fibrillation-induced intracellular Ca2+ overload causes failed electrical defibrillation and post-shock reinitiation of fibrillation. J Mol Cell Cardiol 30:2183–2192. doi:10.1006/jmcc.1998.0777
Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH (2010) Phospholamban ablation rescues sarcoplasmic reticulum Ca2+ handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ Res 106:354–362. doi:10.1161/CIRCRESAHA.109.207423
Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J Jr, Bers DM, Brown JH (2003) The δC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 92:912–919. doi:10.1161/01.RES.0000069686.31472.C5
Zhao Z, Fefelova N, Shanmugam M, Bishara P, Babu GJ, Xie LH (2011) Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol 50:128–136. doi:10.1016/j.yjmcc.2010.11.001
Zhao Z, Wen H, Fefelova N, Allen C, Baba A, Matsuda T, Xie LH (2012) Revisiting the ionic mechanisms of early afterdepolarizations in cardiomyocytes: predominant by Ca waves or Ca currents? Am J Physiol Heart Circ Physiol 302:H1636–H1644. doi:10.1152/ajpheart.00742.2011
Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR (2011) Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med 17:1003–1009. doi:10.1038/nm.2406
Zipes DP (2003) Mechanisms of clinical arrhythmias. J Cardiovasc Electrophysiol 14:902–912. doi:10.1046/j.1540-8167.2003.03228.x
Acknowledgments
The authors’ work is supported by the European Network for Translational Research in Atrial Fibrillation (EUTRAF), the German Federal Ministry of Education and Research (AF Competence Network and DZHK [German Center for Cardiovascular Research]), the Deutsche Forschungsgemeinschaft (Do 769/1-3), the Canadian Institutes of Health Research (MOP 68929), the Quebec Heart and Stroke Foundation, Fondation Leducq (European-North American Atrial Fibrillation Research Alliance, 07CVD03), Mochida Memorial Foundation for Medical and Pharmaceutical Research, Mitsubishi Pharma Research Foundation, Suzuken Memorial Foundation and Medtronic Japan, APEX and CTM Co., Ltd (Nagoya, Japan).
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Tsuji and J. Heijman contributed equally and share first authorship.
Rights and permissions
About this article
Cite this article
Tsuji, Y., Heijman, J., Nattel, S. et al. Electrical storm: recent pathophysiological insights and therapeutic consequences. Basic Res Cardiol 108, 336 (2013). https://doi.org/10.1007/s00395-013-0336-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0336-2