Abstract
Findings from our laboratory indicate that proinflammatory cytokines and their transcription factor, nuclear factor-kappaB (NF-κB), are increased in the hypothalamic paraventricular nucleus (PVN) and contribute towards the progression of heart failure. In this study, we determined whether NF-κB activation within the PVN contributes to sympathoexcitation via interaction with neurotransmitters in the PVN during the pathogenesis of heart failure. Heart failure was induced in rats by left anterior descending coronary artery ligation. Sham-operated control (SHAM) or heart failure rats were treated for 4 weeks through bilateral PVN infusion with SN50, SN50M or vehicle via osmotic minipump. Rats with heart failure treated with PVN vehicle or SN50M (inactive peptide for SN50) had increased levels of glutamate, norepinephrine (NE), tyrosine hydroxylase (TH), superoxide, gp91phox (a subunit of NAD(P)H oxidase), phosphorylated IKKβ and NF-κB p65 activity, and lower levels of gamma-aminobutyric acid (GABA) and the 67-kDa isoform of glutamate decarboxylase (GAD67) in the PVN compared with those of SHAM rats. Plasma levels of cytokines, norepinephrine, epinephrine and angiotensin II, and renal sympathetic nerve activity (RSNA) were increased in heart failure rats. Bilateral PVN infusion of SN50 prevented the decreases in PVN GABA and GAD67, and the increases in RSNA and PVN glutamate, norepinephrine, TH, superoxide, gp91phox, phosphorylated IKKβ and NF-κB p65 activity observed in vehicle or SN50M-treated heart failure rats. A same dose of SN50 given intraperitoneally did not affect neurotransmitters concentration in the PVN and was similar to vehicle-treated heart failure rats. These findings suggest that NF-κB activation in the PVN modulates neurotransmitters and contributes to sympathoexcitation in rats with ischemia-induced heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is the end-stage manifestation of cardiac syndromes in which the heart fails to pump an adequate supply of blood to the body, often due to left ventricular dysfunction. Neurohumoral mechanisms play important roles in the pathophysiology of HF with sympathoexcitation considered as the hallmark of HF. Plasma levels of proinflammatory cytokines (PICs), such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1beta (IL-1β) and IL-6, are also increased in HF patients, with their levels rising concomitantly with cardiac dysfunction [37, 38] and severity of HF [13, 35, 40]. However, the functional role of PIC in HF remains poorly understood, especially within cardioregulatory regions of the brain. Under normal conditions, there is very low expression of PIC in the brain, but during disease conditions such as HF, there is a marked up-regulation of cytokines in the brain [10].
A growing body of evidence suggests that immune-mediated mechanisms play important roles in the pathogenesis of HF. In normal rats, an intracarotid injection of TNF-α elicits a prominent pressor response—characterized by activation of pre-sympathetic neurons in the hypothalamic paraventricular nucleus (PVN) and in the rostral ventrolateral medulla (RVLM)—with associated increases in arterial pressure (AP), heart rate (HR) and renal sympathetic nerve activity (RSNA) [44]. In HF, TNF-α is increased in the heart, plasma and hypothalamus within minutes [11, 14], and the early appearance of TNF-α in the brain can be largely prevented by interrupting the cardiac sympathetic afferent nerve signals [11, 14]. Since PICs are too large to cross the blood–brain barrier, the origin of PIC in the central nervous system (CNS) in HF remains a mystery. A recent study from our laboratory suggests that TNF-α and IL-1β are increased in the hypothalamus, specifically the PVN, of HF rats [13]. Furthermore, anti-cytokine therapy using etanercept, a synthetic TNF-α binding agent, or pentoxifylline, a PIC production inhibitor [13, 15] decreased the expression of PIC in the PVN and attenuated the neurohormonal excitation (NHE) observed in HF [17]. However, due to RENAISSANCE and RECOVER clinical trials, one might argue against the cytokine hypothesis. However, the failure of these two clinical trials could be due to the peripheral targeting of only one cytokine. Hence, in this study, we aimed to target central activation of nuclear factor-kappaB (NF-κB), considered to be the major transcription factor regulating the inflammatory response by mediating the expression of PICs, including TNF-α, IL-1β and IL-6, in various signal transduction pathways, in an attempt to limit the PIC cascade and sympathoexctiation seen in HF.
Increasing evidence demonstrates that NF-κB induced inflammation contributes to the pathophysiology of multiple disease states, including HF [41]. Functional NF-κB complexes are present essentially in all cell types in the CNS and activated NF-κB is the major regulator facilitating the synthesis of several different injury-responsive cytokines in neurons [4]. Both in vivo and in vitro experiments have shown that PICs are effective activators of NF-κB [16]. However, it is not known whether PIC induced within the brain upregulate NF-κB in the PVN, an important central integration site of sympathetic outflow and cardiovascular regulation and contribute to NHE in HF. Based on current findings, NF-κB might be a potential target for the modulation of NHE in HF.
A previous study from our laboratory demonstrated that HF rats had increased neuronal excitation accompanied by higher levels of glutamate and norepinephrine (NE) and lower levels of gamma-aminobutyric acid (GABA) in the PVN when compared with SHAM rats [20, 21]. In this study, we determined whether NF-κB activation in the PVN contributed to sympathoexcitation via interaction with neurotransmitters in the PVN during the pathogenesis of HF. The results from this study will lead to a better understanding of the disease process and aid in designing new therapeutic strategies for the treatment of HF.
Materials and methods
Animals
Adult male Sprague–Dawley rats (275–300 g) were used for all experiments. Rats were housed in temperature- (23 ± 2°C) and light-controlled (12 h light/dark cycle) animal quarters and were provided rat chow and tap water ad libitum. The Institutional Animal Care and Use Committees of Xi’an Jiaotong University and Louisiana State University approved all protocols. This investigation conforms to the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
General experimental protocol
Rats underwent implantation of bilateral PVN cannulae and were allowed a week for recovery. Coronary artery ligation was then performed with the ischemic zone confirmed using echocardiography. Subsequently, osmotic minipumps were implanted subcutaneously and connected to the cannulae for the continuous infusion of SN50 (2 μg/h; a synthetic peptide carrying the nuclear localization sequence of the NF-κB p50 subunit, which competes for the cellular mechanisms mediating nuclear translocation and prevents NF-κB binding to DNA without affecting the level of the inhibitory protein IκB; Enzo Life Sciences), SN50M (2 μg/h; the inactive control peptide for the cell-permeable NF-κB inhibitory peptide SN50; Enzo Life Sciences), or vehicle directly into the PVN. At the conclusion of the study, conscious RSNA measurements were obtained. Another set of HF and SHAM rats were treated with intraperitoneally (IP) infusion of a similar dose of SN50 (2 μg/h) or SN50M (2 μg/h), or vehicle over a 4-week treatment period.
Implantation of bilateral PVN cannulae for chronic infusion studies
The method for PVN cannulation has been previously described [12]. Each rat was anesthetized (ketamine + xylazine, ip), and the head placed into a stereotaxic apparatus. A skin incision was made, the skull opened and the dura carefully dissected parallel to the sinus vein. A stainless steel double cannula (Plastics One, Inc.) with a center-to-center distance of 0.5 mm was implanted into the PVN using an introducer, according to stereotaxic coordinates (1.8 mm posterior to the bregma and 8.5 mm ventral from the skull surface) [36]. The cannula was fixed to the cranium using dental acrylic and two stainless steel screws. Animals received buprenorphine (0.01 mg/kg, sc) immediately following surgery and 12 h post-operation. The success rate of bilateral PVN cannulation is 65%. At the completion of the study, brains were sectioned to verify location of cannulae, and only animals with verifiable bilateral PVN injection sites were used in the final analysis.
Coronary ligation
Rats underwent sterile surgery under anesthesia (ketamine + xylazine, ip) for induction of HF by ligation of the left anterior descending coronary artery, or the same surgery without vessel ligation (SHAM), as previously described [19, 21, 22, 24, 25].
Echocardiographic assessment of left ventricular function
Echocardiography was performed under ketamine sedation to assess left ventricular (LV) function as previously described [22, 24, 25]. From these measurements, LV ejection fraction (LVEF) and LV end-diastolic volume (LVEDV) were reported.
Drug infusion
Within 24 h of coronary ligation or sham operation, anesthetized (ketamine + xylazine, ip) rats underwent subcutaneous implantation of osmotic minipumps (Alzet, Model #1004). Minipumps were connected to the bilateral PVN cannulae for continuous infusion (0.11 μl/h/side) of SN50, which inhibits nuclear translocation of NF-κB, at a total dose of 2 μg/h; SN50M (2 μg/h) or vehicle (0.11 μl/h/side) over a 4-week period. Another set of HF and SHAM rats were treated with IP infusion of a similar dose of SN50 (2 μg/h) or SN50M (2 μg/h), or vehicle over a 4-week treatment period. The doses used in this study were determined from preliminary studies based on a previous report from our laboratory [22].
Tissue microdissection
Palkovits’s microdissection procedure was used to isolate the PVN, as previously described [21, 33]
Measurement of PVN tissue levels of glutamate, GABA and NE, and of plasma NE and epinephrine
Tissue concentrations of glutamate and GABA were measured using HPLC with electrochemical detection (ECD-300, Eicom Corporation, Japan) as previously described [21, 42]. Tissue NE concentration was measured using HPLC with electrochemical detection (HTEC-500, Eicom Corporation, Japan) as previously described [3, 21, 42]. Plasma NE and epinephrine (EPI) were measured using HPLC as previously described [18, 19].
Western blot
Measurement of PVN protein was performed as previously described [21, 31]. Briefly, protein extracted from the PVN was used for measurements of tyrosine hydroxylase (TH, Abcam) and the 67-kDa isoform of glutamate decarboxylase (GAD67, Abcam) expression by western blot. Protein loading was controlled by probing all blots with β-actin antibody (Santa Cruz Biotechnology) and normalizing their protein intensities to that of β-actin. The bands were analyzed using NIH Image J software.
Immunohistochemistry and Immunofluorescence
Coronal sections from brains were obtained from the region approximately 1.80 mm from the bregma. Immunohistochemical labeling was performed in floating sections as previously described [20, 21] to identify Fra-like (Fra-LI, a marker of chronic neuronal activation; Santa Cruz Biotechnology) expression.
Immunofluorescence studies were performed as previously described [22, 23]. Superoxide generation was determined by fluorescent-labeled dihydroethidium (DHE; Molecular Probes) staining, as previously described [18]. The primary gp91phox antibodies were from Santa Cruz Biotechnology, and the phosphorylated IKKβ (p-IKKβ) antibody was from Cell Signaling Technology. DAPI for nuclear staining was from Molecular Probes. Positive immunofluorescent-staining cells were counted under confocal microscopy in 4 view fields (equal area) randomly selected from bilateral PVN transverse sections at about −1.80 mm from bregma. One sample consisted of the average of 4 view fields from a section.
Quantification of NF-κB p65 activity in the PVN
The NF-κB/p65 Active ELISA (Active Motif, USA) kit was used to measure the binding activity of free NF-κB p65 in nuclear extracts as described previously [1, 9]. The analysis was done using a sandwich ELISA method according to the manufacturer’s instructions.
ELISA studies
Plasma and tissue cytokine levels were measured using ELISA (Biosource International Inc.) techniques, as previously described [21, 22, 25]. Plasma angiotensin II (ANGII) was measured using an EIA kit (Cayman Chemical Company) as previously described [22].
Electrophysiological recordings and anatomical measurements
AP, HR and RSNA were recorded. The general methods for recording and data analysis have been described previously [21, 23]. Maximum RSNA was detected using an intravenous bolus administration of sodium nitroprusside (SNP) [21, 34]. At the end of the experiment, the background noise, defined as the signal-recorded post-mortem, was subtracted from actual RSNA and subsequently expressed as percent of maximum (in response to SNP) [18, 19, 32]. The left ventricular end-diastolic pressure (LVEDP), the right ventricle (RV)/body weight (BW) ratio and lung/BW ratio were measured as previously described [21, 22].
Statistical analysis
All data are expressed as mean ± SEM. Data were analyzed by two-way ANOVA. Multiple testing was corrected for using Tukey’s post hoc test. Echocardiography data were analyzed with repeated measures ANOVA. A P value of 0.05 was considered significant.
Results
Echocardiography
At 24-h post-coronary artery ligation or sham operation, rats assigned to treatment with SN50, SN50M and vehicle were grouped based on echocardiographically defined LV function (Table 1). The infarct sizes in this study ranged from 40 to 50% of the LV. LVEF was significantly reduced, and LVEDV and LVEDV/mass ratio were significantly increased in rats with ischemic injury assigned to SN50, SN50M or vehicle treatments, when compared with sham-operated rats assigned to those same treatments. At 24 h, however, there were no differences in LVEF, LVEDV, LVEDV/mass ratio or percent ischemic zone (%IZ) among rats with ischemic injury assigned to SN50, SN50M or vehicle treatment. At 4 weeks, LVEDV and LVEDV/mass ratio were significantly higher than the 24-h baseline values in the SN50-, SN50M- and vehicle-treated HF rats, and LVEF was significantly lower in the SN50M- or vehicle-treated HF rats (Table 1). Moreover, LVEF was higher in the HF rats that received SN50 when compared with the HF rats that received SN50M or vehicle. However, there were no significant differences in LVEDV, LVEDV/mass ratio or %IZ among the SN50-, SN50M- and vehicle-treated HF rats at 4 weeks.
PVN Neurotransmitters
HF rats had elevated levels of NE and glutamate, and lower levels of GABA in the PVN. Four-week bilateral infusions of SN50 into the PVN prevented the decrease in PVN GABA and the increases in PVN glutamate and NE in HF rats (Fig. 1). However, IP treatment with the same dose of SN50 did not alter NE, glutamate, or GABA in the PVN of HF rats.
PVN levels of norepinephrine (NE), glutamate (GLU) and GABA in heart failure (HF) and sham-operated (SHAM) rats treated for 4 weeks with SN50, SN50M or vehicle. HF rats had higher levels of NE (a) and glutamate (b), and lower levels of GABA (c) in the PVN. Four-week bilateral infusions of SN50 into the PVN prevented the decrease in PVN GABA and the increases in PVN glutamate and NE in HF rats. PVN infusions of SN50M for 4 weeks did not alter NE, glutamate, or GABA in the PVN of HF rats. IP treatment with the same dose of SN50 did not alter NE, glutamate, or GABA in the PVN of HF rats. *P < 0.05 versus SHAM groups. † P < 0.05 HF + PVN SN50 versus HF + PVN vehicle or HF + PVN SN50M
TH and GAD67 protein expression in the PVN
Western blot showed that HF rats had higher TH and lower GAD67 levels in the PVN when compared with SHAM rats (Fig. 2). Bilateral PVN infusion of SN50 for 4 weeks prevented the decrease in GAD67 and the increase in TH in the PVN of HF rats (Fig. 2).
Western blot of TH and GAD67 in the PVN showed that HF rats had higher levels of TH and lower levels of GAD67 when compared with SHAM rats. Bilateral PVN infusions of SN50 for 4 weeks decreased expression of TH, and increased GAD67 expression in the PVN of HF rats. *P < 0.05 versus SHAM groups. † P < 0.05 HF + PVN SN50 versus HF + PVN vehicle
Fra-LI activity, an indicator of chronic neuronal activation, in the PVN
Compared with SHAM rats, Fra-LI activity was higher in the PVN of HF rats. Bilateral PVN infusions of SN50 prevented the increases in Fra-LI of HF rats (Fig. 3).
Fra-LI activity immunoreactivity in the PVN. HF rats had higher PVN levels of Fra-LI (black dots) immunoreactivity when compared with SHAM rats. Bilateral PVN infusions of SN50 for 4 weeks decreased Fra-LI expression in the PVN of HF rats. PVN infusions of SN50M for 4 weeks did not alter Fra-LI expression in the PVN of HF rats. *P < 0.05 versus SHAM groups. † P < 0.05 HF + PVN SN50 versus HF + PVN vehicle or HF + PVN SN50M
NF-κB activation in the PVN
HF rats showed increases in NF-κB p65 activity and p-IKKβ in the PVN. Four-week bilateral infusion of SN50 into the PVN prevented the increase in PVN NF-κB p65 activity and p-IKKβ in HF rats (Fig. 4).
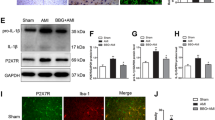
NF-κB activation in the PVN. HF rats had higher level of NF-κB p65 activity and p-IKKβ in the PVN. Four-week bilateral infusions of SN50 into the PVN prevented the increases in NF-κB p65 activity and p-IKKβ in the PVN of HF rats. a Immunofluorescence for p-IKKβ (red) and neuronal nuclei (DAPI, blue) in the PVN. b Group data showing that effects of PVN infusions of SN50 on numbers of p-IKKβ positive neurons in the PVN of HF and SHAM rats. c Group data showing that effects of PVN infusions of SN50 on NF-κB p65 activity in the PVN of HF and SHAM rats. PVN infusions of SN50M for 4 weeks did not alter NF-κB p65 activity in the PVN of HF rats (c). *P < 0.05 versus SHAM groups. † P < 0.05 HF + PVN SN50 versus HF + PVN vehicle or HF + PVN SN50M
Superoxide and NAD(P)H Oxidase in the PVN
Immunofluorescence revealed that HF rats had more superoxide in the PVN, as determined by fluorescent-labeled dihydroethidium (DHE) and the NAD(P)H oxidase subunit gp91phox, when compared with SHAM rats (Fig. 5). Bilateral PVN infusion of SN50 for 4 weeks decreased gp91phox and DHE in the PVN of HF rats (Fig. 5).
Superoxide and NAD(P)H oxidase in the PVN. a Immunofluorescence for the NAD(P)H oxidase subunit gp91phox and superoxide as determined by fluorescent-labeled dihydroethidium (DHE) in the PVN in different groups. b Comparison of gp91phox positive neurons in the PVN in different groups. c immunofluorescent intensity of DHE in the PVN of different groups of rats. *P < 0.05 versus SHAM groups. † P < 0.05 HF + PVN SN50 versus HF + PVN vehicle
PVN levels of proinflammatory cytokines
PVN levels of TNF-α, IL-1β and IL-6 were higher in HF rats than in SHAM rats. PVN levels of TNF-α, IL-1β and IL-6 were lower in HF rats that received bilateral PVN infusion of SN50 when compared with SN50M- or vehicle-treated HF rats (Table 2). Bilateral PVN infusion of SN50 prevented the increases in PVN PIC seen in HF rats.
Plasma levels of proinflammatory cytokines, epinephrine and ANGII
Humoral indicators of HF paralleled the PVN findings. Plasma levels of epinephrine, ANGII, TNF-α, IL-1β and IL-6 were all higher in HF rats than in SHAM rats. Bilateral PVN infusion of SN50 prevented the increases in plasma levels of epinephrine, ANGII, TNF-α, IL-1β and IL-6 in HF rats (Table 2; Fig. 7).
Renal sympathetic nerve activity (RSNA)
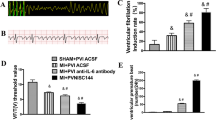
In conclusion, conscious RSNA was measured 5 h after rats recovered from anesthesia, HF rats exhibited higher RSNA (% of max) when compared with SHAM rats; bilateral PVN infusion of SN50 inhibited RSNA in HF rats (Fig. 6). Plasma NE, a marker of sympathetic activity, was also higher in HF rats than in SHAM rats. Bilateral PVN infusion of SN50 prevented the increase in plasma NE of HF rats (Fig. 7).
Renal sympathetic nerve activity (RSNA) was increased in HF rats compared with SHAM rats. Treatment with bilateral PVN infusions of SN50 for 4 weeks decreased RSNA of HF rats. PVN infusions of SN50M for 4 weeks did not alter RSNA of HF rats. *P < 0.05 versus SHAM groups. † P < 0.05 HF + PVN SN50 versus HF + PVN vehicle or HF + PVN SN50M
Plasma NE and epinephrine (EPI) were higher in HF rats than in SHAM rats. Bilateral PVN infusions of SN50 for 4 weeks prevented the increases in plasma NE and EPI of HF rats. PVN infusions of SN50M for 4 weeks did not alter plasma levels of NE and EPI in HF rats. *P < 0.05 versus SHAM groups. † P < 0.05 HF + PVN SN50 versus HF + PVN vehicle or HF + PVN SN50M
Functional/anatomical indicators of heart failure
Compared with SHAM rats, HF rats had significantly higher LVEDP, RV/BW and lung/BW ratio. SN50-treated HF rats had significantly lower LVEDP and lung/BW ratios than SN50M- or vehicle-treated HF rats (Table 3).
Discussion
The novel finding of the present study is that brain cytokines induce an imbalance between excitatory and inhibitory neurotransmitters in the PVN of HF rats, which contributes to sympathoexcitation. PVN treatment with the NF-κB inhibitor SN50 attenuated this imbalance and decreased the exaggerated sympathetic activity in HF rats. A similar dose of SN50 given peripherally did not restore the imbalance in the neurotransmitters in the PVN of HF animals, suggesting that NF-κB is activated within the PVN, and modulates neurotransmitters thereby contributing to sympathoexcitation in rats with ischemia-induced HF.
Recent evidence suggests that immune-mediated mechanisms play an important role in the pathogenesis of HF. Plasma PICs are increased in HF patients and their levels increase with the severity of HF [2, 35]. Proinflammatory cytokines, including TNF-α, IL-1β and IL-6 [5, 6, 29, 30], are released from the injured site into the circulation post-MI [27, 43]. Recent findings show that cardiac spinal afferent nerves may serve as a potential source of production of brain cytokines [14]. Regardless of the source, an overload of PIC in the brain can induce sympathoexcitation, impact cardiac function, and contribute towards the pathophysiology of cardiovascular diseases [24]. Although increased PICs were found in the brains of HF rats and were considered as contributors to exaggerated sympathetic activity [11, 14], the mechanism by which PIC exert this effect is still unclear.
The hypothalamic PVN plays an important role in the regulation of sympathetic activation [26]. Neurotransmitters (both excitatory and inhibitory) have been demonstrated to contribute to sympathetic activity, including NE, GABA and glutamate [39]. The PVN receives dense catecholaminergic innervations from the caudal medulla, a region which serves mainly as a relay site for sensory information. GABA is the principal inhibitory neurotransmitter in the PVN with inputs originating mainly from local hypothalamic sources. These inputs are thought to impart limbic and cortical influences on PVN mechanisms. Furthermore, GABA has been shown to evoke a sympatho-inhibitory response within the PVN. Microinjection of bicuculline, a GABAA receptor antagonist, into the PVN produced an increase in renal sympathetic nerve activity, indicating that normally there is a strong tonic GABA-mediated inhibition of sympathetic neuronal firing [28]. Conversely, glutamate is the dominant excitatory neurotransmitter involved in neuroendocrine regulation, where injection of glutamate into the PVN causes increases in sympathetic activity [28]. Likewise, the actions of NE mimic those of glutamate, primarily inciting an increase in sympathetic outflow. A number of afferent mediators of PVN responses, including cytokines and neuropeptides, have been identified in HF. However, little attention has been directed towards identifying the neurotransmitter systems involved in sympathetic regulation in HF.
Increased PVN activation in HF results from the interplay of several neurotransmitters and neuromodulators in these sympathetic neurons. In addition, we also recently demonstrated that PIC blockade within the CNS attenuated sympathetic activity in HF rats [8, 25]. Presently, we found that RSNA is increased in rats with HF, along with increases in the PVN levels of the excitatory neurotransmitters glutamate and NE, as well as a decrease in the levels of the inhibitory neurotransmitter GABA. The elevated basal level of NE concentration in the PVN of HF rats may reflect a marked stimulation of ascending noradrenergic drive to the PVN, or alternatively, it may be a result of impaired NE re-uptake.
NF-κB is now considered a major transcription factor regulating the expression of PIC in signal transduction pathways. Functional NF-κB complexes are present essentially in all cell types in the nervous system. In neurons, activated NF-κB is the major regulator facilitating the synthesis of several different injury-responsive cytokines including TNF-α and IL-6 [7]. In this study, PVN treatment with SN50 (a selective inhibitor of NF-κB) reduced PIC expression and NF-κB p65 activity in the PVN of HF rats, indicating that NF-κB is involved in regulating the production of PIC. The present study also demonstrates that PVN treatment with SN50 decreased plasma PICs (TNF-α, IL-1β, and IL-6), ANGII, and NE (a marker of sympathetic activity) in HF. Since we did not see any effect after a similar dose of SN50 given peripherally, we consider the reduction of plasma PIC and ANGII a direct result of decreases in both volume and pressure overload induced by sympathetic activity in the cardiovascular system; we further conclude that the reduction of plasma PIC is likely to be an indirect consequence of brain NF-κB inhibition. Thus, the present findings possibly indicate that increased inflammatory molecule-induced NF-κB activation within the PVN alters the delicate balance between excitatory and inhibitory neurotransmitters within the PVN, thereby contributing to the exaggerated sympathetic activity in HF rats.
In summary, the present study demonstrates that treatment of HF rats with PVN SN50 attenuates the HF-induced increases in PIC, glutamate, and NE, and also attenuates the HF-induced decreases in GABA, in the PVN. Elevated excitatory neurotransmitters and decreased inhibitory neurotransmitters in the PVN contribute to neurohumoral excitation in HF. SN50 could decrease the sympathoexcitation in HF by decreasing excitatory neurotransmitters and increasing inhibitory neurotransmitters in the PVN.
References
Agarwal D, Haque M, Sriramula S, Mariappan N, Pariaut R, Francis J (2009) Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension 54:1393–1400. doi:10.1161/HYPERTENSIONAHA.109.135459
Aker S, Belosjorow S, Konietzka I, Duschin A, Martin C, Heusch G, Schulz R (2003) Serum but not myocardial TNF-alpha concentration is increased in pacing-induced heart failure in rabbits. Am J Physiol Regul Integr Comp Physiol 285:463–469. doi:10.1152/ajpregu.00153.2003
Barber M, Kasturi BS, Austin ME, Patel KP, MohanKumar SM, MohanKumar PS (2003) Diabetes-induced neuroendocrine changes in rats: role of brain monoamines, insulin and leptin. Brain Res 964:128–135. doi:10.1016/S0006-8993(02)04091-X
Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, MacPherson S, Barker PA (2002) Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci 22:8466–8475. doi:0270-6474/02/228466-10
Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF (2009) TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol 104:79–89. doi:10.1007/s00395-008-0749-5
Chorianopoulos E, Heger T, Lutz M, Frank D, Bea F, Katus HA, Frey N (2010) FGF-inducible 14-kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol 105:301–313. doi:10.1007/s00395-009-0046-y
Cowling RT, Gurantz D, Peng J, Dillmann WH, Greenberg BH (2002) Transcription factor NF-kappa B is necessary for up-regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta. J Biol Chem 277:5719–5724. doi:10.1074/jbc.M107515200
Dunn AJ (2000) Cytokine activation of the HPA axis. Ann N Y Acad Sci 917:608–617. doi:10.1111/j.1749-6632.2000.tb05426.x
Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J (2009) Chronic NF-κB blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol 296:298–305. doi:10.1152/ajprenal.90628.2008
Ferrari R (1998) Tumor necrosis factor in CHF: a double facet cytokine. Cardiovasc Res 37:554–559. doi:10.1016/S0008-6363(97)00309-X
Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB (2004) Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 286:H2264–H2271. doi:10.1152/ajpheart.01072.2003
Francis J, MohanKumar SM, MohanKumar PS (2000) Correlations of norepinephrine release in the paraventricular nucleus with plasma corticosterone and leptin after systemic lipopolysaccharide: blockade by soluble IL-1 receptor. Brain Res 867:180–187. doi:10.1016/S0006-8993(00)02311-8
Francis J, Weiss RM, Johnson AK, Felder RB (2003) Central mineralocorticoid receptor blockade decreases plasma TNF-alpha after coronary artery ligation in rats. Am J Physiol Regul Integr Comp Physiol 284:R328–R335. doi:10.1152/ajpregu.00376.2002
Francis J, Zhang ZH, Weiss RM, Felder RB (2004) Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol 287:H791–H797. doi:10.1152/ajpheart.00099.2004
Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH (2005) Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288:H2271–H2279. doi:10.1152/ajpheart.00949.2004
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505. doi:10.1016/j.bcp2006.04.011
Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J (2011) Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol 106:273–286. doi:10.1007/s00395-010-0146-8
Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J (2007) TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol 293:H599–H609. doi:10.1152/ajpheart.00286.2007
Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J (2008) Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail 10:625–634. doi:10.1016/j.ejheart.2008.05.004
Kang YM, Zhang AQ, Zhao XF, Cardinale J, Elks C, Cao XM, Zhang ZW, Francis J (2011) Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol 106(3):473–483. doi:10.1007/s00395-011-0155-2
Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J (2009) Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 83:737–746. doi:10.1093/cvr/cvp160
Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J (2008) Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB. Cardiovasc Res 79:671–678. doi:10.1093/cvr/cvn119
Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J (2009) Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82:503–512. doi:10.1093/cvr/cvp073
Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB (2006) Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99:758–766. doi:10.1161/01.RES.0000244092.95152.86
Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB (2008) Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 295:H227–H236. doi:10.1152/ajpheart.01157.2007
Kenney MJ, Weiss ML, Haywood JR (2003) The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerveoutflow. Acta Physiol Scand 177(1):7–15. doi:10.1046/j.1365-201X.2003.01042.x
Kleinbongard P, Heusch G, Schulz R (2010) TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther 127:295–314. doi:10.1016/j.pharmthera.2010.05.002
Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP (2006) Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291:H2847–H2856. doi:10.1152/ajpheart.00625.2005
Lacerda L, McCarthy J, Mungly SF, Lynn EG, Sack MN, Opie LH, Lecour S (2010) TNFα protects cardiac mitochondria independently of its cell surface receptors. Basic Res Cardiol 105:751–762. doi:10.1007/s00395-010-0113-4
Li S, Zhong S, Zeng K, Luo Y, Zhang F, Sun X, Chen L (2010) Blockade of NF-kappaB by pyrrolidine dithiocarbamate attenuates myocardial inflammatory response and ventricular dysfunction following coronary microembolization induced by homologous microthrombi in rats. Basic Res Cardiol 105:139–150. doi:10.1007/s00395-009-0067-6
Lupia E, Spatola T, Cuccurullo A, Bosco O, Mariano F, Pucci A, Ramella R, Alloatti G, Montrucchio G (2010) Thrombopoietin modulates cardiac contractility in vitro and contributes to myocardial depressing activity of septic shock serum. Basic Res Cardiol 105:609–620. doi:10.1007/s00395-010-0103-6
Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH (2000) Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation 102:1854–1862. doi:10.1161/01.CIR.102.15.1854
MohanKumar SM, MohanKumar PS, Quadri SK (1998) Specificity of interleukin-1beta-induced changes in monoamine concentrations in hypothalamic nuclei: blockade by interleukin-1 receptor antagonist. Brain Res Bull 47:29–34. doi:10.1016/S0361-9230(98)00037-9
Nagura S, Sakagami T, Kakiichi A, Yoshimoto M, Miki K (2004) Acute shifts in baroreflex control of renal sympathetic nerve activity induced by REM sleep and grooming in rats. J Physiol 558:975–983. doi:10.1113/jphysiol.2004.064527
Nozaki N, Yamaguchi S, Shirakabe M, Nakamura H, Tomoike H (1997) Soluble tumor necrosis factor receptors are elevated in relation to severity of congestive heart failure. Jpn Circ J 61:657–664. doi:10.1253/jcj.61.657
Paxinos G, Watson CR, Emson PC (1987) The rat brain in stereotaxic coordinates, 2nd edn. Academic, San Diego
Schulz R, Heusch G (2009) Tumor necrosis factor-alpha and its receptors 1 and 2: Yin and Yang in myocardial infarction? Circulation 119:1355–1357. doi:10.1161/CIRCULATIONAHA.108.846105
Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G (2007) Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res 100:140–146. doi:10.1161/01.RES.0000255031.15793.86
Swanson LW, Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31:410–417
Thielmann M, Dörge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Büchert A, Krüger A, Schulz R, Heusch G (2002) Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res 90:807–813. doi:10.1161/01.RES.0000014451.75415.36
Valen G, Yan ZQ, Hansson GK (2001) Nuclear factor kappa-B and the heart. J Am Coll Cardiol 38:307–314. doi:10.1016/s0735-1097(01)01377-8
Yang LM, Hu B, Xia YH, Zhang BL, Zhao H (2008) Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res 188:84–90. doi:10.1016/j.bbr.2007.10.022
Zhang C, Wu J, Xu X, Potter BJ, Gao X (2010) Direct relationship between levels of TNF-alpha expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol 105:453–464. doi:10.1007/s00395-010-0083-6
Zhang ZH, Wei SG, Francis J, Felder RB (2003) Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284:R916–R927. doi:10.1152/ajpregu.00406.2002
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 81070199, 81170248, 81025001), U.S. National Institutes of Health (NIH) Grant RO1-HL-080544, and National Basic Research Program of China (Nos. 2012CB517805, 2007CB512106).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y.-M. Kang, F. Gao and H.-H. Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kang, YM., Gao, F., Li, HH. et al. NF-κB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic Res Cardiol 106, 1087–1097 (2011). https://doi.org/10.1007/s00395-011-0215-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-011-0215-7