Abstract
Electrophysiological properties of implanted mesenchymal stem cells (MSCs) in infarcted hearts remain unclear, and their proarrhythmic effect is still controversial. The intent of this study was to investigate electrophysiological properties and proarrhythmic effects of MSCs in infarcted hearts. Rats were randomly divided into a myocardial infarction (MI) group, a MI-DMEM group (received DMEM medium injection) and MI-MSCs group (received MSCs injection). Survival analysis showed that the majority of engrafted MSCs died at day 9 after transplantation. Engrafted MSCs expressed cardiac markers (MYH, cTnI, Cx43), cardiac ion channel genes (Kv1.4, Kv4.2 and Kir2.1) and potassium currents (I to, I K1 and I KDR), but did not express Nav1.5, Cav1.2, Na+ current and Ca2+ current during their survival. When induced by Ca2+, implanted MSCs exhibited no contraction ability after being isolated from the heart. Following 8-week electrocardiography monitoring, the cumulative occurrence of ventricular arrhythmias (VAs) was not different among the three groups. However, the prolonged QRS duration in infarcted rats without VAs was significantly decreased in the MI-MSCs group compared with the other two groups. The inducibility of VAs in the MI-MSCs group was much lower than that in the MI and MI-DMEM groups (41.20 vs. 86.67 % and 92.86 %; P < 0.0125). The ventricular effective refractory period in MI-MSCs group was prolonged in comparison with that in the MI and MI-DMEM groups (56.0 ± 8.8 vs. 47.7 ± 8.8 ms and 45.7 ± 6.2 ms; P < 0.01). These results demonstrate that MSCs do not acquire the electrophysiological properties of mature cardiomyocytes during the survival period in the infarcted hearts. However, they can alleviate the electrical vulnerability and do not promote ventricular arrhythmias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is a major cause of morbidity and mortality worldwide, and post-MI heart failure has emerged as a global epidemic [57]. Many factors are involved in the process of cardiac injury and repair [26, 36–38]. Experimental studies and clinical trials have demonstrated that treatment with MSCs [14, 16, 20, 21, 35], cardiac stem cells [4, 17, 24, 31, 59], or other cytokines [43] can improve cardiac function after MI. However, the occurrence of ventricular arrhythmias in both experiments [6, 12] and clinical trials [44, 45] suggests the potential risks of proarrhythmias by MSCs or bone marrow cell (BMC) therapy.
The proarrhythmic effect of cell therapy was initially observed during clinical trials of skeletal myoblast therapy [15, 33]. Subsequently, arrhythmias were also reported in BMC therapy [5, 11, 48]. However, these studies are nonrandomized and the number of cases was limited. Although recent randomized placebo-controlled trials [2, 7, 16, 19, 27, 28, 58] indicate that there is no enhanced risk of clinical arrhythmias associated with MSC or BMC transplantation, these studies were not designed specifically to evaluate the arrhythmogenic effects of cell transplantation. In addition, the methods for evaluating arrhythmic risks in these studies are generally not comprehensive. Inducibility of arrhythmia by programmed ventricular stimulation was not determined in most of the previous studies [2, 7, 16, 19, 27, 28], while some studies never reported any rhythm monitoring during follow-up after transplantation. Two recent studies [3, 22] specifically evaluated arrhythmogenic effects of autologous bone marrow-derived progenitor cell transplantation and the results suggested a potential antiarrhythmic effect. However, due to the small case number and nonrandomization, antiarrhythmic potential of these cells is still inconclusive. Therefore, proarrhythmia or antiarrhythmia of BMC or MSC therapy is still controversial in recent clinical and animal studies [12, 29, 30, 34, 39, 53]. Further systematic evaluation of the arrhythmic potential of MSCs is necessary.
On the other hand, it is well known that transplanted cells must possess cardiomyocytes with electrophysiological properties in order to execute their electrical functions when engrafted into infarcted hearts. If MSCs do not differentiate into electrically functional cardiomyocytes, they might create a fixed heterogeneity among host tissues in the implanted region, and possibly predispose the heart to ventricular arrhythmia [47]. However, the electrophysiological properties of MSCs in infarcted hearts remain unknown.
In this regard, we conducted a randomized and controlled study to investigate the electrophysiological properties and proarrhythmic effects of MSCs in infarcted hearts. Our results indicate that MSCs can alleviate the electrical vulnerability and do not promote ventricular arrhythmias after transplantation into infarcted hearts, although they do not acquire the electrophysiological properties of mature cardiomyocytes.
Materials and methods
Animals
Adult Sprague–Dawley rats were obtained from the Experimental Animal Center of Xi’an Jiaotong University. All animals received humane care, which is in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publication NO. 85-23, revised 1996). All the experimental procedures were approved by the Care of Experimental Animals Committee of the First Affiliated Hospital of Xi’an Jiaotong University's School of Medicine.
Culturing and labeling of MSCs
MSCs were prepared as we reported previously [55]. Briefly, MSCs were isolated from the bone marrow of a male rat by density gradient separation of mononuclear cells, and seeded in a culture flask containing Dulbecco’s modified Eagle’s medium–low glucose (DMEM–LG) (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS) (Invitrogen). The cells were cultured at 37 °C in a humidified atmosphere containing 5 % CO2. Once 90 % confluency was achieved, cells were passaged by 0.25 % trypsin (Amresco, Solon, OH, USA). In order to observe the distribution of implanted MSCs in vivo, MSCs were labeled with GFP in vitro before transplantation. Briefly, MSCs were transfected with lentiviral vector expressing green fluorescent protein (GFP) at a multiplicity of infection (MOI) of 200 for 24 h. The cells were subsequently washed three times and incubated in fresh complete medium for 72 h. The transfection efficiency was evaluated by cytometry analysis.
In vitro proliferation assay
The MTT assay was performed at each time point in triplicate. A total of 1 × 103 cells were seeded in 96-well flat bottom plates and incubated at 37 °C for 24 h. At the indicated time points, 20 μL of 5 mg/mL MTT solution was added to each well. After 3 h of incubation, the medium was removed and 200 μL of DMSO was added to each well. The absorbance at 490 nm was measured using a microplate reader (Beckman Coulter, Brea, CA, USA).
Flow cytometric analysis
MSCs were trypsinized (with 0.25 % trypsin), washed twice with PBS, and incubated with rabbit against CD29, CD34, CD44, CD45, CD90 and CD105 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 20 min at room temperature. After being washed two times with PBS, cells were stained with goat anti-rabbit IgG-PerCP-Cy5.5 antibody (Santa Cruz Biotechnology, Inc.) at room temperature for 20 min. Cells in the control group were incubated with normal rabbit IgG (Santa Cruz Biotechnology, Inc.). After incubation, cells were washed with PBS two times. Quantitative analysis was performed using a flow cytometer (Beckman Coulter).
Trilineage differentiation
MSCs were grown in six-well plates until 50 % confluency was reached. Adipogenic, osteogenic and chondrogenic differentiation were induced as described previously [42]. After 3 weeks of differentiation, MSCs were fixed in 4 % paraformaldehyde for 25 min and stained with 1 % Alizarin Red S (Sigma) for 30 min to detect calcified extracellular matrix. Lipid accumulation and matrix deposition of sulfated glycosaminoglycans were detected by staining the MSCs with 0.3 % Oil Red O (Sigma) in 0.6 % isopropanol for 1 h, and 1 % Alcian Blue 8-GX (Sigma) overnight at 25 °C, respectively.
Induction of myocardial infarction
Myocardial infarction was developed as described previously [13]. Briefly, a total of 114 eight-week-old male rats (200–230 g) were prepared for surgical occlusion of the left anterior descending (LAD) coronary artery without ventilation. The rats were anesthetized by an intraperitoneal injection of 300 mg/kg of chloral hydrate. A skin cut (2.5 cm) was made over the left chest. After dissection of the pectoral major and minor muscles, a small hole was made at the fourth intercostal space with a mosquito clamp to open the pleural membrane and pericardium. With the clamp slightly open, the heart was smoothly and gently “popped out” through the hole. The LAD was ligated at a site 2 mm from its origin using a 6-0 silk suture. Successful ligation was confirmed when the anterior wall of the left ventricle turned pale. After ligation, the heart was immediately placed back into the intrathoracic space followed by manual evacuation of air and closure of muscle and the skin. The rat was then allowed to breathe air and monitored during the recovery period. During the whole operation, electrocardiography showed significant changes after LAD ligation (supplemental information Fig. 1).
Transplantation of MSCs
After 2 weeks of MI, 91 rats were randomly divided into three groups (MI, MI-DMEM and MI-MSCs). For transplantation in MI-MSCs group, a total of 6×106 GFP-labeled MSCs were suspended in 150 μL of DMEM and injected at five injection sites into anterior and lateral aspects of the viable myocardium bordering the infarcted region (with pale appearance, showed in supplemental information Fig. 2) using 1 mL sterile insulin syringe with a 30-gauge needle. In the MI-DMEM group, 150 μL of DMEM medium was injected into the myocardium at the same sites. In the MI group, the rats did not receive any treatment.
Left ventricular catheterization
Cardiac hemodynamics was assessed for six rats in each group before transplantation. For invasive hemodynamics, left ventricular catheterization was performed at 14 days after MI. A Millar Mikro-tip 2 F pressure transducer (Millar Instruments, Houston, TX, USA) was introduced into the left ventricle via the right carotid artery. Hemodynamic parameters were recorded using BL-420 biological and functional experimental system (Tai Meng, LinYin, Chengdu). All data were analyzed with BL-New Century software (Tai Meng).
Surface ECG
A surface six-lead ECG (lead II is shown in figures) was obtained from six rats in the three groups 1 day before injection and during the peri-operative period, followed by monitoring at 1, 3, 7, 14, 21, 28, 35, 42, 49 and 56 days after cell injection. The occurrence of ventricular arrhythmias, including premature ventricular contractions and ventricular tachycardias, was counted throughout the study.
Detection of fluorescence in vivo
Fluorescence was detected using IVIS-SPECTRUM (Xenogen, Hopkinton, MA, USA). An excitation filter of 490 nm and an emission filter of 520 nm were used to detect GFP with the exposure time of 1 s. During the measurement, six rats in each group were anesthetized by 2 % isoflurane in 100 % O2 at a flow rate of 2 L/min. The same rats were scanned for GFP at 3, 7 and 11 days after transplantation. Region-of-interest (ROI) measurements on the images were used to quantify the fluorescence in units of total photons per second per centimeter squared per steradian (p/s/cm2/sr).
Immunofluorescence staining
Frozen heart sections were prepared for three rats in each group. For immunofluorescence staining, cells were fixed for 20 min with 4 % paraformaldehyde and permeabilized for 30 min with 0.2 % Triton X-100. After being blocked with goat serum, cells were incubated with rabbit against Kv1.4, Kv4.2, Kir2.1, Nav1.5, Cav1.2 (Alomone Labs, Jerusalem, Israel), cTnI, MYH, α-actin, Cx43 (Santa Cruz Biotechnology, Inc.) antibodies at a dilution of 1:100 overnight at 4 °C. The cells were then washed three times with PBS and were incubated with Cy3-conjugated goat anti-rabbit IgG/rabbit anti-goat IgG (Santa Cruz Biotechnology, Inc.) at a dilution of 1:100 for 1 h at room temperature. The cells were visualized using fluorescence microscope.
Laser capture microdissection
Three sections from each rat were used for laser microdissection, which was performed as reported previously [1]. Briefly, fresh frozen heart sections were mounted on polyethylenenaphthalate (PEN) membrane slides (Leica Microsystems, Wetzlar, Germany), and immediately placed on ice. Single engrafted MSCs were cut and placed in 0.5 ml sterile PCR tubes using a Laser Microdissection (AS-LMD) System (Leica), and lysed in standard lysis solution.
Real-time quantitative PCR
Real-time quantitative PCR was performed as reported previously [9, 56]. Briefly, cells were collected by laser microdissection. Reverse transcription was performed by incubation at 25 °C for 10 min and 50 °C for 20 min. Real-time quantitative polymerase chain reaction (PCR) was performed on a Bio-Rad iQ5 (Bio-Rad, Hercules, CA, USA) with an initial cycle of 95 °C for 2 min, followed by 50 cycles of 95 °C for 15 s and 60 °C for 30 s. Primers were listed in supplemental information (Table 1). A relative quantification method 2−ΔΔCt (Ct, cycle threshold) was chosen for quantitative analysis. The PCR products were analyzed by electrophoresis on 2 % agarose gel.
Isolation of engrafted MSCs
Six rats in each group were anesthetized by an intraperitoneal injection of 300 mg/kg of chloral hydrate. The heart was excised and perfused in a retrograde Langendorff mode with a modified calcium-free Tyrode buffer. Subsequently, fresh buffer supplemented with 20 mg of collagenase II and 20 mg of bovine serum albumin (BSA) was recirculated for 12–15 min. MSCs in the transplanted region were gently dissociated in the Kraft-Brühe (KB) solution. All cells, including most cardiomyocytes, were filtered through a 200-mm mesh. Ca2+ concentration was increased to 1.0 mM before MSCs were prepared for electrophysiological recording by patch-clamp technology.
Measurement of electrophysiological parameters
After acute isolation from the infarcted heart, engrafted MSCs were prepared for electrophysiological recording by patch-clamp technology in vitro. The whole-cell patch-clamp technique was used as described previously [23]. Borosilicate glass electrodes were pulled with a P-97 puller (Sutter Instrument Company, Novato, CA, USA). The tip resistances were 2–5 MΩ when filled with pipette solution. The tip potentials were compensated before the pipette touched the cell. The GΩ-seal was obtained after a gentle negative suction. By gentle suction, cell membrane was ruptured to establish the whole-cell configuration. Experimental data were acquired with a software pCLAMP 10.2 (Axon Instruments, Sunnyvale, CA, USA). Tyrode’s solution contained 140 mM of NaCl, 5.4 mM of KCl, 1.8 mM of MgCl2, 1.8 mM of CaCl2, 10 mM of glucose and 10 mM of HEPES, and pH was adjusted to 7.35 with NaOH. The pipette solution contained 20 mM of KCl, 110 mM of K-aspartate, 10 mM of HEPES, 5.0 mM of Na2–phosphocreatine, 5.0 mM of Mg2–ATP, 1.0 mM of MgCl2 and 0.05 mM of EGTA, and pH was adjusted to 7.2 with KOH. K+ in pipette solutions and superfusion was replaced by equimolar Cs+ when sodium current or L-type Ca2+ current was recorded. All experiments were conducted at 22–23 °C.
In vivo ventricular arrhythmias study
A total of 46 rats were used for the induced ventricular arrhythmias study. Ventricular pacing was performed according to the previous studies [53]. Briefly, the chest of each rat was reopened for induction of ventricular arrhythmias (VAs) in the in situ heart after 8 weeks of MSCs implantation. A pair of electrodes was positioned 2 mm apart at the noninfarcted epicardial base of the left ventricular anterior wall. ECGs were recorded from three electrodes at both upper limbs and the left leg.
Programmed electrical stimulation (PES) was performed as follows: after a train of eight stimuli at 120 ms drive cycle length, single, double and triple extra stimuli were applied using a standard PES protocol. The coupling interval of the last extra stimulus was decreased in 2 ms steps beginning at 80 ms and finishing at the ventricular effective refractory period (VERP). Ventricular tachycardia (VT) was defined as equal to or more than three consecutive premature ventricular contractions (PVCs). Sustained VT was defined as a fast ventricular rhythm over 15 or more PVCs, according to Lambeth Conventions [52].
Statistical analysis
All the data were expressed as mean ± SD. The statistical analyses of two groups were conducted using Student’s t test. Comparison for more than two groups was conducted using one-way analysis of variance (ANOVA). A χ2 test was used to compare the data on cumulative occurrence and inducibility of VAs. Statistical analysis was performed using SPSS version 13.0. P < 0.05 was considered statistically significant.
Results
GFP-labeling does not affect the biological characteristics of MSCs
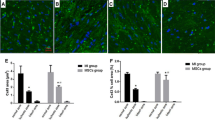
After three passages, unlabeled MSCs were distributed uniformly and presented typical fibroblast-like morphology (Fig. 1a). Six days after infection with lentiviral vectors containing GFP, almost all MSCs expressed GFP and typical morphology was well maintained (Fig. 1b). Flow cytometry analysis revealed that GFP was expressed in 99.38 % of MSCs (Fig. 1c). Importantly, the expression level of GFP was not decreased during the entire in vitro culturing period. MTT analysis showed that the proliferation rate of the GFP-labeled MSCs was similar to that of the unlabeled MSCs (Fig. 1d). Typical surface markers of MSCs including CD29, CD44, CD90 and CD105 were also expressed by the GFP-labeled MSCs, while CD34 and CD45 were negative (Fig. 1e). After induction, adipogenesis, osteogenesis and chondrogenesis occurred in GFP-labeled MSCs (Fig. 1f). These characteristics of GFP-labeled MSCs are similar to those of unlabeled MSCs.
The effect of labeling on biological characteristics of MSCs. a The morphology of the MSCs with three passages before labeling. b Fluorescent microscopic analysis of MSCs after 6 days of GFP labeling. c Cytometric analysis of GFP expression in labeled MSCs. d Proliferation of labeled MSCs as determined by MTT assay. e Expression of surface markers on GFP-labeled MSCs. f Trilineage differentiation of GFP-labeled MSCs. Scale bar 10 μm
Engrafted MSCs did not survive over long time periods in infarcted hearts
GFP-labeled MSCs were injected into the myocardium bordering the infarcted region 2 weeks after MI was developed. A large number of GFP-positive cells were observed in the tissue sections after 3 days of MSCs injection. The number of GFP-positive cells decreased sharply from day 3 to day 9 after engraftment. Almost all transplanted MSCs died after 9 days of engraftment (Fig. 2a). Quantitative analysis demonstrated that the in vivo fluorescence intensity declined significantly with time following transplantation (Fig. 2b). At day 3 after engraftment, the fluorescence intensity was 3.43 × 1010 ± 1.0 × 1010 P/sec/cm2/sr, which was decreased to 0.39 % (1.35 × 108 ± 3.6 × 107 P/sec/cm2/sr) at day 7 (Fig. 2c). At day 11, the specific fluorescence signals were not detected (Fig. 2b). Immunofluorescent staining showed that there were no CD4 or CD8 positive cells in the transplanted region (Fig. 2d). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining showed that apoptosis occurred in some transplanted MSCs (Fig. 2e). These results indicated that apoptosis, not immunorejection, may be one of the factors that are responsible for the death of engrafted MSCs.
Survival of MSCs in infarcted hearts. a Survival of MSCs after transplantation by tissue sections. b Survival of MSCs after transplantation by fluorescence detection in vivo. c Quantitative analysis of survived MSCs after transplantation. d Immunofluorescent staining of CD4 and CD8 in the tissues collected from MSCs-transplanted region. e Apoptosis of engrafted MSCs by TUNEL (arrowheads). Representative data are shown in each panel. Scale bar 100 μm
Engrafted MSCs did not differentiate into mature cardiomyocytes
To determine whether MSCs could differentiate into cardiomyocytes in infarcted myocardium, we investigated the protein and mRNA expressions of cardiac markers (cTnI, MYH, α-actin and Cx43) on engrafted MSCs after 3, 5 and 7 days of transplantation. Immunofluorescent staining showed that engrafted MSCs expressed MYH and Cx43, but did not express α-actin from day 3 to day 7 (Fig. 3a). Expression of cTnI in the engrafted MSCs was positive at day 5 and day 7, but was negative at day 3 (Fig. 3a). Real-time PCR analysis (Fig. 3b) showed that transcription of all these markers was not detected in MSCs before transplantation. After implantation, transcription of cTnI, MYH and Cx43 was detected, but the transcription level was lower than that in the mature cardiomyocytes. Transcription of cTnI at day 7 was significantly increased compared to that at day 5. Transcription of MYH at day 3 was significantly lower than that at day 5 or day 7. Transcription of Cx43 at day 7 was significantly higher than that at day 3 or day 5.
Expression of cardiac markers in transplanted MSCs. a Immunofluorescent staining of cardiac markers (cTnI, MYH, α-actin and Cx43) after 3, 5 and 7 days of transplantation; red fluorescence indicated the cells with expression of each marker; green fluorescence indicated the GFP-labeled MSCs; merged yellow fluorescence indicated expression of cardiac markers on MSCs (arrowheads). b Transcription of cardiac markers (cTnI, MYH and Cx43) after 3, 5 and 7 days of transplantation as determined by real-time PCR. Representative data are shown in each panel. All data are presented as mean ± SD. *P < 0.01 vs. CM; ★ P < 0.05. Scale bar 25 μm. CM cardiomyocyte, iMSCs induced MSCs (engrafted MSCs were induced in vivo)
To investigate whether engrafted MSCs had a function like cardiomyocytes, the morphology and response to Ca2+ were observed among MSCs, engrafted MSCs and cardiomyocytes (supplemental information Fig. 3). After acute isolation, cells were treated with 1.8 mmol/L Ca2+. MSCs showed a round morphology, without contraction. Most of the engrafted MSCs also exhibited a round shape and no contraction, while few of the engrafted MSCs showed a rod-shaped morphology, but without contraction. In addition, the size of those rod-shaped engrafted MSCs was smaller than the cardiomyocytes. Cardiomyocytes exhibited a rod shape and had contraction ability.
Engrafted MSCs did not acquire electrophysiological properties of mature cardiomyocytes
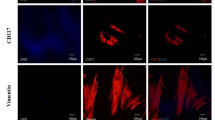
The electrophysiological properties of engrafted MSCs were first investigated by determining expression and transcription of cardiac ion channel genes, including Kv1.4, Kv4.2, Kir2.1, Nav1.5 and Cav1.2 after 3, 5 and 7 days of transplantation. Immunofluorescent staining showed that transplanted MSCs expressed Kv1.4, Kv4.2 and Kir2.1, but did not express Nav1.5 and Cav1.2 from day 3 to day 7 (Fig. 4a). Real-time PCR revealed that transcription of Nav1.5 and Cav1.2 was not detected in both engrafted MSCs and the MSCs before transplantation. Transcription of Kir2.1 was not detected in MSCs before transplantation. Transcription of Kv1.4, Kv4.2 and Kir2.1 in the engrafted MSCs was significantly increased compared with the MSCs before transplantation, but was still lower than that in the mature cardiomyocytes. Transcription of Kv1.4, Kv4.2 and Kir2.1 at day 5 or day 7 was higher than that at day 3. At day 7, transcription of Kv1.4 was also upregulated compared to day 5 (Fig. 4b).
Expression of cardiac ion channel genes in transplanted MSCs. a Immunofluorescent staining of cardiac ion channel genes (Kv1.4, Kv4.2, Kir2.1, Nav1.5 and Cav1.2) after 3, 5 and 7 days of transplantation; red fluorescence indicated the cells with expression of cardiac ion channel genes; green fluorescence indicated the GFP-labeled MSCs; merged yellow fluorescence indicated expression of cardiac ion channel genes on MSCs (arrowheads). b Transcription of cardiac ion channel genes (Kv1.4, Kv4.2 and Kir2.1) after 3, 5 and 7 days of transplantation as determined by real-time PCR. Representative data are shown in each panel. All data are presented as mean ± SD. *P < 0.01 vs. CM; # P < 0.05 vs. MSCs; ★ P < 0.05. Scale bar 25 μm. CM cardiomyocyte, iMSCs induced MSCs (engrafted MSCs were induced in vivo)
To further understand the electrophysiological function of the engrafted MSCs, we determined the functional current (I Na.TTX, I Ca,L, I K) of transplanted MSCs by using patch-clamp technology. Three types of membrane currents were observed in the transplanted MSCs. The first type of current activated by a voltage range between −140 and 40 mV in 10 mV increments from a holding potential of −40 mV was characteristic of inwardly rectifying K+ current (I K1) (Fig. 5a). I K1 was observed in 63.64 % (7/11) of the transplanted MSCs. The second type of current elicited by a voltage range between −20 and 50 mV in 10 mV increments from a holding potential of −50 mV was an outward delayed rectifier current (Fig. 5b). This current was activated at −20 mV, and can be significantly suppressed by 20 mM tetraethylammonium, suggesting that it was likely a delayed rectifier K+ current (I KDR). I KDR was observed in 54.55 % (6/11) of the transplanted MSCs. The third type of current was observed when voltage steps between −40 and 70 mV from a holding potential of −90 mV were applied, and displayed properties of the transient outward K+ current (I to) (Fig. 5c). It was significantly inhibited by 5 mM 4-aminopyridine (4-AP). Furthermore, the average current density of I to (6.27 ± 0.84 pA/pF) was similar to that in adult rat ventricular cardiomyocytes (5.83 ± 0.24 pA/pF) at 60 mV, although the membrane capacitance was much lower than that in the mature cardiomyocytes (12.64 ± 1.19 vs. 114.69 ± 9.26 pF, P < 0.05) (Fig. 5d). I to was observed in 90.91 % (10/11) of the transplanted MSCs. Potassium currents I KDR and I to were recorded in 53.85 % (7/13) and 84.62 % (11/13), respectively, of MSCs before transplantation. However, the average current density of I to in the MSCs before transplantation was significantly different from that in the engrafted MSCs and cardiomyocytes (35.74 ± 3.82 vs. 6.27 ± 0.84 pA/pF or 5.83 ± 0.24 pA/pF; P < 0.05) (Fig. 5d). Moreover, the average membrane capacitance of MSCs before transplantation was significantly lower than that in the engrafted MSCs and cardiomyocytes (2.32 ± 0.31 vs. 12.64 ± 1.19 pF, 114.69 ± 9.26 pF; P < 0.05).
Expression of functional currents in transplanted MSCs. a Expression of inwardly rectifying K+ current (I K1) in transplanted MSCs. I K1 current was recorded in a representative cell with the protocol shown in the inset. The I–V curve showed a strong inward rectification, which is characteristic of I K1. b Expression of delayed rectifier K+ current (I KDR) in transplanted MSCs. I KDR was recorded in a representative engrafted MSC with the protocol shown in the inset in the absence (control) and presence of 20 mM TEA. The I–V relationship was shown in the absence and presence of 20 mM TEA. I KDR was substantially inhibited by TEA (P < 0.05 at 10–50 mV vs. control), and the effect was reversed after washout. c Expression of transient outward K+ current (I to) in transplanted MSCs. I to was recorded in a representative engrafted MSC, with a voltage step protocol shown in the inset, in the absence (control) and presence of 5 mM 4-AP. The I–V relationship was shown in the absence and presence of 5 mM 4-AP. I to was significantly suppressed by 4-AP (P < 0.05 at 0–60 mV vs. control), and the effect was reversed after washout. d Expression of I to in mature cardiomyocytes. I to was recorded in a representative adult rat ventricular cardiomyocyte with the same protocol used in engrafted MSCs. Membrane capacitance and I to current density were compared among MSCs, engrafted MSCs, and cardiomyocytes at 60 mV. Representative data are shown in each panel. All data are presented as mean ± SD. ★ P < 0.05, ★★ P < 0.01. CMs cardiomyocytes, iMSCs induced MSCs (engrafted MSCs were induced in vivo)
Engrafted MSCs did not promote ventricular arrhythmias in infarcted heart
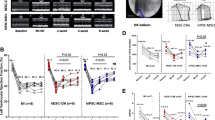
To determine the arrhythmogenic effect of MSCs on the infarcted heart, we treated MI rats with MSCs suspended in DMEM (MI-MSCs group). MI rats treated with DMEM (MI-DMEM group) or untreated rats (MI group) were used as a control. Before treatment, there were no differences in cardiac hemodynamics and heart rate among the three groups (Supplemental information, Table 2). The ventricular arrhythmias (VAs) recorded by ECG displayed premature ventricular contractions (PVCs) or nonsustained ventricular tachycardias (NSVTs) (Fig. 6a). During 8-week monitoring of arrhythmias by ECG, the cumulative occurrence of VAs in the MI, MI-DMEM and MI-MSCs groups was 66.67 % (10/15), 64.29 % (9/14) and 58.82 % (10/17), respectively. Statistical analysis showed no significant differences in the occurrence of VAs among the three groups (Fig. 6b). We further measured the average QRS width in rats with abnormal QRS duration but without VAs in the MI group (n = 5), MI-DMEM group (n = 4) and MI-MSCs group (n = 7). The results showed that prolonged QRS in some rats of the MI-MSCs group was shortened compared with rats in the MI group (Fig. 6c). The average QRS width in the MI-MSCs group was significantly shorter than that in the MI or MI-DMEM group (18.2 ± 0.4 vs. 24.5 ± 1.0 or 24.7±1.0 ms; P < 0.01) from 1 to 8 weeks after treatment (Fig. 6d). These suggest that MSCs transplantation can improve the intraventricular conductions at least in some infarcted hearts.
Occurrence of ventricular arrhythmias (VAs) after transplantation of MSCs. a Representative ECGs showed VAs (arrowheads), including premature ventricular contractions (PVCs) and nonsustained ventricular tachycardias (NSVTs). b Comparison of cumulative occurrence of VAs among the three groups. c Representative ECGs showed prolonged ORS in infarcted rats without PVCs and NSVTs. d Comparison of the average QRS width in infarcted rats without PVCs and NSVTs among the three groups. Representative data are shown in each panel. All data are presented as mean ± SD. ★ p < 0.01 versus MI or MI-DMEM
Electrical vulnerability in infarcted heart was alleviated by the engrafted MSCs
In order to evaluate the effect of MSCs on the electrical vulnerability of the infarcted heart, we utilized PES to induce VAs. In the electrical vulnerability test, ventricular tachycardia (VT) or ventricular fibrillation (VF) were induced in many infarcted rats, whereas VT or VF was not induced in most normal rats (Fig. 7a). The inducibility of VAs in the MI-MSCs group (41.20 %, n = 17) was significantly lower than that in the MI group (86.67 %, n = 15) and the MI-DMEM group (92.86 %, n = 14), suggesting that inducible VAs could be prevented by MSCs transplantation. We further examined the ventricular effective refractory period (VERP) in normal rats and each group of rats after 8 weeks of treatment. The representative ECGs were shown in Fig. 7c. The VERP in both the MI group or MI-DMEM groups was significantly shorter than that in the normal animals (47.7 ± 8.8 or 45.7 ± 6.2 ms vs. 72.5 ± 2.1 ms, P < 0.01) (Fig. 7d). However, VERP in the MI-MSCs group was significantly longer than that in the MI or MI-DMEM groups (56.0 ± 8.8 vs. 47.7 ± 8.8 ms or 45.7 ± 6.2 ms, P < 0.01), although it was still shorter than that in normal animals (56.0 ± 8.8 vs. 72.5 ± 2.1 ms, P < 0.01). These results indicated that VERP in infarcted hearts was shortened and could be prolonged by MSCs transplantation.
Effects of MSCs on the electrical vulnerability of the infarcted heart. a Representative ECGs of induced ventricular tachycardia (VT) or ventricular fibrillation (VF) in infarcted rats. Arrhythmias were not induced in normal rats. b Comparison of induced VAs among the three groups after 8 weeks of MSCs transplantation. c Representative ECGs of ventricular effective refractory period (VERP) in normal rats and three experimental groups. d Comparison of VERP in normal rats and three experimental groups. Representative data are shown in each panel. All data are presented as mean ± SD. N normal group, ERP effective refractory period
Discussion
In this study, we demonstrated that implanted MSCs could not fully acquire the electrophysiological properties of mature cardiomyocytes during the survived period in infarcted hearts, as these cells only expressed parts of functional currents (I to, I K1 and I KDR) and related ion channel genes (Kv1.4, Kv4.2 and Kir2.1), but did not express functional Na+ current and Ca2+ current, as well as without response to Ca2+. However, transplanted MSCs did not promote ventricular arrhythmias. In contrast, they can alleviate the electrical vulnerability by reducing inducibility of ventricular arrhythmias, prolonging the VERP and shortening abnormal QRS width when implanted into infarcted hearts.
Survival of MSCs in infarcted hearts
Consistent with previous studies [8, 18, 49, 53], our data demonstrated that MSCs did not survive effectively in infarcted hearts. After 7 days of engraftment, the number of survived MSCs was decreased sharply to 0.39 % of the count at day 3 post-engraftment, and almost all MSCs died after 9 days of transplantation. Multiple factors contribute to the poor viability of MSCs in the infarcted heart. For instance, the ischemic environment lacks nutrients and oxygen, which is unfavorable for the survival of MSCs. In addition, loss of the survival signals from the matrix attachment and cell–cell interactions may result in the death of transplanted MSCs [10]. These speculations can be supported by previous studies showing that hypoxic preconditioning [41], or activation of the survival signaling pathway (PI3K/Akt) by IGF-1 [10], or overexpressing Akt [32] can promote survival of MSCs in ischemic myocardium. In this study, we showed that apoptosis occurred in the engrafted MSCs, suggesting that apoptosis induced by ischemic or hypoxic environment plays an important role in the poor survival of MSCs. Therefore, anti-apoptosis might be an efficient approach to improving the survival of MSCs in infarcted hearts. This has been partially demonstrated by previous studies in which Bc1-2 engineered MSCs can inhibit apoptosis and improve heart function [25]. In addition, our results showed that expression of Cx43 (gap junction protein) in MSCs was significantly upregulated after transplantation, while the number of the survived MSCs decreased significantly. These results suggest that connection with host cardiomyocytes is not sufficient for the survival of MSCs. Thus, inhibition of ischemia or hypoxia-induced apoptosis might be an important strategy to improve the viability of engrafted MSCs.
Electrophysiological properties of MSCs in infarcted hearts
For treatment of heart injury by cell transplantation, it is expected that implanted cells could acquire the electrophysiological properties of mature cardiomyocytes, and thus form electrical coupling with the host myocardium. Although differentiation of MSCs into cardiomyocytes is generally identified by immunostaining using cardiac markers, e.g., α-actin, cTnT and cTnI [46, 50, 51], the electrophysiological properties of implanted MSCs in infarcted hearts remain unclear. Here, our results showed that engrafted MSCs expressed cardiac markers (MYH, cTnI and Cx43), cardiac ion channel genes (Kv1.4, Kv4.2 and Kir2.1) and functional currents (I to, I K1 and I KDR) during their 7 day-survival in vivo. The expression of cardiac ion channel genes and specific cardiac markers was increased gradually in a time dependent manner. Additionally, consistent with previous studies [23, 54], our results showed that MSCs expressed functional currents I to and I KDR in vitro before transplantation. But after engraftment, MSCs initially expressed I k1 current besides I to and I KDR. Furthermore, the current density of I to in the engrafted MSCs was more similar to cardiomyocytes compared with MSCs before transplantation. These results indicate that microenvironment of infarcted myocardium can promote MSCs to acquire some electrophysiological properties of mature cardiomyocytes. However, engrafted MSCs did not express cardiac functional Na+ current and Ca2+ current during their 7-day-survival in vivo, as well as without reaction to Ca2+. All mentioned above suggest that MSCs have a potential but do not have the opportunity to fully acquire the electrophysiological properties of mature cardiomyocytes in infarcted hearts. We speculate that limited survival hinders MSCs to further acquire electrophysiological properties of mature cardiomyocytes. This is also supported by a previous study [40] showing that preconditioning enhanced the survival and differentiation of MSCs after transplantation into the infarcted myocardium.
Evaluation of proarrhythmic effect of implanted MSCs in infarcted hearts
We initially hypothesized that MSCs could not obtain electrophysiological properties of mature cardiomyocytes in infarcted hearts, and thus might act as arrhythmogenic substrates to promote arrhythmias. However, our results demonstrated that MSCs do not promote spontaneous ventricular arrhythmias after being implanted into infarcted hearts, although they did not acquire the electrophysiological properties of mature cardiomyocytes. In addition, they can even alleviate the electrical vulnerability and reduce the inducibility of ventricular arrhythmias in the infarcted hearts. These can be explained naturally. First of all, our results showed that implanted MSCs did not express functional Na+ current and Ca2+ current, suggesting that transplanted MSCs are unlikely to possess excitability, not to mention action potential and abnormal impulse initiation. Furthermore, the number of our transplanted MSCs per injection site was limited and decreased sharply during 3–9 days after transplantation, which might not be enough to form a cell cluster and to generate a clear reentry substrate to form abnormal impulse conduction. Chang et al. [6] reported that reentrant arrhythmias can be induced in a monolayer co-cultures containing 10 or 20 % human MSCs and neonatal rat ventricular myocytes, but not in the group containing 1 % MSCs. So non-proarrhythmias by MSC transplantation in our study may be partly due to the small proportion of engrafted MSCs in implanted heart. Evidence from other clinical trials [2, 7, 16, 19, 27, 28, 58] and preclinical studies [34, 53] suggested that MSC implantation did not seem to intrinsically create significant local reentry substrates and did not facilitate ventricular arrhythmias.
Finally, we found that MSCs could prolong the VERP and shorten abnormal QRS width when implanted into infarcted hearts. These might be partially responsible for the reduction of inducible VAs after MSCs transplantation. Moreover, alleviation of fibrosis and attenuations of focal electrophysiological heterogeneity in infarcted hearts may be an important pathophysiological mechanism for MSCs to ameliorate electrophysiological heterogeneity in ischemic hearts [53]. Optical mapping technique has also confirmed that electrical viability could be enhanced in MSCs-treated hearts [34].
Study limitations
Firstly, because of the poor survival of engrafted MSCs, we detected the expression of functional membrane current only at day 3 of MSC transplantation. After 5 or 7 days of implantation, the number of survived MSCs was not sufficient to successfully isolate enough engrafted MSCs for patch-clamp experimentation. Thus, it is unknown whether functional membrane currents of I Na.TTX and I Ca.L are expressed at day 5 or 7 of implantation. However, immunofluorescent staining and real-time PCR revealed no expression of ion channel genes related currents of I Na.TTX and I Ca.L in engrafted MSCs after 5 or 7 days of transplantation. These results indicate that engrafted MSCs are unlikely to express functional currents of I Na.TTX and I Ca.L at day 5 or 7 of implantation. Secondly, although arrhythmias were monitored by ECG for a long time before and after MSCs transplantation, monitoring was not continuous. Future studies are needed to monitor arrhythmias continuously by 24-h Holter ECGs. Finally, we have investigated the effects of MSCs on electrical vulnerability in infarcted hearts. However, the changes of electrical conduction in MSCs engrafted hearts were not examined by optical mapping technology in this study because previous studies [34, 47] have demonstrated that MSC transplantation could enhance electrical conduction post-MI.
In conclusion, although implanted MSCs could not acquire the electrophysiological properties of mature cardiomyocytes during their survived period in infarcted hearts, they do not promote ventricular arrhythmias and can alleviate the electrical vulnerability. Therefore, MSCs transplantation could be a safe and promising approach for the treatment of ischemic hearts.
References
Asp L, Beraki S, Kristensson K, Ogren SO, Karlsson H (2009) Neonatal infection with neurotropic influenza A virus affects working memory and expression of type III Nrg1 in adult mice. Brain Behav Immun 23:733–741. doi:10.1016/j.bbi.2009.04.004
Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM (2006) Transcoronary transplantation of progenitor cells after myocardial infarction. New Engl J Med 355:1222–1232. doi:10.1056/nejmoa051779
Beeres S, Zeppenfeld K, Bax JJ, Dibbets-Schneider P, Stokkel MPM, Fibbe WE, van der Wall EE, Atsma DE, Schalij MJ (2007) Electrophysiological and arrhythmogenic effects of intramyocardial bone marrow cell injection in patients with chronic ischemic heart disease. Heart Rhythm 4:257–265. doi:10.1016/j.hrthm.2006.10.033
Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378:1847–1857. doi:10.1016/s0140-6736(11)61590-0
Briguori C, Reimers B, Sarais C, Napodano M, Pascotto P, Azzarello G, Bregni M, Porcellini A, Vinante O, Zanco P, Peschle C, Condorelli G, Colombo A (2006) Direct intramyocardial percutaneous delivery of autologous bone marrow in patients with refractory myocardial angina. Am Heart J 151:674–680. doi:10.1016/j.ahj.2005.04.033
Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong PH, Marban E, Abraham R (2006) Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation 113:1832–1841. doi:10.1161/circulationaha.105.593038
Chen SL, Fang W, Ye F, Liu YH, Qian J, Shan S, Zhang J, Zhao RCH, Liao LM, Lin S, Sun JP (2004) Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 94:92–95. doi:10.1016/j.amjcard.2004.03.034
Cho J, Zhai P, Maejima Y, Sadoshima J (2011) Myocardial injection with GSK-3 beta-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res 108:478–489. doi:10.1161/circresaha.110.229658
Du Y, Huang X, Wang T, Han K, Zhang J, Xi Y, Wu G, Ma A (2007) Downregulation of neuronal sodium channel subunits Nav1.1 and Nav1.6 in the sinoatrial node from volume-overloaded heart failure rat. Pflug Arch Eur J Phy 454:451–459. doi:10.1007/s00424-007-0216-4
Enoki C, Otani H, Sato D, Okada T, Hattori R, Imamura H (2010) Enhanced mesenchymal cell engraftment by IGF-1 improves left ventricular function in rats undergoing myocardial infarction. Int J Cardiol 138:9–18. doi:10.1016/j.ijcard.2009.04.012
Fuchs S, Kornowski R, Weisz G, Satler LF, Smits PC, Okubagzi P, Baffour R, Aggarwal A, Weissman NJ, Cerqueira M, Waksman R, Serrruys P, Battler A, Moses JW, Leon MB, Epstein SE (2006) Safety and feasibility of transendocardial autologous bone marrow cell transplantation in patients with advanced heart disease. Am J Cardiol 97:823–829. doi:10.1016/j.amjcard.2005.09.132
Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, Barton PJR, Terracciano CMN, Yacoub MH, Suzuki K (2007) Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation 115:2254–2261. doi:10.1161/circulationaha.106.662577
Gao E, Lei YH, Shang XY, Huang ZM, Zuo L, Boucher M, Fan QA, Chuprun JK, Ma XL, Koch WJ (2010) A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107:1445–1453. doi:10.1161/circresaha.110.223925
Grauss RW, van Tuyn J, Steendijk P, Winter EM, Pijnappels DA, Hogers B, Groot A, van der Geest R, van der Laarse A, de Vries AAF, Schalij MJ, Atsmaa DE (2008) Forced myocardin expression enhances the therapeutic effect of human mesenchymal stem cells after transplantation in ischemic mouse hearts. Stem Cells 26:1083–1093. doi:10.1634/stemcells.2007-0523
Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasche P (2006) Skeletal myoblast transplantation in ischemic heart failure—long-term follow-up of the first phase I cohort of patients. Circulation 114:I108–I113. doi:10.1161/circulationaha.105.000521
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Reisman MA, Schaer GL, Sherman W (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54:2277–2286. doi:10.1016/j.jacc.2009.06.055
Heusch G (2011) SCIPIO brings new momentum to cardiac cell therapy. Lancet 378:1827–1828. doi:10.1016/s0140-6736(11)61648-6
Huang J, Zhang ZP, Guo JA, Ni AG, Deb A, Zhang LN, Mirotsou M, Pratt RE, Dzau VJ (2010) Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 106:1753–1762. doi:10.1161/circresaha.109.196030
Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kolantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F (2006) Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367:113–121. doi:10.1016/S0140-6736(05)67861-0
Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Dong A, Du Y, Huang X, Wang J, Lei X, Zheng X (2006) Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflug Arch Eur J Phy 453:43–52. doi:10.1007/s00424-006-0117-y
Jin JY, Jeong SI, Shin YM, Lim KS, Shin HS, Lee YM, Koh HC, Kim KS (2009) Transplantation of mesenchymal stem cells within a poly(lactide-co-epsilon-caprolactone) scaffold improves cardiac function in a rat myocardial infarction model. Eur J Heart Fail 11:147–153. doi:10.1093/eurjhf/hfn017
Katritsis DG, Sotiropoulou P, Giazitzoglou E, Karvouni E, Papamichail M (2007) Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace 9:167–171. doi:10.1093/europace/eul184
Li GR, Deng XL, Sun HY, Chung SSM, Tse HF, Lau CP (2006) Ion channels in mesenchymal stem cells from rat bone marrow. Stem Cells 24:1519–1528. doi:10.1634/stemcells.2005-0307
Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O’Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R (2011) Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol 106:849–864. doi:10.1007/s00395-011-0180-1
Li WZ, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G (2007) Bc1-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25:2118–2127. doi:10.1634/stemcells.2006-0771
Lorenzen JM, Martino F, Thum T (2012) Epigenetic modifications in cardiovascular disease. Basic Res Cardiol 107:245. doi:10.1007/s00395-012-0245-9
Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K (2006) Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. New Engl J Med 355:1199–1209. doi:10.1056/nejmoa055706
Lunde K, Solheim S, Forfang K, Arnesen H, Brinch L, Jornerheim R, Ragnarsson A, Egeland T, Endresen K, Ilebekk A, Mangschau A, Aakhus S (2008) Anterior myocardial infarction with acute percutaneous coronary intervention and intracoronary injection of autologous mononuclear bone marrow cells—safety, clinical outcome, and serial changes in left ventricular function during 12-months’ follow-up. J Am Coll Cardiol 51:674–676. doi:10.1016/j.jacc.2007.10.032
Ly HQ, Nattel S (2009) Stem cells are not proarrhythmic: letting the genie out of the bottle. Circulation 119:1824–1831. doi:10.1161/circulationaha.108.812701
Macia E, Boyden PA (2009) Stem cell therapy is proarrhythmic. Circulation 119:1814–1823. doi:10.1161/circulationaha.108.779900
Madonna R, Rokosh G, De Caterina R, Bolli R (2010) Hepatocyte growth factor/Met gene transfer in cardiac stem cells—potential for cardiac repair. Basic Res Cardiol 105:443–452. doi:10.1007/s00395-010-0102-7
Mangi AA, Noiseux N, Kong DL, He HM, Rezvani M, Ingwall JS, Dzau VJ (2003) Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9:1195–1201. doi:10.1038/nm912
Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D (2003) Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 41:1078–1083. doi:10.1016/S0735-1097(03)00092-5
Mills W, Mal N, Kiedrowski M, Unger R, Forudi F, Popovic Z, Penn M, Laurita K (2007) Stem cell therapy enhances electrical viability in myocardial infarction. J Mol Cell Cardiol 42:304–314. doi:10.1016/j.yjmcc.2006.09.011
Numasawa Y, Kimura T, Miyoshi S, Nishiyama N, Hida N, Tsuji H, Tsuruta H, Segawa K, Ogawa S, Umezawa A (2011) Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells 29:1405–1414. doi:10.1002/stem.691
Oerlemans MI, Goumans MJ, van Middelaar B, Clevers H, Doevendans PA, Sluijter JP (2010) Active Wnt signaling in response to cardiac injury. Basic Res Cardiol 105:631–641. doi:10.1007/s00395-010-0100-9
Ohtani K, Dimmeler S (2011) Control of cardiovascular differentiation by microRNAs. Basic Res Cardiol 106:5–11. doi:10.1007/s00395-010-0139-7
Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S (2010) Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol 105:703–712. doi:10.1007/s00395-010-0109-0
Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS, Makkar R (2003) Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a swine model of myocardial infarction. J Cardiovasc Electr 14:841–848. doi:10.1046/j.1540-8167.2003.03124.x
Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M (2008) Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res 77:134–142. doi:10.1093/cvr/cvm025
Rosova I, Dao M, Capoccia B, Link D, Nolta JA (2008) Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26:2173–2182. doi:10.1634/stemcells.2007-1104
Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC (2010) In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 28:788–798. doi:10.1002/stem.312
Sanganalmath SK, Abdel-Latif A, Bolli R, Xuan YT, Dawn B (2011) Hematopoietic cytokines for cardiac repair: mobilization of bone marrow cells and beyond. Basic Res Cardiol 106:709–733. doi:10.1007/s00395-011-0183-y
Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu JT, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM, Investigators R-A (2006) Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. New Engl J Med 355:1210–1221. doi:10.1056/nejmoa060186
Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu JT, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM, Investigators RA (2006) Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J 27:2775–2783. doi:10.1093/eurheartj/ehl388
Shinmura D, Togashi I, Miyoshi S, Nishiyama N, Hida N, Tsuji H, Tsuruta H, Segawa K, Tsukada Y, Ogawa S, Umezawa A (2011) Pretreatment of human mesenchymal stem cells with pioglitazone improved efficiency of cardiomyogenic transdifferentiation and cardiac function. Stem Cells 29:357–366. doi:10.1002/stem.574
Song H, Hwang HJ, Chang W, Song BW, Cha MJ, Kim IK, Lim S, Choi EJ, Ham O, Lee CY (2011) Cardiomyocytes from phorbol myristate acetate-activated mesenchymal stem cells restore electromechanical function in infarcted rat hearts. Proc Natl Acad Sci USA 108:296–301. doi:10.1073/pnas.1015873107
Stamm C, Kleine HD, Westphal B, Petzsch M, Kittner C, Nienaber CA, Freund M, Steinhoff G (2004) CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac Cardiov Surg 52:152–158. doi:10.1055/s-2004-817981
Tang YL, Tang Y, Zhang YC, Qian KP, Shen LP, Phillips I (2005) Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol 46:1339–1350. doi:10.1016/j.jacc.2005.05.079
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105:93–98. doi:10.1161/hc0102.101442
Tsuji H, Miyoshi S, Ikegami Y, Hida N, Asada H, Togashi I, Suzuki J, Satake M, Nakamizo H, Tanaka M, Mori T, Segawa K, Nishiyama N, Inoue J, Makino H, Miyado K, Ogawa S, Yoshimura Y, Umezawa A (2010) Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res 106:1613–1623. doi:10.1161/circresaha.109.205260
Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DWG, Higgins AJ, Julian DG, Lab MJ, Manning AS, Northover BJ, Parratt JR, Riemersma RA, Riva E, Russell DC, Sheridan DJ, Winslow E, Woodward B (1988) The lambeth conventions-guidelines for the study of arrhythmias in ischemia, infarction, and reperfusion. Cardiovasc Res 22:447–455. doi:10.1093/cvr/22.7.447
Wang D, Zhang F, Shen W, Chen M, Yang B, Zhang Y, Cao K (2010) Mesenchymal stem cell injection ameliorates the inducibility of ventricular arrhythmias after myocardial infarction in rats. Int J Cardiol 152:314–320. doi:10.1016/j.ijcard.2010.07.025
Wang SP, Wang JA, Luo RH, Cui WY, Wang H (2008) Potassium channel currents in rat mesenchymal stem cells and their possible roles in cell proliferation. Clin Exp Pharmacol Physiol 35:1077–1084. doi:10.1111/j.1440-1681.2008.04964
Wang TZ, Xu ZY, Jiang WH, Ma AQ (2006) Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol 109:74–81. doi:10.1016/j.ijcard.2005.05.072
Wei F, Wang TZ, Liu JJ, Du Y, Ma AQ (2011) The subpopulation of mesenchymal stem cells that differentiate toward cardiomyocytes is cardiac progenitor cells. Exp Cell Res 317:2661–2670. doi:10.1016/j.yexcr.2011.08.011
White HD, Chew DP (2008) Acute myocardial infarction. Lancet 372:570–584. doi:10.1016/S0140-6736(08)61237-4
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H (2004) Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364:141–148. doi:10.1016/S0140-6736(04)16626-9
Wu J, Li J, Zhang N, Zhang C (2011) Stem cell-based therapies in ischemic heart diseases: a focus on aspects of microcirculation and inflammation. Basic Res Cardiol 106:317–324. doi:10.1007/s00395-011-0168-x
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No: 30800455, 81000063) and Research Fund for the Doctoral Program of Higher Education of China (No: 200806981027). We thank Prof. Zhiquan Liu and Dr. Min Gong for their helpful suggestions in study design.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Wei and T.-Z. Wang contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, F., Wang, TZ., Zhang, J. et al. Mesenchymal stem cells neither fully acquire the electrophysiological properties of mature cardiomyocytes nor promote ventricular arrhythmias in infarcted rats. Basic Res Cardiol 107, 274 (2012). https://doi.org/10.1007/s00395-012-0274-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0274-4