Abstract
Bone marrow (BM) derived stem and progenitor cells contribute to cardiovascular homeostasis and are affected by cardiovascular risk factors. We devised a clinical data-driven approach to test candidate stem cell mobilizing mechanisms in pre-clinical models. We found that PB and BM CD34+ cell counts were directly correlated, and that most circulating CD34+ cells were viable, non-proliferating and derived from the BM. Thus, we analyzed PB and BM CD34+ cell levels as a two-compartment model in 72 patients with or without cardiovascular disease. Self-organizing maps showed that disturbed compartmentalization of CD34+ cells was associated with aging and cardiovascular risk factors especially diabetes. High activity of DPP-4, a regulator of the mobilizing chemokine SDF-1α, was associated with altered stem cell compartmentalization. For validation of these findings, we assessed the role of DPP-4 in the BM mobilization response of diabetic rats. Diabetes differentially affected DPP-4 activity in PB and BM and impaired stem/progenitor cell mobilization after ischemia or G-CSF administration. DPP-4 activity in the BM was required for the mobilizing effect of G-CSF, while in PB it blunted ischemia-induced mobilization. Indeed, DPP-4 deficiency restored ischemia (but not G-CSF)-induced stem cell mobilization and improved vascular recovery in diabetic animals. In conclusion, the analysis of stem cell compartmentalization in humans led us to discover mechanisms of BM unresponsiveness in diabetes determined by tissue-specific DPP-4 dysregulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone marrow (BM) derived stem and progenitor cells contribute to cardiovascular homeostasis through several mechanisms including differentiation into vascular phenotypes and paracrine signals [3, 22, 23, 30, 31]. The cross-talks between cardiovascular disease (CVD) and the BM system are manifold. Signals from ischemic tissues activate BM metabolism and function [4] and inform the BM for the need of vasculo-regenerative cells through soluble mediators (e.g., VEGF and SDF-1α) produced locally [28]. These cross-talks are disturbed in the presence of cardiovascular risk factors [18], which are associated with reduced and dysfunctional BM-derived circulating progenitor cells, a mechanism that contribute to development and progression of CVD [13]. This is particularly evident in diabetes, as progenitor cell alterations have been described extensively and consistently in animals and humans [12, 19]. BM defects in diabetes are being elucidated and include microangiopathy, neuropathy, altered gene expression and niche dysfunction, which hamper stem cell mobilization [7, 10, 21, 24, 25]. Yet, data on BM function in humans with diabetes or CVD are scant and difficult to collect due to the intrinsic low availability of BM samples.
In this study, we aimed to gather information on the clinical phenotype associated with an altered compartmentalization of stem/progenitor cells in the BM and peripheral blood (PB), which would be indicative of the mobilization capacity. Starting from these phenotypes, we devised a clinical data-driven approach to test the association of candidate regulators of angiogenesis with progenitor cell mobilization, to be validated in animal models.
Materials and methods
An expanded version of the method section can be found in the online supplemental data file.
Patients
Human studies were approved by the ethical committee of the Goethe University of Frankfurt and by the University Hospital of Padova and conducted in accordance to the Declaration of Helsinki, as revised in 2000. All subjects provided informed consent. Patients were recruited at the University Hospital of Frankfurt am Main. All patients undergoing BM aspiration for myocardial cell therapy in the trials NCT00962364 and NCT00284713 were eligible—pending they did not have acute myocardial infarction. Control subjects underwent BM and PB sampling, but did not receive cell therapy. We collected demographic, anthropometric data, cardiovascular risk factors, prevalence of CVD and medications.
Measurement of regulators of angiogenesis and DPP-4 activity
The plasma concentration of angiogenesis-regulating mediators was determined using multiplex suspension arrays (Bio-plex, Biorad). DPP-4 activity was measured with the DPP-4 drug discovery Kit (Enzo Life Sciences, Farmingdale, NY, USA).
Flow cytometry
Human circulating progenitor cells were quantified using flow cytometry by staining for CD34, CD133 and KDR. The proliferation rate of circulating CD34+ cells was determined by staining fixed and permeabilized cells with propidium iodide (PI) for DNA content estimation. The apoptotic rate of circulating CD34+ cells was analyzed by the Annexin-V binding assay and PI staining. The levels of rat stem and progenitor cells were quantified by flow cytometry by staining for Sca-1 and c-kit (hematopoietic stem/progenitor cells) or Sca-1 and CD31 (EPCs).
Animal experiments
The animal protocol was approved by the ethic committee for animal experiments of the University of Padova and from the Italian Ministry of health. All experiments were conducted according to the “Principles of laboratory animal care” (NIH publication no. 85–23, revised 1985. We used 8-week-old F344 wild type and DPP-4 deficient rats. Diabetes was induced by intraperitoneal injection of streptozotocin 20 mg/kg. Experiments were performed 5–7 weeks after induction of diabetes. Hind limb ischemia was induced by femoral artery and vein excision. Blood samples were obtained before and on days 3 and 7 after ischemia. On day 7, rats were euthanized and for collection of muscles for histologic analysis of capillary density.
For direct bone marrow stimulation, rats were treated with human recombinant G-CSF at the dose of 60 μg/kg/day subcutaneously for four consecutive days.
Statistical analysis
Data are expressed as mean ± standard error or as percentage. Comparison between two or more groups was performed using Student’s t test or ANOVA (for normal variables) and with Mann–Whitney’s U test and Kruskal–Wallis test (for non-normal variables). Linear correlations were analyzed using the Pearson’s r (for normal variables) or the Spearman’s rho (for non-normal data) correlation coefficients.
Groups of patients were created according to their progenitor cell levels as below (termed “low”) or above (termed “high”) the median value in the peripheral blood and in the bone marrow. To render the separation of patients’ groups more extreme, the 50° percentile cutoff was substituted with the 60° percentile cutoff as lower limit of the “high” groups and with the 40° percentile as the higher limit of the “low” groups. The resulting four groups were compared using ANOVA or Kruskal–Wallis test and then with appropriate test comparing couples of groups.
The distribution of clinical characteristics in the four groups was also analyzed using self-organizing-maps (SOM) and statistically verified visualizations in the whole cohort and in patients belonging to the above mentioned groups defined by the median values or by the 40°/60° percentile. The SOM is an unsupervised pattern recognition method used for automated comparisons in a multivariate profile of clinical characteristics. In the map layout, patients with similar clinical characteristics are as close to each other, whereas those who have different characteristics are placed far apart on the map. After computing patients’ positions, the map is colored according to the patients’ characteristics. Statistical significance was accepted at p < 0.05 and SPSS ver. 16.0 was used.
Results
Direct correlations between peripheral blood and bone marrow stem/progenitor cell levels
We quantified the levels of six stem/progenitor cell phenotypes in the PB and BM of 72 subjects, including 12 healthy controls and 60 patients with heart disease (online Table 1S). For each cell phenotype, there was a significant direct linear correlation between PB and BM levels, which was stronger for CD34+ cells (r = 0.43, Fig. 1a), CD133+CD34+ cells (r = 0.44) and CD133+KDR+ cells (r = 0.50) (Table 2S). These direct correlations suggest that PB stem cell levels are informative of the BM status. The coefficient of variation was lower for both PB and BM CD34+ cells, the distribution of which was normal and less affected by extreme data. Therefore, we focused on CD34+ cells for further analyses.
Most circulating CD34+ cells are viable, non-proliferating and bone marrow-derived. a Correlation between bone marrow and peripheral blood CD34+ cell counts. Dashed lines indicate the 95 % CI of the regression line. b Fluorescence in situ hybridization (FISH) analysis of circulating CD34+ cell origin. The upper panels show positive controls of male cells (hybridization efficiency). The lower panels show Y-chromosome signal in sorted circulating CD34+ cells from females transplanted with a male bone marrow (zoom from ×40 original magnification). The table summarizes the percentage of chimerism of three transplanted patients, after correcting for the Y-chromosome hybridization efficiency. c Circulating CD34+ cells were analyzed for DNA content: the CD34 versus PI plot shows very few CD34+ cells above the PI threshold for the G2-M phase. The proliferating rate of three replicates is shown. d Apoptosis of circulating CD34+ cells: the CD34 versus Annexin-V plot shows that very few CD34+ cells stained positive for the early apoptotic marker Annexin-V. The apoptotic rate of six replicates is shown
Most circulating CD34+ cells are viable, non-proliferating and originated from the bone marrow
As also circulating mature endothelial cells may express CD34 [5], we analyzed the origin of CD34+ cells isolated from three female patients who received a male BM transplantation years before, by detecting the Y-chromosome signal using FISH. After adjusting for hybridization efficiency (89 %) [2], we found that 92.5 ± 3.1 % CD34+ cells were Y+, indicative of a donor BM origin (Fig. 1b). Therefore, the contamination by non-BM-derived cells within the population of circulating CD34+ cells is <10 %. In samples of healthy blood donors, we also show that the apoptotic rate of circulating CD34+ cells is on average 4.0 ± 0.6 % and most are resting (>95 % in G0–G1 phase) (Fig. 1c). Therefore, variations in the number of PB CD34+ cells are mostly attributable to mobilization of BM CD34+ cells.
Distribution of PB and BM CD34+ cells
Despite the significant direct correlation between PB and BM CD34+ cell levels, data were largely dispersed around the regression line, suggesting that circulating CD34+ cell levels are associated with different mobilizing capacity. Thus, we divided patients according to their median values of PB and BM CD34+ cell levels. We defined as “healthy”, patients with high PB and high BM CD34+ cells, because most healthy subjects fell into this subgroup (8/12 of healthy subject, 67 %). By converse, patients with low PB and low BM CD34+ cells were defined as “exhausted”. Patients with high PB and low BM CD34+ cell count were defined as “good mobilizers”, while patients with low PB and high BM CD34+ cells were defined as “poor mobilizers”. When we analyzed the clinical characteristics of these four groups, using conventional statistics, several cardiovascular risk factors were different, but a few reached significance (not shown). Therefore, as cases with values close to the medians of PB and BM cell counts were poorly informative, we excluded those falling within ±1 decile of the median value, to render groups more “extreme”. In other words, the median was substituted with the 40° and 60° percentiles as lower and higher cutoff, respectively (Fig. 2). The 40°/60° rule was a compromise to exclude borderline values without excess reduction of sample size.
Stem cell compartmentalization and the cardiovascular risk profile. Patients were divided into four groups according to their circulating and bone marrow CD34+ cell counts. Cutoffs for definition of groups were based on the 40° and 60° percentile of the distribution. Relevant clinical characteristics of the patients in each group are shown. *p < 0.05 in post hoc test versus the exhausted group after ANOVA or Kruskal–Wallis, as appropriate
Stem cell compartmentalization and the cardiovascular risk profile
The four groups identified by the 40°/60° percentile of PB and BM CD34+ cell counts were first analyzed for demographics, cardiovascular risk factors and therapies using conventional statistics. We found that subjects in the “healthy” group were (not significantly) younger, had a low number of cardiovascular risk factors (p = 0.02), and a low prevalence of diabetes, obesity and hypertension compared with patients in the “exhausted” group (post-ANOVA tests). The other groups, in which the compartmentalization of CD34+ cells was altered, were associated with higher prevalence of cardiovascular risk factors, and the “exhausted” group showed the worst risk profile. Among therapies, use of beta-blockers and insulin were higher in the group of “good mobilizers”, consistent with the previous findings that these drugs can increase circulating progenitor cell levels [20, 27] (Fig. 2).
As stem cell compartmentalization is a bi-dimensional variable, we used SOM to analyze the association between clinical characteristics of the patients and their belonging group in 2D planes. This visual and statistical approach shows colocalization of several high-risk features with the group of “exhausted” patients (age, diabetes, hypertension and obesity) or poor mobilizers (age, hypertension and obesity). Overlapping maps that represent groups with those that represent clinical features allow to describe the phenotype of a typical patient of each group. In compliance with its higher statistical power, SOM show significant and almost identical final results in the whole study cohort (Fig. 3) and in patients of the 40°/60° percentile groups (online Fig. 1S). A multivariable analysis runs on SOM revealed that diabetes was associated with group distribution independently of age, obesity, hypertension and therapies. We also found that diabetes was a strong determinant of reduced PB CD34+ cells independently of BM CD34+ cells, suggesting that diabetes compromises CD34+ cell mobilization (online Table 3S). These data indicate that altered CD34+ cell compartmentalization is associated with diabetes and a high cardiovascular risk profile.
Self-organizing maps showing clinical phenotypes associated with stem cell compartmentalization. a Self-organizing maps coloring of clinical features in patients analyzed for stem cell compartmentalization. The color scale indicates deviation from the population mean compared to random fluctuations that can be expected by chance. Numbers on selected hexagons indicate the prevalence (from 0 to 1) or the mean of each variable for that region. The p values indicate the probability to observe equal color distribution from random fluctuations. b Topological localization of stem cell compartmentalization groups can be visually overlaid to maps in panel a to derive a representation of the clinical typical phenotype of each group. c Each histogram of this figure represents a multivariate clinical profile in which bars can be interpreted as regression coefficients. The topological organization is the same as in the maps of panels a and b. Thus, bar plots illustrate the averaged profile for patients that reside on a given hexagon. The legends aids interpretation of the histograms: for instance, the upper-right region contains a typical patient with advanced age, diabetes, hypertension and obesity, belonging to the “exhausted” group. d Typical phenotypes associated with stem cell compartmentalization groups are shown. Numbers in the hexagons represent sample size. The analysis was conducted in the whole cohort of 72 patients
Compartmentalization was then repeated for other progenitor cell phenotypes (online Table 4S). Altered PB versus BM CD133+ and CD34+CD133+ cell compartmentalization was associated with a high cardiovascular risk profile similar to what obtained with CD34+ cell compartmentalization. However, the discriminative capacity of CD34+ cells was higher, as there were more clinical features differing among groups. These data confirm that CD34+ cell compartmentalization offers the best correlate of cardiovascular risk.
Stem cell compartmentalization and regulators of angiogenesis
We quantified the plasma concentrations of a panel of angiogenesis-regulating factors (online Table 4S). When patients were divided according to CD34+ cell compartmentalization, plasma VEGF concentrations and DPP-4 activity were lower in the “healthy” group, while other factors were not significantly different. SOM showed that high activity of the enzyme DPP-4 colocalizes with the “exhausted” phenotype (Fig. 2S).
The “healthy” group according to CD133+ and CD34+CD133+ cell compartmentalization had higher concentrations of the pro-angiogenic factors Angiopoietin-2 and PDGF-BB. Compartmentalization of CD34+CD133+KDR+ cells revealed high SDF-1α concentrations in the “healthy” and “good mobilizer” groups, consistent with the notion that circulating SDF-1α mobilizes EPCs [8]. These data indicate that some mobilizing factors may explain progenitor cell compartmentalization in this cohort of patients. We also suggest that this clinical data-driven approach is a suitable platform to identify new strategies of progenitor cell mobilization.
We then aimed at validating one of these factors in diabetes, as a model of altered progenitor cell mobilization.
DPP-4 deficiency restores ischemia- but not G-CSF-induced mobilization in diabetes
DPP-4 has been shown to cleave pro-angiogenic factors including SDF-1α [32]. Consistently with this finding, in the whole cohort of subjects, we found a negative correlation between DPP-4 activity and plasma SDF-1α concentrations (r = −0.27; p = 0.017). Plasma DPP-4 activity was also directly correlated to the BM/PB ratio of CD34+ cells (r = 0.25, p = 0.028), supporting the hypothesis that DPP-4 regulates CD34+ cell mobilization. DPP-4 activity is increased in diabetic patients and its inhibition restores EPC levels in type 2 diabetes [15, 16]. Taking into account that stem cell compartmentalization was associated with both diabetes and DPP-4 activity in our study, we hypothesized that DPP-4 may be a suitable target to restore progenitor cell mobilization in diabetes. We first analyzed DPP-4 activity in rats and found increased activity in PB and decreased activity in BM of diabetic compared to non-diabetic rats (Fig. 4). Then, we tested the bone marrow progenitor cell mobilization in wild type and DPP-4 deficient rats after either G-CSF stimulation or hind limb ischemia.
A 5-day course of G-CSF administration increased circulating Sca-1+c-Kit+cells in non-diabetic wild type rats, while this effect was markedly blunted in streptozotocin diabetic animals. In addition, DPP-4 activity in the BM increased in non-diabetic but not in diabetic animals (Fig. 4). DPP-4 deficiency completely abolished Sca-1+c-Kit+and Sca-1+ CD31+ progenitor cell mobilization in diabetic and non-diabetic animals (Fig. 5). These results confirm previous findings in non-diabetic mice that DPP-4 is required for the mobilization effect of G-CSF [9] and indicate that the reduced DPP-4 activity in the BM contributes to unresponsiveness to G-CSF in diabetes.
DPP-4 deficiency does not restore the defective G-CSF response in diabetic rats. The levels of circulating Sca-1+c-kit+ and Sca-1+CD31+ progenitor cells were determined by FACS at baseline, and after 5-day administration of G-CSF. a The gating strategy used to enumerate Sca-1+c-kit+ and Sca-1+CD31+ cells. Data from a representative wild type (WT) non-diabetic animal are shown. b Effects of G-CSF on circulating Sca-1+c-kit + and Sca-1+CD31+ cells in WT and DPP4null diabetic and non-diabetic (control) rats. *p < 0.05 post- versus pre-G-CSF. Bars indicate SEM. Logarithmic scale
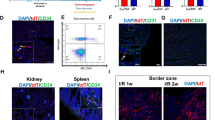
In wild type non-diabetic animals, hind limb ischemia induced a transient increase in Sca-1+c-Kit+ progenitor cells and Sca-1+CD31+ EPCs on day 3, which returned to baseline on day 7. This surge in progenitor cell levels after ischemia was completely abolished in wild type diabetic animals, which showed a paradoxical decrease in Sca-1+c-Kit+ and Sca-1+CD31+ cells on day 3 (Fig. 6a). In DPP-4 deficient rats, diabetes did not impair post-ischemic increase in Sca-1+c-Kit+ progenitor cells, which was similar to non-diabetic rats. Rather, the increase of Sca-1+CD31+ EPCs on day 3 in diabetic DPP-4 deficient rats was even higher than that of non-diabetic DPP-4 deficient rats. As a counterpart of progenitor cell mobilization, we analyzed microvascular density, blood flow, degree of ischemia and apoptosis in muscles on day 7. Recovery of capillary density was slightly impaired in wild type diabetic versus non-diabetic rats, while it was not in DPP4null diabetic versus non-diabetic rats (Fig. 6b). The degree of tissue ischemia, as assessed by staining with the hypoxic probe pimonidazole, was higher in diabetic compared to non-diabetic animals and was significantly lower in DPP4null rats (Fig. 6c). Therefore, DPP-4 deficiency abolished the detrimental effects of diabetes on development of ischemia and skeletal muscle microvascular recovery. We found no significant differences in superficial skin perfusion as assessed by laser Doppler imaging (online Fig. 3S), suggesting that differences in blood flow recovery among groups occur earlier than day 7. Finally, the number of apoptotic myofibers, assessed by the ApopTag kit (in situ TUNEL labeling), was very low in all samples (average < 2/field) and there was a non-significant trend toward reduced apoptosis in DPP4null rats (online Fig. 4S). Muscle DPP-4 activity was low (about 10 % of that in PB) and unaffected by diabetes or ischemia (not shown), ruling out that local DPP-4 targets are responsible for the effects observed in DPP4null rats.
DPP-4 deficiency restores post-ischemic progenitor cell mobilization in diabetic rats. The levels of circulating Sca-1+c-kit+ and Sca-1+CD31+ progenitor cells were determined at baseline, day 3 and day 7 after hind limb ischemia. a The gating strategy used to enumerate Sca-1+c-kit+ and Sca-1+CD31+ cells is shown on the left. Data from a representative wild type (WT) non-diabetic animal are shown. Trends over time of circulating Sca-1+c-kit+ and Sca-1+CD31+ cells in WT and DPP4null diabetic and non-diabetic (control) rats are shown on the right. *p < 0.05 diabetic versus controls. Bars indicate SEM. b Capillary density in ischemic muscles on day 7 in wild type and DPP4null diabetic and non-diabetic rats. Capillaries are stained in green with lectin (×40). c Degree of ischemia assessed by staining with the hypoxia marker pimonidazole. Pimonidazole adducts formed in tissue areas with pO2 < 10 mmHg were detected by immunohistochemistry *p < 0.05 diabetic versus non-diabetic
Discussion
This study, by combining a clinical data-driven discovery approach with animal models, shows that altered mobilization of stem/progenitor cells is associated with a high cardiovascular risk profile and, in the setting of diabetes, is driven by a tissue-specific DPP-4 dysregulation.
Progenitor cell mobilization, diabetes and cardiovascular risk
Investigation into the mechanisms of reduced progenitor cell levels has led to several hypothesis including excess apoptosis and senescence. In type 2 diabetes, we have previously shown that apoptosis is not responsible for the observed low level of circulating CD34+ cells [17]. Rather, animal models suggest that BM alterations account for depressed circulating progenitor cells in diabetes [7, 21, 24]. Unfortunately, the poor availability of BM samples limits our understanding of BM function in human diabetes. Herein, we analyzed BM and PB samples from patients undergoing cell therapy for heart disease and healthy volunteers. The direct correlation between BM and PB progenitor cell levels, although scattered, indicates that circulating progenitor cell levels are informative of the BM status. We also show that most circulating CD34+ cells are BM-derived, non-apoptotic and non-proliferating. As long as homing to the target tissues is not increased [1], these data indicate that variations in circulating CD34+ cells among patients are attributable to different capacity of BM mobilization. This allowed to categorize patients according to their BM and PB progenitor cell status into distinct mobilizer phenotypes. This original approach, although based on a snapshot of a dynamic system, led to the discovery that individuals with an altered distribution of CD34+ cells in the BM and PB display a strikingly high cardiovascular risk profile, characterized by aging, diabetes, obesity and hypertension. Using self-organizing maps, we visually and statistically analyzed cardiovascular risk factors in relation to the mobilizer phenotype: DM was strongly associated with exhaustion of PB and BM CD34+ cells independently of the distribution of other risk factors. While reduction of circulating progenitors is a consistent finding in animal and human DM, the effect of DM on the amount of BM progenitors is debated. Long-term diabetic mice appear to have reduced BM Sca-1+c-kit+ hematopoietic stem cells [24, 25], while short-term diabetic rats showed no reduction of BM Sca-1+c-kit+ [20] and short-term diabetic mice had normal [29] or even increased BM Sca-1+c-kit+ [21]. While methodological issues can explain these discrepancies, we suggest that the low CD34+ cell count in BM aspirates of DM patients indicates a reduced accessibility of stem cell niches to the aspiration manoeuver, in compliance with the observation that the diabetic BM niche is sticky and more prone to stem cell retention than mobilization [21].
Among other clinical features, it appears that use of beta-blockers and insulin was associated with a good mobilizer phenotype. Experimental studies support a role for beta-blockers [27] and insulin therapy [11, 20] in improving progenitor cell mobilization, further indicating that the clinical phenotype associated with stem cell compartmentalization is biologically plausible.
Thanks to their instructing spatial networks, SOM provide complementary and incremental information over classic statistical group comparison, also indicating that the “good mobilizer” and, to a lesser extent, the “poor mobilizer” are less well-defined phenotypes than the “healthy” and “exhausted”, as evidence from the uneven spatial distribution and a high p value. Importantly, SOM allowed description of relevant clinical associations in the whole study cohort and is independent of the method of patients’ partitioning (the 40°/60° percentile rule).
DPP-4 in the diabetic stem cell mobilopathy
We tested the plasma concentration of a selected range of regulators of angiogenesis to evaluate whether our stem cell compartmentalization analysis is a suitable benchmark for the discovery of stem cell mobilizing factors. Plausible associations were indeed found such as between the CD34+CD133+KDR+ EPCs “healthy” and “good mobilizer” phenotypes and high concentrations of the mobilizing chemokine SDF-1α. An in-depth analysis of the plasma proteome could allow the identification of new mobilizing factors.
In addition, we found low activity of DPP-4 in the CD34+ “healthy” phenotype and high activity in patients with disturbed stem cell compartmentalization and mobilization. As peripheral DPP-4 activity is increased in DM [14], we performed pre-clinical experiments to validate causality of this association in the defective stem cell mobilization observed in DM. We employed two assays of stem/progenitor cell mobilization because the molecular mechanisms involved are different and may be differently affected by DPP-4. First, we confirm that both stimuli fail to mobilize hematopoietic (Sca-1+c-Kit+) and endothelial (Sca-1+CD31+) progenitor cells in diabetic rats, lending further support to the diabetic bone marrow failure. There are important implications of these findings, as BM stem/progenitor cell mobilization after ischemia is a physiologic response, while responsiveness to pharmacologic stimulation could be used therapeutically [26]. The phenotypes used to define rat and human progenitor cells differ because there is no rat homology of human CD34 and CD133. Moreover, Sca-1+c-Kit+ cells contain hematopoietic progenitor cells in mice and rats, while co-expression of the endothelial antigen CD31 on Sca-1+ cells is compatible with a EPC phenotype [18]. In addition, previous studies have found that these rat progenitor cell phenotypes are responsive to mobilizing stimuli [6, 20]. Interestingly, DPP-4 deficiency restored ischemia-induced progenitor cell mobilization in diabetic rats, reduced the degree of ischemia and preserved from the detrimental effects of diabetes on microvascular recovery. These data suggest that DPP-4 deficiency improves the outcome of ischemic tissues, although the number of capillaries in genetically deficient mice might be influenced by the resulting phenotype and the link with progenitor cell mobilization is indirect.
We have previously demonstrated that impaired post-ischemic progenitor cell mobilization in diabetic rats is attributable to an altered activation of the hypoxia-sensing system HIF-1α leading to a blunted release of the chemokine SDF-1α, a circulating mobilizing stimulus [20]. As SDF-1α is a natural substrate of DPP-4 and is increased in diabetic patients after treatment with a DPP-4 inhibitor [16], excess DPP-4 activity is, therefore, one mechanism for reduced of SDF-1α in diabetes. Thus, we hypothesize that restored peripheral SDF-1α concentrations in DPP4null rats preserved the ability to mobilize BM progenitors. The measure of rat intact versus cleaved SDF-1α is challenging because traditional sandwich ELISA poorly discriminate between the two forms. However, proof-of-concept mass spectrometry analysis of mouse tissues confirmed that absence of DPP-4 reduces cleavage of native SDF-1α [32].
Differently from the post-ischemic assay, DPP-4 deficiency did not restore G-CSF induced HSC and EPC mobilization in diabetes, confirming data obtained in non-diabetic DPP4-/- mice [9]. The mobilizing effect of G-CSF relies on the establishment of a SDF-1α chemotactic gradient toward the vasculature, by reducing intra-marrow concentrations of SDF-1α, through DPP-4 induction and suppression of stromal cell activity. We now report that diabetes reduces BM DPP-4 activity, which was not stimulated by G-CSF, likely leading to high intra-marrow SDF-1α levels, a retention signal for stem cells. This is consistent with the previous observation of a failed SDF-1α switch in the BM in response to tissue injury in diabetes [29]. Indeed, while DPP-4 deficiency restored the peripheral signal from the ischemic tissue to the BM, response to G-CSF remained deficient. Altogether these data indicate that tissue-specific DPP-4 dysregulation accounts for both defective post-ischemic and G-CSF induced mobilization (Fig. 7).
The role of DPP-4 in diabetic stem cell mobilopathy. Bone marrow stem cell mobilization is governed by SDF-1α gradients across the osteoblast/mesenchymal compartment and the vasculature within the niche. In the non-diabetic condition, tissue ischemia upregulates circulating SDF-1α, which is partly cleaved by DPP-4. G-CSF activates bone marrow DPP-4 and reduces SDF-1α. As a result, both stimuli create a mobilizing gradient toward the vasculature. In diabetes, circulating DPP-4 activity in increased and ischemia-derived SDF-1α is reduced, thereby impairing post-ischemic mobilization. In addition, diabetes reduces DPP-4 activity in the bone marrow and prevents G-CSF from suppressing SDF-1α concentrations. Thus, tissue-specific DPP-4 maladaptation in diabetes is responsible for both G-CSF and ischemia-induced impaired mobilization. DPP-4 deficiency in diabetes protects circulating SDF-1α from degradation and restores post-ischemic mobilization, but not G-CSF induced mobilization, which requires stimulation of DPP-4
Conclusion
We have conducted a reverse translational study that stems from the observation of the clinical phenotype in patients with disturbed progenitor cell mobilization and leads to the discovery of a central role for DPP-4 in the diabetic stem cell mobilopathy.
In this clinical data-driven discovery approach for regenerative medicine, we used a targeted strategy to screen soluble factors potentially involved in progenitor cell regulation, but future development into the human plasma proteome analysis may allow an unbiased approach.
The tissue-specific regulation of DPP-4 in diabetes sheds light on the different mechanisms of ischemia- and GCSF-induced mobilization and has therapeutic implications based on clinically available DPP-4 inhibitors [15].
References
Albiero M, Menegazzo L, Boscaro E, Agostini C, Avogaro A, Fadini GP (2011) Defective recruitment, survival and proliferation of bone marrow-derived progenitor cells at sites of delayed diabetic wound healing in mice. Diabetologia 54:945–953. doi:10.1007/s00125-010-2007-2
Angelini A, Castellani C, Tona F, Gambino A, Caforio AP, Feltrin G, Della Barbera M, Valente M, Gerosa G, Thiene G (2007) Continuous engraftment and differentiation of male recipient Y-chromosome-positive cardiomyocytes in donor female human heart transplants. J Heart Lung Transplant 26:1110–1118. doi:10.1016/j.healun.2007.08.004
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967. doi:10.1126/science.275.5302.964
Assmus B, Iwasaki M, Schachinger V, Roexe T, Koyanagi M, Iekushi K, Xu Q, Tonn T, Seifried E, Liebner S, Kranert WT, Grunwald F, Dimmeler S, Zeiher AM (2011) Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur Heart J 33:1911–1919. doi:10.1093/eurheartj/ehr388
Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 6:835–845. doi:10.1038/nrc1971
Bolego C, Rossoni G, Fadini GP, Vegeto E, Pinna C, Albiero M, Boscaro E, Agostini C, Avogaro A, Gaion RM, Cignarella A (2010) Selective estrogen receptor-alpha agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J 24:2262–2272. doi:10.1096/fj.09-139220
Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Li Calzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB (2009) Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 206:2897–2906. doi:10.1084/jem.20090889
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864. doi:10.1038/nm1075
Christopherson KW, Cooper S, Hangoc G, Broxmeyer HE (2003) CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26-/- mice. Exp Hematol 31:1126–1134. doi:S0301472X0300256X
DiPersio JF (2011) Diabetic stem-cell “mobilopathy”. N Engl J Med 365:2536–2538. doi:10.1056/NEJMcibr1112347
Dong L, Kang L, Ding L, Chen Q, Bai J, Gu R, Li L, Xu B (2011) Insulin modulates ischemia-induced endothelial progenitor cell mobilization and neovascularization in diabetic mice. Microvasc Res 82:227–236. doi:10.1016/j.mvr.2011.09.006
Fadini GP (2008) An underlying principle for the study of circulating progenitor cells in diabetes and its complications. Diabetologia 51:1091–1094. doi:10.1007/s00125-008-1021-0
Fadini GP, Agostini C, Sartore S, Avogaro A (2007) Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis 194:46–54. doi:10.1016/j.atherosclerosis.2007.03.046
Fadini GP, Albiero M, Menegazzo L, de Kreutzenberg SV, Avogaro A (2011) The increased dipeptidyl peptidase-4 activity is not counteracted by optimized glucose control in type 2 diabetes, but is lower in metformin-treated patients. Diabetes Obes Metab 14:518–522. doi:10.1111/j.1463-1326.2011.01550.x
Fadini GP, Avogaro A (2011) Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol 55:10–16. doi:10.1016/j.vph.2011.05.001
Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A (2010) The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care 33:1607–1609. doi:10.2337/dc10-0187
Fadini GP, Boscaro E, de Kreutzenberg S, Agostini C, Seeger F, Dimmeler S, Zeiher A, Tiengo A, Avogaro A (2010) Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 33:1097–1102. doi:10.2337/dc09-1999
Fadini GP, Losordo D, Dimmeler S (2012) Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110:624–637. doi:10.1161/CIRCRESAHA.111.243386
Fadini GP, Sartore S, Agostini C, Avogaro A (2007) Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 30:1305–1313. doi:10.2337/dc06-2305
Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A (2006) Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia–reperfusion injury in rats. Diabetologia 49:3075–3084. doi:10.1007/s00125-006-0401-6
Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, Mangoni M, Rizzoli V, Sykes SM, Lin CP, Frenette PS, Quaini F, Scadden DT (2011) Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med 3:104ra101. doi:10.1126/scitranslmed.3002191
Iekushi K, Seeger F, Assmus B, Zeiher AM, Dimmeler S (2012) Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. Circulation 125:1765–1773. doi:10.1161/CIRCULATIONAHA.111.079699
Ohtani K, Dimmeler S (2011) Control of cardiovascular differentiation by microRNAs. Basic Res Cardiol 106:5–11. doi:10.1007/s00395-010-0139-7
Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, Madeddu P (2010) Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol 30:498–508. doi:10.1161/ATVBAHA.109.200154
Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S (2010) Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol 105:703–712. doi:10.1007/s00395-010-0109-0
Sanganalmath SK, Abdel-Latif A, Bolli R, Xuan YT, Dawn B (2011) Hematopoietic cytokines for cardiac repair: mobilization of bone marrow cells and beyond. Basic Res Cardiol 106:709–733. doi:10.1007/s00395-011-0183-y
Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, Lobysheva I, Mohmand W, Akbar R, Bahlmann F, Besler C, Schaefer A, Hilfiker-Kleiner D, Luscher TF, Balligand JL, Drexler H, Landmesser U (2011) Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1-blockade. J Am Coll Cardiol 57:601–611. doi:10.1016/j.jacc.2010.09.037
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5:434–438. doi:10.1038/7434
Tepper OM, Carr J, Allen RJ Jr, Chang CC, Lin CD, Tanaka R, Gupta SM, Levine JP, Saadeh PB, Warren SM (2010) Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1 alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes 59:1974–1983. doi:10.2337/db09-0185
Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S (2005) Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 39:733–742. doi:10.1016/j.yjmcc.2005.07.003
Wu J, Li J, Zhang N, Zhang C (2011) Stem cell-based therapies in ischemic heart diseases: a focus on aspects of microcirculation and inflammation. Basic Res Cardiol 106:317–324. doi:10.1007/s00395-011-0168-x
Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM (2009) Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell 4:313–323. doi:10.1016/j.stem.2009.02.013
Acknowledgments
This work was supported by a European Foundation for the Study of Diabetes (EFSD)/Lilly Fellowship grant to GPF. There are no relationships with industry.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fadini, G.P., Albiero, M., Seeger, F. et al. Stem cell compartmentalization in diabetes and high cardiovascular risk reveals the role of DPP-4 in diabetic stem cell mobilopathy. Basic Res Cardiol 108, 313 (2013). https://doi.org/10.1007/s00395-012-0313-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0313-1