Abstract
Nitric oxide (NO)-dependent soluble guanylate cyclase (sGC) activation is an important component of cardiac signal transduction pathways, including the cardioprotective signaling cascade induced by ischemic preconditioning (IPC). The sGCα subunit, which binds to the common sGCβ1 subunit, exists in two different isoforms, sGCα1 and sGCα2, but their relative physiological roles remain unknown. In the present study, we studied Langendorff-perfused isolated hearts of genetically engineered mice lacking functional sGCα1 (sGCα1KO mice), which is the predominant isoform in the heart. Our results show that the loss of sGCα1 has a positive inotropic and lusitropic effect on basal cardiac function, indicating an important role for sGCα1 in regulating basal myocardial contractility. Surprisingly, IPC led to a similar 35–40% reduction in infarct size and concomitant protein kinase Cε (PKCε) phosphorylation in both wild-type (WT) and sGCα1KO hearts subjected to 40 min of global ischemia and reperfusion. Inhibition of the activation of all sGC isoforms by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ, 10 μmol/L) completely abolished the protection by IPC in WT and sGCα1KO hearts. NO-stimulated cGMP production was severely attenuated in sGCα1KO hearts compared to WT hearts, indicating that the sGCα2 isoform only produces minute amounts of cGMP after NO stimulation. Taken together, our results indicate that although sGCα1 importantly regulates cardiac contractility, it is not required for cardioprotection by IPC. Instead, our results suggest that possibly only minimal sGC activity, which in sGCα1KO hearts is provided by the sGCα2 isoform, is sufficient to transduce the cardioprotective signal induced by IPC via phosphorylation of PKCε.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac ischemia is the leading cause of death in the world. The preferred treatment is the rapid restoration of blood flow to the myocardium, but reperfusion of the tissue is in itself responsible for significant additional tissue damage [59]. A large number of studies have confirmed that ischemic preconditioning (IPC) can significantly reduce infarct size after myocardial ischemia–reperfusion [58]. IPC was shown to have two phases of cardioprotection against ischemic injury: the initial or “early” phase of preconditioning lasting approximately 2–3 h after the IPC stimulus [36], and a second window of protection, or “delayed” preconditioning, occurring 12–24 h after the IPC stimulus and lasting for 2–3 days [32]. A similar cardioprotection is observed after ischemic postconditioning (IPostC) [61]. Studies examining the role of nitric oxide (NO) in myocardial ischemia and reperfusion have shown that endogenous NO is not only involved in the adaptation to cardiac ischemia [20, 22], but also plays a major role in cardioprotection after both IPC and IPostC [8, 21, 35, 49, 51]. Delayed preconditioning was shown to rely on both endothelial and inducible NO synthase [16, 55], while a recent study confirms that early IPC also requires endothelial NO synthase [53]. The precise mechanisms of cardioprotection, however, remain incompletely understood. Elucidation of the mechanisms responsible for cardioprotection induced by IPC and IPostC might lead to the development of new therapeutic strategies to improve the outcome of patients with ischemic heart disease [19, 40].
One of the most important cardiovascular receptors for NO is soluble guanylate cyclase (sGC) [39, 47]. sGC has been shown to be required for the infarct size reduction afforded by IPostC [43, 57], and it has been suggested that a crucial step in the trigger phase of cardioprotective IPC signaling is the stimulation of sGC activity [9, 45]. sGC-derived cGMP is believed to exert its cytoprotective effects by activating cGMP-dependent protein kinase (PKG) [15], which then directly or indirectly phosphorylates the ε isoform of protein kinase C (PKCε) [10], leading to the opening of mitochondrial ATP-sensitive K+-channels (mitoKATP) and subsequent reactive oxygen species production [2, 18, 38, 44]. This ultimately leads to inhibition of the mitochondrial permeability transition at reperfusion, which protects mitochondria from rupturing and prevents cardiomyocyte death [11, 35].
In addition, some of the effects of NO on the regulation of cardiac contractility have also been proposed to be mediated by sGC [27]. It has been suggested that sGC, via a PKG-dependent mechanism, can inhibit the influx of Ca2+ into cardiomyocytes [1] and attenuate the myofilament sensitivity to Ca2+ [30]. cGMP can also stimulate phosphodiesterase 2 activity, which counteracts cAMP generation and in this way blunts positive inotropic signaling [34].
sGC is a heterodimeric enzyme, consisting of an α and a β subunit, which exist in different isoforms. sGCα1 and sGCα2, bound to the common β1 subunit, have been characterized as the catalytically active forms. The sGCα1 subunit has been shown to be the most abundant isoform in most tissues, including the heart [3, 33]. However, the relative physiological and pathophysiological importance of sGCα1 versus sGCα2 signaling has not yet been revealed. We have previously described the generation of genetically modified mice deficient in functional sGCα1 (sGCα1KO mice) [5, 6] and found that male sGCα1KO mice on a 129/S6 genetic background are hypertensive, although this phenotype is lost after backcrossing the mutation to the C57BL/6J background. Interestingly, 129/S6 sGCα1KO mice also display an enhanced cardiac contractility phenotype.
In this study, we used both sGCα1KO mice as well as total pharmacological sGC inhibition to elucidate the role of sGC and its isoforms in the signal transduction mechanisms responsible for the early phase of cardioprotection afforded by IPC.
Materials and methods
Mice
All animal procedures were performed in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996) and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Mice lacking a functional sGCα1 subunit (sGCα1KO mice) were generated previously [6] and were backcrossed for eight generations on the C57BL/6J genetic background. Wild-type (WT) mice on the C57BL/6J background were obtained from the Jackson Laboratory (Bar Harbor, ME). Male mice, matched for age (range 2–4 months) and body weight (range 23–30 g), were used throughout the study.
Langendorff preparation
Mice were administered 200 U heparin by intraperitoneal injection and anesthetized with 50 mg/kg pentobarbital. The heart was excised and quickly transferred to ice-cold perfusion buffer (a modified Krebs-Henseleit buffer, containing, in mmol/L: NaCl 118.5; NaHCO3 25; glucose 11; KCl 4; MgSO4 1.2; KH2PO4 1.2; pyruvate 1; CaCl2 1.8; gassed and equilibrated with 95% O2 and 5% CO2 at 37°C). The aorta was cannulated immediately, and the heart was mounted on the Langendorff apparatus and perfused at a constant pressure of 70 mmHg. Hearts were paced at 7 Hz. The left ventricular (LV) diastolic pressure was initially set at 5–10 mmHg using a fluid-filled balloon inserted into the LV, which also contained the tip of a Millar SPR-671 pressure transducer (ADInstruments, Colorado Springs, CO). Coronary flow rate was measured using an N1 in-line flow probe and a T106 flow meter (Transonic Systems, Ithaca, NY). Coronary flow rate and LV pressure were constantly measured, and heart rate, LV developed pressure (LVDevP), and maximum and minimum rate of LV pressure change (dP/dt max and dP/dt min, respectively) were calculated from the LV pressure signal using a Powerlab 8/30 data acquisition system and Chart Pro software (ADInstruments, Colorado Springs, CO).
Experimental protocols
After a stabilization period of approximately 20 min perfusion on the Langendorff system, hearts were subjected to a preconditioning protocol of four cycles of 5 min each of global ischemia followed by 5 min of reperfusion. Hearts were then subjected to 40 min of global ischemia, followed by 60 min of reperfusion. Hearts were then removed from the Langendorff apparatus and their infarct size was determined as described below. Control hearts were subjected to the 40-min global ischemia and subsequent reperfusion without preconditioning. In a separate group of experiments, hearts were frozen in liquid nitrogen after 20 min of reperfusion after the prolonged ischemia for immunoblot analysis. For a subgroup of hearts, the perfusion buffer was supplemented with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ, Cayman Chemical, Ann Arbor, MI) at a final concentration of 10 μmol/L, a dose that was previously shown to inhibit all sGC activation [23, 46].
In a separate set of experiments, after stabilization on the Langendorff setup, the NO donor compound diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA/NO, Cayman Chemical, Ann Arbor, MI) or its vehicle was infused into the perfusion buffer. Hearts were frozen in liquid nitrogen immediately after 90 s of infusion of DEA/NO (100 μmol/L) or vehicle.
Infarct size measurements
At the end of reperfusion, hearts were removed from the Langendorff apparatus, and 1 mm cardiac slices were stained with 2,3,5-triphenyltetrazolium chloride (1% wt/vol) as described previously [37]. Computer-assisted planimetry (NIH ImageJ 1.43 h) was used to quantify the myocardial infarction size as a percentage of total myocardial area.
Western blot
Cardiomyocytes were obtained from adult mouse hearts as described previously [24]. Using a Dounce tissue grinder, the isolated cardiomyocytes were then homogenized in buffer containing, in mmol/L: sucrose 250, EDTA 0.1, Tris–HCl (pH 7.4) 50, dithiotreitol 1, and protease inhibitors. Frozen whole hearts were homogenized in buffer containing, in mmol/L: sucrose 250, EDTA 1, Tris–HCl (pH 7.4) 10, and phosphatase and protease inhibitors. After clearing the homogenates by ultracentrifugation at 100,000g, protein concentration in the supernatant was measured with the BCA protein assay (Thermo Scientific, Rockford, IL). Protein samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. The blots were then probed with primary antibodies against sGCα1 (1:3,000; Abcam, Cambridge, MA), sGCα2 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), phosphoPKCε (Ser729) (1:5,000; Upstate, Lake Placid, NY), total PKCε (1:10,000; Millipore, Temecula, CA), and β-tubulin (1:20,000; Cell Signaling Technology, Danvers, MA). To assess the specificity of the sGCα2 antibody, a parallel experiment was performed where the antibody was first preincubated for 1 h at room temperature with a fivefold excess of the blocking peptide (Santa Cruz Biotechnology, Santa Cruz, CA). Next, the membranes were incubated with the appropriate secondary antibody coupled to HRP (for sGCα2: anti-goat, 1:100,000; Santa Cruz Biotechnology, Santa Cruz, CA; for all others: anti-rabbit, 1:50,000; Cell Signaling Technology, Danvers, MA), developed with the ECL Advance chemiluminescence kit (GE Healthcare, Piscataway, NJ) and exposed to X-ray film. The film was then scanned and densitometric analysis was performed using NIH ImageJ 1.43 h. Experiments were repeated at least twice for each sample. Phospho-PKCε levels are presented as the ratio of the level of the phosphorylated protein to total protein, normalized to the respective values of control samples without IPC.
cGMP measurements
Frozen cardiac tissue was homogenized in 1 mL ice-cold 10% trichloroacetic acid. The protein pellet obtained after centrifugation was saved to measure original protein concentration in the sample using the BCA protein assay. The supernatants were extracted with water-saturated ether and dried by vacuum centrifugation. After resuspending the samples in assay buffer, cGMP concentration was determined using an enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s recommendations. The obtained results were normalized to the protein concentration in the original sample.
Statistics
All data are presented as mean ± SEM. Statistical differences among groups were evaluated using the appropriate test as indicated in the text. A value of P < 0.05 was considered as significant.
Results
Both sGCα isoforms are expressed in cardiomyocytes
Western blot analysis of homogenates of perfused whole hearts and isolated cardiomyocytes demonstrated the expression of both the sGCα1 and sGCα2 isoform in WT samples (Fig. 1). In samples from sGCα1KO mice, the sGCα1-specific band was absent, while sGCα2 was still detectable. Densitometric analysis of sGCα2 levels showed that there were no significant differences between WT and sGCα1KO samples.
Immunoblot analysis of the sGCα1 and sGCα2 isoforms, as well as tubulin in whole hearts and cardiomyocytes of WT and sGCα1KO mice. a Representative immunoblots of sGC isoforms in homogenates of isolated cardiomyocytes (CM) and perfused whole hearts (heart). The sGCα2-immunoreactive band was blocked by the corresponding immunization peptide, confirming the specificity of the immunostaining. b Quantitative analysis of sGCα2 expression levels in WT (N = 4) and sGCα1KO (N = 5) samples, normalized to the respective WT samples. No significant differences were found between groups
sGCα1 deficiency enhances LV contractility at baseline
Measurements of the functional parameters of Langendorff-perfused hearts, after stabilization and before ischemia or other experimental interventions, indicated an altered basal cardiac function in sGCα1KO hearts as compared to WT hearts. Both LVDevP and dP/dt max were higher in isolated sGCα1KO hearts than in WT hearts (Fig. 2a, b), demonstrating an increase in contractile activity and inotropy in hearts lacking functional sGCα1. Pharmacological inhibition of sGC also significantly increased inotropy as evidenced by elevated LVDevP and dP/dt max in hearts perfused with 10 μmol/L ODQ. In addition, both the genetic ablation of sGCα1 and sGC inhibition had a positive lusitropic effect, as measured by a more negative dP/dt min (Fig. 2c). However, coronary flow rate was similar in all groups, demonstrating that the changes in contractility are not secondary to an increase in substrate or oxygen availability to the heart (Fig. 2d).
Basal functional parameters in isolated hearts from male WT (N = 49), sGCα1KO (N = 32), and WT mice treated with 10 μmol/L ODQ (N = 40). a LV developed pressure (LVDevP); b maximum and c minimum rate of LV pressure change (dP/dt max and dP/dt min, respectively); d coronary flow rate. **P < 0.01 and ***P < 0.001 versus WT by Bonferroni’s post hoc test after one-way ANOVA
Ischemia–reperfusion and cardioprotection by IPC has similar effects in isolated hearts from both wild-type and sGCα1KO mice
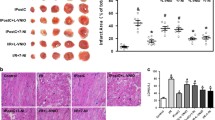
Infarct sizes measured after 40 min of global ischemia and 1 h of reperfusion were comparable between both genotypes (41 ± 4% in WT and 40 ± 2% in sGCα1KO). Interestingly, IPC markedly reduced infarct size to a similar extent in both genotypes (26 ± 2% in WT and 24 ± 2% in sGCα1KO; for both: P < 0.01 versus control without IPC, by Bonferroni’s post hoc test after two-way ANOVA) (Fig. 3). Although IPC improved contractile function after 40 min of global ischemia and 20 min of reperfusion, there was no significant difference in functional parameters within or between genotypes (Supplementary Table 1).
Infarct size measured after 40 min of global ischemia and 1 h of reperfusion with (IPC) or without (Control I/R) ischemic preconditioning. N = 6–8 per group; **P < 0.01 versus control I/R without IPC and ## P < 0.01 versus respective group without ODQ by Bonferroni’s post hoc test after two-way ANOVA
Total sGC inhibition abolishes the cardioprotective effect of IPC
In a subgroup of hearts, ODQ (10 μmol/L) was added to the perfusion buffer to inhibit the activation of all sGC isoforms [23, 46] (Fig. 3). Although ODQ administration did not have an effect on the infarct size of control WT hearts without IPC, it completely abolished the ability of IPC to reduce infarct size in WT hearts (WT control + ODQ: 42 ± 4%; WT IPC + ODQ: 42 ± 3%). ODQ administration had a similar effect on sGCα1KO hearts, leading to inhibition of IPC-induced cardioprotection in these hearts as well (infarct sizes: sGCα1KO control + ODQ: 40 ± 4%; sGCα1KO IPC + ODQ: 44 ± 3%).
Protection by IPC is associated with sGC-dependent PKCε phosphorylation
Serine residue 729 (Ser729) phosphorylation of PKCε was investigated by immunoblot after 40 min of ischemia and 20 min of reperfusion (Fig. 4). Both in WT and sGCα1KO hearts, the level of PKCε phosphorylation was increased after IPC, as compared to control hearts that underwent ischemia and reperfusion without IPC. However, in WT hearts that were perfused with ODQ, IPC did not increase PKCε Ser729 phosphorylation.
Western blots showing the phosphorylation of the Ser729 residue on PKCε after IPC in WT and sGCα1KO hearts. a Representative blots are shown for Ser729 phosphorylated PKCε (p-PKCε), total PKCε, and tubulin. Control samples subjected to 40 min ischemia and 20 min of reperfusion without IPC; IPC samples subjected to ischemia and reperfusion with IPC. b Graph showing the average ratio of the relative intensity of Ser729 phosphorylated PKCε to total PKCε (N = 4–6 samples per group). *P < 0.05 versus respective control by unpaired t test
cGMP production after NO stimulation is severely attenuated in sGCα1KO hearts
To examine the relative contribution of both sGCα isoforms to cardiac cGMP production, we stimulated isolated WT and sGCα1KO hearts with a high dose (100 μmol/L) of the NO donor compound DEA/NO or vehicle and measured the cGMP content in tissue homogenates. In WT hearts, we observed a 72-fold increase in cGMP concentration in NO-stimulated samples versus control samples, while we only found a sevenfold increase in sGCα1KO hearts (Fig. 5). Similarly, we found a significant increase in cGMP secretion in the effluent of WT hearts stimulated with 1 μmol/L DEA/NO, while we found no significant NO-dependent increases in sGCα1KO hearts or hearts treated with ODQ (Supplementary Fig. 1).
Discussion
The data presented here describe the phenotype of isolated sGCα1KO hearts perfused in the Langendorff mode and subjected to ischemia and reperfusion with or without IPC. Firstly, we confirmed the presence of both the sGCα1 and sGCα2 isoform in both whole hearts and isolated cardiomyocytes, and demonstrated the absence of the former while the latter remained unchanged in sGCα1KO tissue. We found that sGCα1KO hearts exhibited a higher basal contractility than WT hearts, but had a similar infarct size after ischemia and reperfusion as WT hearts. Moreover, IPC had a similar protective effect in both WT and sGCα1KO hearts, as demonstrated by a comparable reduction of infarct size after ischemia and reperfusion, whereas inhibition of all sGC isoforms abolished cardioprotection afforded by IPC. The IPC-induced cardioprotection was shown to correlate with phosphorylation of PKCε at the Ser729 residue. Finally, we demonstrated that sGCα1 deficiency severely attenuated the NO-induced cGMP production in the heart. These observations underscore the important role of the sGCα1 isoform in the regulation of basal contractility. Our results also confirm its role as the predominant cardiac isoform responsible for the bulk of cGMP production, while surprisingly demonstrating that it is not required for cardioprotection. Instead, our results suggest that in sGCα1KO hearts, the sGCα2 isoform, which only produces low amounts of cGMP after NO stimulation, is sufficient to transduce the IPC-induced cardioprotective signal to PKCε.
To the best of our knowledge, this is the first study to examine contractile function and IPC in hearts of sGCα1KO mice using the Langendorff setup. We observed that sGCα1 deficiency led to enhanced inotropy (higher LVDevP and dP/dt max) and lusitropy (more negative dP/dt min) in the heart, an effect that was at least partly mimicked by acute pharmacological inhibition of sGC by ODQ. Of note, we previously observed an increased cardiac contractile phenotype in vivo as well in sGCα1KO mice, albeit on the 129/S6 genetic background [6]. This increased cardiac contractility was, however, absent in sGCα1KO mice on a C57BL/6J background [5]. Nonetheless, the results presented here suggest that the higher contractility in sGCα1KO mice at baseline is caused by an inherent cardiac phenotype, which might be masked in the sGCα1KO mice on the C57BL/6J background in vivo due to the influence of circulating neurohumoral factors. Furthermore, our data are in line with other studies suggesting a negative inotropic effect of sGC-derived cGMP in the myocardium [42, 54]. The absence of an effect of ODQ on basal coronary flow, as previously observed in another study using a similar isolated heart perfusion setup [14], most likely reflects the negligible contribution of basal vascular NO-mediated sGC activation in this model.
Several mechanisms have been proposed to be involved in the effects of cGMP on cardiac contractility. One possibility is the PKG-dependent phosphorylation of the L-type Ca2+ channel [48, 56], leading to decreased Ca2+ influx into the cardiomyocyte. PKG activation can also increase the phosphorylation of troponin I, thereby decreasing the myofilament sensitivity to Ca2+ [30, 50], although this does not explain the lusitropic effect we observed. Alternatively, the PKG-independent activation of cAMP-catabolizing phosphodiesterase might be responsible for the negative inotropic and lusitropic effects of cGMP [60]. Decreased activation of this phosphodiesterase in sGCα1KO hearts may increase cAMP-dependent protein kinase (PKA) activity, leading to the phosphorylation of phospholamban and troponin I [31], which would increase both inotropy and lusitropy. This effect of PKA is supported by a recent study showing that disruption of PKA localization and activity leads to a decrease in inotropy and lusitropy in isolated perfused hearts [41].
Importantly, our data demonstrate that the α1 isoform of sGC is not required for the infarct size reduction afforded by IPC in the heart. However, administration of ODQ, which has previously been shown to inhibit both sGC isoforms [46], to the perfusion buffer, abolished the cardioprotective effects of IPC. This finding underscores the key role of sGC in IPC signaling, and is consistent with previous studies indicating a role for sGC in cardioprotection [17, 43, 57]. Furthermore, the observation that ODQ inhibits IPC-induced cardioprotection in sGCα1KO hearts also suggests that the sGCα2 isoform is responsible for mediating the cardioprotective signal transduction pathways initiated by IPC in sGCα1KO hearts.
Moreover, our finding that ODQ has similar effects on cardioprotection in WT and sGCα1KO hearts demonstrates that there is no compensatory upregulation of alternative, non-sGC-dependent cardioprotective pathways in the sGCα1KO hearts. Nevertheless, our data do not contradict other studies that indicate a cardioprotective role for non-sGC-dependent effects of NO (e.g., S-nitrosylation [51]) or non-sGC-derived cGMP (e.g., B-type natriuretic peptide signaling [4]). It is likely that these alternative pathways play a role in another phase of cardioprotective signaling. Indeed, Cohen et al. [9] recently proposed that an sGC-independent signaling step is activated in the mediator phase of cardioprotection, while sGC is suggested to play a role in the trigger phase of IPC, and another group observed that natriuretic peptide-induced cardioprotection still requires sGC activation [12].
In this study, we also show that phosphorylation of the Ser729 residue of PKCε is correlated with cardioprotection by IPC, confirming that this kinase is an essential component of the IPC signaling cascade downstream of sGC [10]. Our results imply that cGMP produced by sGCα2 in sGCα1KO hearts is sufficient to activate downstream mechanisms that increase PKCε phosphorylation, leading to opening of mitoKATP and other subsequent signaling pathways that confer protection from cardiac ischemia–reperfusion injury [8, 11, 38, 45]. In WT hearts, the cGMP that mediates the cardioprotective signal might be derived from either sGCα1 or sGCα2.
Interestingly, administration of a high dose of an NO donor stimulated cGMP production to a significantly higher level in WT hearts than in sGCα1KO hearts (approximately, 70-fold vs. 7-fold increase as compared to vehicle control, respectively). Similarly, a lower dose of the NO donor significantly increased cGMP release from WT hearts, but not from sGCα1KO hearts. These results demonstrate that sGCα1 is the predominant isoform in the heart responsible for the bulk of cGMP production, and that sGCα2 activity is still detectable in sGCα1KO hearts, albeit after administration of a high dose of exogenous NO. These observations are consistent with our previously published results showing that left ventricular extracts from sGCα1KO mice display a severely attenuated NO-induced increase in sGC activity [6]. Nevertheless, inhibition of all sGC isoforms by ODQ, but not loss of the sGCα1 subunit, impaired the cardioprotection by IPC, suggesting that the sGCα2 isoform can be of functional importance despite producing very low amounts of cGMP.
In the current study, we did not detect upregulation of the sGCα2 subunit in whole heart or isolated cardiomyocyte samples of sGCα1KO mice, confirming our observations in other tissue types we examined previously [5, 6]. This supports our hypothesis that only very small amounts of sGC-derived cGMP, as provided by sGCα2 in the sGCα1KO hearts, are sufficient to transduce an NO-dependent signal in IPC. It is possible that sGCα2 has a more restricted subcellular localization than sGCα1, leading to sGC isoform-specific compartmentation of NO-dependent cGMP signaling. Activation of sGCα2 (or sGCα1) in this theoretical compartment might be sufficient to transduce the cardioprotective signal induced by IPC. In support of this hypothesis, localized pools of cGMP with differing biological effects have already been shown to exist in cardiomyocytes [7], explaining why cGMP derived from sGC can have different effects than cGMP produced by particulate guanylate cyclase (e.g., cGMP produced by sGC specifically blunts the cardiac contractile response to β-adrenergic signaling [52], while cGMP derived from particulate guanylate cyclase only modulates the contractile response to angiotensin II [25]). Definitive confirmation of the specific role of sGCα2 in IPC, however, will require further investigation of genetically modified mice lacking a functional sGCα2 isoform.
Taken together, our data confirm that cardioprotection afforded by IPC is mediated by sGC. However, our study surprisingly shows that the major cardiac isoform, sGCα1, is not required for this effect. Instead, our results suggest that the much less prominent sGCα2 isoform is sufficient to mediate the IPC-induced cardioprotective signaling cascade in sGCα1KO mice. Since several recent studies showed the ability of sGC stimulators, which activate both sGCα isoforms [13, 26], to protect the heart from ischemic injury [28, 29], it might be of clinical value to develop sGCα2-specific stimulators. It is conceivable that inducing only very low levels of sGC activity, such as via specific stimulation of the sGCα2 isoform, might be sufficient to protect the heart. This might have therapeutical benefits over non-specific stimulation of both sGC isoforms, which can be expected to have negative inotropic and lusitropic effects due to the higher levels of cGMP produced by the sGCα1 isoform.
References
Abi-Gerges N, Fischmeister R, Mery PF (2001) G protein-mediated inhibitory effect of a nitric oxide donor on the L-type Ca2+ current in rat ventricular myocytes. J Physiol 531(Pt 1):117–130. doi:10.1111/j.1469-7793.2001.0117j.x
Andrukhiv A, Costa AD, West IC, Garlid KD (2006) Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol 291(5):H2067–H2074. doi:10.1152/ajpheart.00272.2006
Budworth J, Meillerais S, Charles I, Powell K (1999) Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun 263(3):696–701. doi:10.1006/bbrc.1999.1444
Burley D, Baxter G (2007) B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic Res Cardiol 102(6):529–541. doi:10.1007/s00395-007-0672-1
Buys ES, Cauwels A, Raher MJ, Passeri JJ, Hobai I, Cawley SM, Rauwerdink KM, Thibault H, Sips PY, Thoonen R, Scherrer-Crosbie M, Ichinose F, Brouckaert P, Bloch KD (2009) sGC{alpha}1{beta}1 attenuates cardiac dysfunction and mortality in murine inflammatory shock models. Am J Physiol Heart Circ Physiol 297(2):H654–H663. doi:10.1152/ajpheart.00367.2009
Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P (2008) Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res 79(1):179–186. doi:10.1093/cvr/cvn068
Castro LR, Verde I, Cooper DM, Fischmeister R (2006) Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113(18):2221–2228. doi:10.1161/circulationaha.105.599241
Cohen MV, Yang XM, Downey JM (2006) Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70(2):231–239. doi:10.1016/j.cardiores.2005.10.021
Cohen MV, Yang XM, Liu Y, Solenkova NV, Downey JM (2010) Cardioprotective PKG-independent NO signaling at reperfusion. Am J Physiol Heart Circ Physiol 299(6):H2028–H2036. doi:10.1152/ajpheart.00527.2010
Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD (2005) Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97(4):329–336. doi:10.1161/01.res.0000178451.08719.5b
Costa ADT, Pierre SV, Cohen MV, Downey JM, Garlid KD (2008) cGMP signaling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res 77(2):344–352. doi:10.1093/cvr/cvm050
D’Souza SP, Davis M, Baxter GF (2004) Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther 101(2):113–129. doi:10.1016/j.pharmthera.2003.11.001
Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HHHW, Stasch J-P (2006) NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5(9):755–768. doi:10.1038/nrd2038
Favaloro JL, Kemp-Harper BK (2007) The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res 73(3):587–596. doi:10.1016/j.cardiores.2006.11.018
Gorbe A, Giricz Z, Szunyog A, Csont T, Burley DS, Baxter GF, Ferdinandy P (2010) Role of cGMP-PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation. Basic Res Cardiol 105(5):643–650. doi:10.1007/s00395-010-0097-0
Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R (1999) The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA 96(20):11507–11512. doi:10.1073/pnas.96.20.11507
Hamid SA, Totzeck M, Drexhage C, Thompson I, Fowkes RC, Rassaf T, Baxter GF (2010) Nitric oxide/cGMP signaling mediates the cardioprotective action of adrenomedullin in reperfused myocardium. Basic Res Cardiol 105(2):257–266. doi:10.1007/s00395-009-0058-7
Hausenloy D, Wynne A, Yellon D (2007) Ischemic preconditioning targets the reperfusion phase. Basic Res Cardiol 102(5):445–452. doi:10.1007/s00395-007-0656-1
Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM (2010) Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol 105(6):677–686. doi:10.1007/s00395-010-0121-4
Heinzel FR, Gres P, Boengler K, Duschin A, Konietzka I, Rassaf T, Snedovskaya J, Meyer S, Skyschally A, Kelm M, Heusch G, Schulz R (2008) Inducible nitric oxide synthase expression and cardiomyocyte dysfunction during sustained moderate ischemia in pigs. Circ Res 103(10):1120–1127. doi:10.1161/circresaha.108.186015
Heusch G, Boengler K, Schulz R (2008) Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation 118(19):1915–1919. doi:10.1161/circulationaha.108.805242
Heusch G, Post H, Michel MC, Kelm M, Schulz R (2000) Endogenous nitric oxide and myocardial adaptation to ischemia. Circ Res 87(2):146–152
Hoenicka M, Becker EM, Apeler H, Sirichoke T, Schroder H, Gerzer R, Stasch JP (1999) Purified soluble guanylyl cyclase expressed in a baculovirus/Sf9 system: stimulation by YC-1, nitric oxide, and carbon monoxide. J Mol Med 77(1):14–23. doi:10.1007/s001090050292
Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD (2007) Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res 100(1):130–139. doi:10.1161/01.res.0000253888.09574.7a
Klaiber M, Kruse M, Volker K, Schroter J, Feil R, Freichel M, Gerling A, Feil S, Dietrich A, Londono JE, Baba HA, Abramowitz J, Birnbaumer L, Penninger JM, Pongs O, Kuhn M (2010) Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol 105(5):583–595. doi:10.1007/s00395-010-0098-z
Koglin M, Stasch JP, Behrends S (2002) BAY 41–2272 activates two isoforms of nitric oxide-sensitive guanylyl cyclase. Biochem Biophys Res Commun 292(4):1057–1062. doi:10.1006/bbrc.2002.6764
Kojda G, Kottenberg K (1999) Regulation of basal myocardial function by NO. Cardiovasc Res 41(3):514–523. doi:10.1016/S0008-6363(98)00314-9
Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, Veres G, Pali S, Seidel B, Zollner S, Karck M, Szabo G (2009) Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation 120(8):677–686. doi:10.1161/circulationaha.109.870774
Krieg T, Liu Y, Rutz T, Methner C, Yang XM, Dost T, Felix SB, Stasch JP, Cohen MV, Downey JM (2009) BAY 58–2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically post-conditions rabbit and rat hearts. Eur Heart J 30(13):1607–1613. doi:10.1093/eurheartj/ehp143
Layland J, Li JM, Shah AM (2002) Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol 540(Pt 2):457–467. doi:10.1113/jphysiol.2001.014126
Leineweber K, Bohm M, Heusch G (2006) Cyclic adenosine monophosphate in acute myocardial infarction with heart failure: slayer or savior? Circulation 114(5):365–367. doi:10.1161/circulationaha.106.642132
Marber MS, Latchman DS, Walker JM, Yellon DM (1993) Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88(3):1264–1272
Mergia E, Russwurm M, Zoidl G, Koesling D (2003) Major occurrence of the new alpha(2)beta(1) isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal 15(2):189–195. doi:10.1016/S0898-6568(02)00078-5
Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M (2006) Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98(2):226–234. doi:10.1161/01.res.0000200178.34179.93
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia–reperfusion injury. Physiol Rev 88(2):581–609. doi:10.1152/physrev.00024.2007
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74(5):1124–1136
Nagasaka Y, Fernandez BO, Garcia-Saura MF, Petersen B, Ichinose F, Bloch KD, Feelisch M, Zapol WM (2008) Brief periods of nitric oxide inhalation protect against myocardial ischemia–reperfusion injury. Anesthesiology 109(4):675–682. doi:10.1097/aln.0b013e318186316e
Oldenburg O, Qin Q, Krieg T, Yang X-M, Philipp S, Critz SD, Cohen MV, Downey JM (2004) Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol 286(1):H468–H476. doi:10.1152/ajpheart.00360.2003
Oppermann M, Suvorava T, Freudenberger T, Dao VT, Fischer JW, Weber M, Kojda G (2011) Regulation of vascular guanylyl cyclase by endothelial nitric oxide-dependent posttranslational modification. Basic Res Cardiol. doi:10.1007/s00395-011-0160-5
Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R (2010) Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 87(3):406–423. doi:10.1093/cvr/cvq129
Patel HH, Hamuro LL, Chun BJ, Kawaraguchi Y, Quick A, Rebolledo B, Pennypacker J, Thurston J, Rodriguez-Pinto N, Self C, Olson G, Insel PA, Giles WR, Taylor SS, Roth DM (2010) Disruption of protein kinase A localization using a trans-activator of transcription (TAT)-conjugated A-kinase-anchoring peptide reduces cardiac function. J Biol Chem 285(36):27632–27640. doi:10.1074/jbc.M110.146589
Pellegrino D, Shiva S, Angelone T, Gladwin MT, Tota B (2009) Nitrite exerts potent negative inotropy in the isolated heart via eNOS-independent nitric oxide generation and cGMP-PKG pathway activation. Biochim Biophys Acta 1787(7):818–827. doi:10.1016/j.bbabio.2009.02.007
Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, Losano G, Pagliaro P (2006) Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol 101(2):168–179. doi:10.1007/s00395-005-0543-6
Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, Losano G, Pagliaro P (2006) Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol 101(2):180–189. doi:10.1007/s00395-006-0584-5
Qin Q, Yang X-M, Cui L, Critz SD, Cohen MV, Browner NC, Lincoln TM, Downey JM (2004) Exogenous NO triggers preconditioning via a cGMP- and mitoKATP-dependent mechanism. Am J Physiol Heart Circ Physiol 287(2):H712–H718. doi:10.1152/ajpheart.00954.2003
Russwurm M, Behrends S, Harteneck C, Koesling D (1998) Functional properties of a naturally occurring isoform of soluble guanylyl cyclase. Biochem J 335(Pt 1):125–130
Russwurm M, Koesling D (2004) NO activation of guanylyl cyclase. EMBO J 23(22):4443–4450. doi:10.1038/sj.emboj.7600422
Schroeder F, Klein G, Fiedler B, Bastein M, Schnasse N, Hillmer A, Ames S, Gambaryan S, Drexler H, Walter U, Lohmann SM, Wollert KC (2003) Single L-type Ca2+ channel regulation by cGMP-dependent protein kinase type I in adult cardiomyocytes from PKG I transgenic mice. Cardiovasc Res 60(2):268–277. doi:10.1016/s0008-6363(03)00546-7
Schulz R, Kelm M, Heusch G (2004) Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res 61(3):402–413. doi:10.1016/j.cardiores.2003.09.019
Su J, Scholz PM, Weiss HR (2005) Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood) 230(4):242–250
Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E (2007) Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101(11):1155–1163. doi:10.1161/circresaha.107.155879
Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA (2007) Compartmentalization of cardiac {beta}-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation 115(16):2159–2167. doi:10.1161/circulationaha.106.643536
Talukder MAH, Yang F, Shimokawa H, Zweier JL (2010) eNOS is required for acute in vivo ischemic preconditioning of the heart: effects of ischemic duration and sex. Am J Physiol Heart Circ Physiol 299(2):H437–H445. doi:10.1152/ajpheart.00384.2010
Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F, Feil R (2002) cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res 90(1):18–20. doi:10.1161/hh0102.103222
Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R (2007) Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription 1/3 pathway. Circulation 116(5):535–544. doi:10.1161/circulationaha.107.689471
Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO (2007) Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res 101(5):465–474. doi:10.1161/circresaha.107.156976
Yang X-M, Philipp S, Downey JM, Cohen MV (2005) Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol 100(1):57–63. doi:10.1007/s00395-004-0498-4
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83(4):1113–1151. doi:10.1152/physrev.00009.2003
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357(11):1121–1135. doi:10.1056/NEJMra071667
Zaccolo M (2006) Phosphodiesterases and compartmentalized cAMP signaling in the heart. Eur J Cell Biol 85(7):693–697. doi:10.1016/j.ejcb.2006.01.002
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285(2):H579–H588. doi:10.1152/ajpheart.01064.2002
Acknowledgments
This work was supported by the Resuscitation Fellowship Award from the American Heart Association and Philips (P.Y.Sips) and by National Institute of Health grant GM079360 (F.Ichinose). The authors would like to thank Kentaro Tokuda for technical assistance in harvesting isolated cardiomyocytes.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sips, P.Y., Brouckaert, P. & Ichinose, F. The alpha1 isoform of soluble guanylate cyclase regulates cardiac contractility but is not required for ischemic preconditioning. Basic Res Cardiol 106, 635–643 (2011). https://doi.org/10.1007/s00395-011-0167-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-011-0167-y