Abstract
Endothelial progenitor cells (EPCs) contribute to the process of reendothelialization and prevent neointimal formation after vascular injury. The present study was designed to investigate whether the cysteine-rich 61 (CYR61, CCN1), an important matricellular component of local vascular microenvironment, has effect on EPCs differentiation and reendothelialization in response to vascular injury in rat. Following balloon injury, CCN1 was rapidly induced and dynamically changed at vascular lesions. Overexpression of CCN1 by adenovirus (Ad-CCN1) accelerated reendothelialization and inhibited neointimal formation in the early phase (day 14) after vascular injury (p < 0.05), while no effect was shown on day 21. Ad-CCN1 treatment increased the adhering EPCs on the surface of injured vessels on day 7, and the ratio of GFP- and vWF-positive area to the total luminal length on day 14 was 2.3-fold higher in the Ad-CCN1-EPC-transplanted group than in controls. Consistent with these findings, CCN1-stimulated EPC differentiation in vitro and 20 genes were found differentially expressed during CCN1-induced EPC differentiation, including Id1, Vegf-b, Vegf-c, Kdr, Igf-1, Ereg, Tgf, Mdk, Ptn, Timp2, etc. Among them, negative transcriptional regulator Id1 was associated with CCN1 effect on EPC differentiation. Our data suggest that CCN1, from the microenvironment of injured vessels, enhances reendothelialization via a direct action on EPC differentiation, revealing a possible new mechanism underlying the process of vascular repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelial cell (EC) damage is an important pathophysiological step of atherosclerosis and restenosis following percutaneous coronary intervention (PCI) such as angioplasty and stenting [31]. Accelerated reendothelialization effectively inhibits smooth muscle cell (SMC) migration, proliferation, and resulting neointimal formation, and is therefore of special interest with regard to prevention of the early stages of atherosclerosis and restenosis [19]. EPCs, which can differentiate into mature ECs, are increasingly recognized to play a key role in the maintenance of vascular integrity and to act as “repair” cells in response to endothelial injury [20, 36, 37, 39]. Transfusion of EPCs to balloon-injured arteries leads to accelerated reendothelialization and reduced neointimal formation after vascular injury. However, the underlying molecular mechanisms, especially in the microenvironment of the injured vessel wall that regulates EPC differentiation and reendothelialization during the vascular repair process remain poorly understood.
Cysteine-rich 61 (CYR61, CCN1), a secreted matricellular protein belonging to the CCN family, is expressed by all types of vascular cells [1]. Accumulating evidences indicate that CCN1 is required for vascular development and is a potent regulator of angiogenesis. CCN1-deficient mice exhibit embryonic lethality as a result of placental vascular insufficiency and compromised vessel integrity [30]. CCN1 induces angiogenesis both in vitro and in vivo and has been implicated in pathophysiology contexts in which angiogenesis and vascularization play important roles, such as tumorigenesis, cutaneous wound healing [3], bone fracture repair [15], and liver regeneration [34]. Moreover, it is noteworthy that expression of CCN1 is associated with vascular disorders, such as atherosclerosis [19], mechanical injury [14], and hypertension [38]. However, whether and what impact CCN1 may play on EPC-mediated vascular repair is largely unknown at present.
Interestingly, recent evidence showed that CCN1 plays an important role in the migration and differentiation of bone marrow-derived mesenchymal stem cells (MSCs) [35]. MSCs, a population of pluripotent progenitors, are capable of accelerating repair of EC damage in adult blood vessels as well as EPCs. Furthermore, CCN1, in the plasma and endothelium surface, promotes the recruitment of circulating CD34+ progenitor cells to the endothelial monolayer, trans-endothelial migration and their contribution to endothelial regeneration [12]. We recently reported that CCN1, in an autocrine and paracrine manner, modulates the adhesion, proliferation, and in vitro capillary formation of EPCs and, thus, play an important role in microenvironment-mediated biological properties of EPCs [44]. Herein, we hypothesized that CCN1 has a major impact on EPC-mediated vascular repair through induction of EPC differentiation.
The aim of this study was to investigate the role of CCN1 in EPC differentiation and reendothelialization in response to vascular injury. We found that the expression of CCN1 was dynamically changed in the balloon-injured rat carotid artery. CCN1 overexpression was shown to promote reendothelialization and inhibit neointimal formation at an early stage after vascular injury. In vitro experiments revealed that CCN1 directly stimulated the EPC–EC differentiation associated with transcription factor Id1 and the expression of genes such as Vegf-b, Vegf-c, Kdr, Igf-1, Ereg, Tgf, Mdk, and Ptn. These findings suggest that CCN1, as a matricellular protein expressed in injured vessel walls, enhances reendothelialization via a promotion of EPC differentiation and thus play a critical role in vascular repair mechanisms.
Methods
Materials and methods are described in detail in the supplement.
Gene transfer using adenoviral vector
Two adenovirus vectors were constructed using the AdEasy system which contains the GFP gene. The cDNA was first TA-cloned into pMD19-T simple vector and then subcloned into adenoviral shuttle vector pAdTrack-CMV. Recombinant adenoviruses Ad-CCN1 and Ad-Id1 were generated and purified according to the manufacturer’s protocol.
EPCs culture and characterization
Culture and characterization of EPCs was performed previously in our laboratory [43]. To determine the endothelial phenotype of EPCs, cells were incubated with acLDL-DiI (10 mg/ml) for 4 h, fixed with 4% paraformaldehyde and then incubated with FITC-labeled lectin (BS-1 Isolectin B4, 10 μg/ml) for 1 h. Dual-stained cells positive for both acLDL-DiI and BS-1 Isolectin B4 were identified as differentiated EPCs. In addition, the expression of EC markers (vWF, VEGFR-2, CD144, and eNOS) and progenitor cell markers (CD34, CD133) was evaluated by RT-PCR and flow cytometry (FCM) analysis.
Microarray analysis
To characterize the expression profile of 113 genes important in angiogenesis, the GEArray (SuperArray Bioscience, Bethesda, MD) was used according to the manufacturer’s protocol. More than twofold changes in gene expression were considered to be significant.
Rat carotid balloon-injury model
Male SD rats (180–220 g, Chongqing) were anesthetized with 75 mg/kg pentobarbital and heparinized with 100 U/kg heparin sodium. Balloon injury was performed in the left common carotid artery by a 1.5-French catheter (Cordis; USA) three times as previously described. After balloon injury, viral infusion mixtures with 5 × 108 plaque-forming units of indicated adenoviral vectors, diluted to a total volume of 30 μL in l-DMEM were then instilled into the arterial segment and incubated for 20 min. For cell transplantation, rats were lethally irradiated with a total dose of 9.5 Gy 24 h before vascular injury, and then immediately after balloon injury, EPCs (3 × 106) suspended in 100 μL l-DMEM and transduced with either Ad-CCN1 or Ad-GFP were incubated in the arterial lumen as above. All animal procedures were approved by the Care of Experimental Animals Committee of the Third Military Medical University and complied with the National Research Council’s Guidelines for the Care and Use of Laboratory Animals (revised 1996).
Statistical analysis
Data from independent experiments were expressed as mean ± SD of at least three experiments. Comparisons between groups were analyzed by two-tailed Student’s t test or ANOVA, as appropriate. P value < 0.05 was considered statistically significant.
Results
Expression of CCN1 during vascular injury following rat carotid artery balloon angioplasty
To evaluate the role of CCN1 in vascular repair in vivo, we first examined the expression of CCN1 in balloon-injured rat carotid artery. As shown in Fig. 1a, CCN1 mRNA expression was detected at low levels in normal, uninjured control arteries (0 h), whereas following vascular injury CCN1 mRNA level was rapidly enhanced at 12 h (sevenfold), which began gradually declining 24 h later and remained elevated for up to at least 21 days. CCN1 protein expression was assessed by western blotting and was consistently up-regulated. Figure 1b shows that the protein level started to increase at 12 h (2.2-fold), peaked at 24 h (2.9-fold), and then decreased to basal levels around day 7 and reached another peak at day 14 (1.8-fold) after balloon injury.
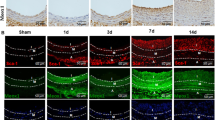
Expression of CCN1 during vascular injury following rat carotid artery balloon angioplasty. a Real-time PCR revealed that CCN1 was rapidly induced and dynamically changed in rat carotid arteries after balloon injury. Representative images from semi-quantitative RT-PCR are shown. b Protein level of CCN1 was assessed by western blot analysis. c Immunohistochemical staining for CCN1 was performed on artery sections before (day 0) and after (days 2, days 14) vascular injury. Scale bar = 100 μm
Then, the accumulation pattern of vascular CCN1 was analyzed by immunohistochemistry before and after vascular injury. While weak staining for CCN1 was detected in the intima and media of uninjured vessels, enhanced CCN1 staining in the media and adventitia was detectable 2 days after balloon injury, and by day 14 CCN1 was mainly detected in the media and neointima (Day 0, 2, 14 in Fig. 1c). No staining was observed with unspecific IgG, demonstrating the specificity of the CCN1 staining (data not shown). The observed dynamic expression pattern during vascular injury implied a potential role of CCN1 in vascular regeneration.
Effect of CCN1 on reendothelialization and neointimal formation
Next, an adenoviral vector expressing exogenous CCN1 was constructed and injected into balloon-injured arteries to investigate CCN1 gene function during vascular injury. As described previously [14], we first examined the efficiency of adenovirus transfection in rat carotid arteries by delivering control adenovirus expressing GFP (Ad-GFP) to balloon-injured carotid arteries. The level of GFP expression increased to a maximum at day 3, remaining elevated until day 7–14 after adenoviral infection, suggesting that the adenovirus-mediated delivery system was effective in rat carotid artery. We then delivered Ad-CCN1 to rat carotid arteries. Overexpression of CCN1 protein was confirmed by western blotting, and more importantly, no observable adverse side-effect (mortality or any other clinical signs of distress/morbidity) was found after adenovirus infection.
To evaluate reendothelialization, Evans blue dye was administered premortem at 4, 7, 14, and 21 days after injury. Non-endothelialized lesions were marked by blue staining, whereas the reendothelialized area appeared white. At the 4-, 7-, and 14-day time points, the reendothelialized area in the Ad-CCN1-infected arteries was significantly larger than in Ad-GFP–infected arteries (2.3-, 1.7-, and 2.1-fold on days 4, 7, and 14, respectively; n = 6; p < 0.05 for each), while no difference was shown on the 21-day (85.2 ± 10.1 vs. 82.17 ± 11%; n = 6; p > 0.05; Fig. 2a). Morphometric analysis of vWF immunostaining along the luminal surface of injured arteries showed a significant increase in the rate of reendothelialization of the denuded vascular surface with Ad-CCN1 infection as compared with control (4 days: 33.82 ± 12.39 vs. 12.31 ± 4.06%; 7 days: 53.49 ± 10.68 vs. 34.17 ± 7.93%; 14 days: 83.21 ± 14.96 vs. 45.97 ± 11.2%; p < 0.05 for each; 21 days: 84.52 ± 10.8 vs. 80.98 ± 12.2%; p > 0.05), consistent with the result from Evans blue dye experiment.
The effect of CCN1 on reendothelialization and neointimal formation. a Quantification of Evans blue dye staining showed that Ad-CCN1 accelerated reendothelialization at 4, 7, and 14 days after balloon injury while no difference was observed at 21 days. b The ratio of vWF+ length to total luminal surface was evaluated and compared at day 4, 7, 14, and 21 after gene delivery. c Sections of carotid arteries at 14 and 21 days following injury and gene delivery were stained with H&E. Arrows point to where the internal elastic lamina (IEL) was. Bar graphs show the intima to media ratio (I/M ratio). *p < 0.05 compared with the Ad-GFP
Moreover, as rapid reendothelialization is well known to inhibit neointimal formation, we then investigated whether Ad-CCN1 treatment affected neointimal formation at 14 and 21 days following vascular injury. As depicted in Fig. 2c, there was a marked decrease in the neointimal area and I/M ratio in Ad-CCN1-treated rats compared with controls at day 14 (0.26 ± 0.17 vs. 0.58 ± 0.18%; n = 9; p < 0.05). However, no difference was found at 21 days (1.18 ± 0.14 vs. 1.23 ± 0.19%; n = 9; p > 0.05), and medial thickness did not significantly differ between either group (data not shown), suggesting that CCN1 effectively prevented neointimal hyperplasia at an early stage.
Contribution of EPCs to CCN1-induced reendothelialization
The above observations indicated that CCN1 effectively promote reendothelialization and inhibit neointimal formation at early stage after vascular injury. Herein, to understand whether the observed effect of CCN1 on accelerated reendothelialization and reduced neointimal formation would be ascribed to its impact on EPCs, we performed EPC transfusion experiments. Cultured EPCs were isolated from rat bone marrow, effectively transduced with either Ad-CCN1 or control Ad-GFP, and then locally transfused into balloon-injured arteries. As expected, no GFP-positive cells were observed in the uninjured vessels, whereas scanning of the injured vessels revealed GFP-positive cells attached at the injured site, forming islets of transplanted cells within the neo-endothelium area. Notably, the number of attached GFP-positive cells on the surface of injured vessels was significantly higher in the group treated with Ad-CCN1-EPC transfusion, when compared with the controls on day 7 (25.6 ± 3.5 vs. 4.2 ± 1.2 cells/field; n = 9; p < 0.05), suggesting that the accelerated reendothelialization induced by CCN1 was contributed by recruited EPCs (Fig. 3a).
Contribution of EPCs to CCN1-induced reendothelialization. a, b EPCs infected with Ad-CCN1 or Ad-GFP were transfused into the irradiated recipient rats after vascular injury and attached to the vascular injury site on day 7. The number of attaching cells was significantly higher in rats transfused with Ad-CCN1-infected EPCs (a) compared with rats transfused with Ad-GFP-infected EPCs (b). c–e The 14-day samples were attached with GFP + transfused EPCs (green fluorescence) and stained-positive for EC marker vWF (red fluorescence), and the ratio of GFP- and vWF-positive area to the total luminal area were compared. *p < 0.05 compared with that of Ad-GFP. Scale bar = 100 μm
In addition, to confirm that the attaching transfused cells in neo-endothelium represent ECs, we assessed the expression of EC-specific marker vWF. Interestingly, the luminal GFP-positive cells were also positive for vWF by immunofluorescence analysis, revealing that the transfused exogenous EPCs, at least in part, underwent the differentiation process into EC-lineage cells and participated in EC regeneration. Moreover, the ratio of GFP- and vWF-positive cell area to the total luminal length was 5.2-fold higher in Ad-CCN1-EPC-transplanted rats than controls on day 14 (Fig. 3b), suggesting that CCN1 promoted the differentiation of EPCs in vivo and thus contributed to accelerated reendothelialization after vascular injury.
Effect of CCN1 on EPC differentiation in vitro
The findings described above supposed an impact of CCN1 on EPC differentiation during vascular repair. To further clarify the effect of CCN1 on EPC-EC differentiation, in vitro experiments were performed by overexpression of CCN1 in cultured EPCs as we reported before. One week after Ad-CCN1 transduction, a noticeable amount of EPCs transformed into a spindle- or polygonal-shaped morphology, which was not observed in control Ad-GFP-transduced EPCs. Additionally, the expression of the progenitor marker CD34 was drastically decreased (53.58 ± 9.81 vs. 15.21 ± 4.33%; n = 3; p < 0.05), whereas the expressions of the EC marker VE-cadherin (CD144) was increased (27.36 ± 5.26 vs. 78.35 ± 8.45%; n = 3; p < 0.05), as shown in Fig. 4a. As a representative control of EC phenotype, rat aortic endothelial cells (RAEC) were used, and lineage markers for hematopoietic cells (CD45) and mesenchymal stem cells (CD90) were also analyzed by FCM, showing virtually no or fairly weak expression in differentiated EPCs (data not shown). Because other EC markers were not detected by FCM analysis, we examined their expressions by reverse-transcription PCR. Although mRNA expression of vWF and eNOS was barely observed in untreated control or Ad-GFP-infected EPCs, 7-day culturing of EPCs infected with Ad-CCN1 significantly induced vWF and eNOS expression (Fig. 4b). Inversely, mRNA expression of CD133 was decreased to 20% of the controls (p < 0.05). Furthermore, those differentiated EPCs bound to murine endothelial-specific lectin from Bandeiraea simplicifolia (BS-1 Isolectin B4) and incorporated fluorescent DiI-acLDL intracellularly. Immunofluorescence experiments showed that Ad-CCN1 significantly increased the number of DiI-acLDL- and lectin-positive cells from 54.5 ± 8.7 to 92.1 ± 6.9% per field (n = 9; p < 0.05), corresponding to a 1.7-fold increase (Fig. 4c). The effect of Ad-CCN1 on those differentiation events was comparable to that of VEGF, a strong stimulator of EPC differentiation, and moreover, it was abrogated significantly in the presence of anti-CCN1 antibody (p < 0.01), while no obvious changes were seen in the control groups (data with control IgG and VEGF not shown). These findings confirm a role for CCN1 in the differentiation of EPCs to ECs.
The effect of CCN1 on EPC differentiation in vitro. a Representative pictures for EPC/EC morphology: CCN1 transduction transformed EPCs into a spindle or polygonal-shaped morphology. b Representative flow cytometry histograms show expression of surface markers CD34 (upper line), CD144 (VE-cadherin, lower line), and their isotype IgG controls (overlay). Significant differences between groups were assessed with a two-tailed unpaired t test. c PCR analysis of eNOS, vWF, and CD133 of cells treated with Ad-GFP, Ad-CCN1, and Ad-CCN1 plus anti-CCN1 blocking antibody (CCN1-Ab). d Immunofluorescence analysis showed that Ad-CCN1 increased the number of DiI-acLDL- and lectin-positive cells, which was significantly reversed by CCN1-Ab. *p < 0.05 versus Ad-GFP; #p < 0.05 versus Ad-CCN1. Scale bar = 200 μm
Gene expression during CCN1-induced EPC differentiation
To further elucidate the mechanism by which CCN1 may impact on the differentiation of EPCs, we performed microarray analysis using the SuperArray ORN-024 gene chips. Genes that were either up-regulated to at least twofold or down-regulated by at least 0.5-fold in Ad-CCN1 infected EPCs compared to controls were examined and listed in Table 1. As a result, we observed 20 genes of 113 analyzed cDNAs differentially expressed in EPCs transfected with Ad-CCN1. Among these changes, we focused on genes predominantly involved in cell differentiation. We found that two endothelial-specific genes, Kdr and Pecam, were concordantly up-regulated upon Ad-CCN1 transfection, which strengthened our demonstration that CCN1 promoted the differentiation of EPCs to ECs. In addition, we observed that Ad-CCN1 transfected EPCs displayed a selective down regulation of growth factor genes, including Vegf-b, Tgfα, Tgfβ, Mdk, Ptn, and Plau; in contrast, genes encoding growth factors such as Vegf-c, Igf-1, and Ereg were significantly up-regulated, revealing a potential involvement of those growth factors in CCN1-induced EPC differentiation. Moreover, interestingly, the microarray results identified the gene Id1, which encodes inhibitor of differentiation/DNA binding 1, a transcription factor belonging to the helix-loop-helix (HLH) family important for cell processes, was noticeably down-regulated upon CCN1-induced EPC-EC differentiation. However, the role of Id1 in EPC differentiation remains largely unknown.
Impact of Id1 on the effect of CCN1
Id1 was of particular interest because it was reported to be a key regulator of EPC recruitment [10] and our observations previously showed that Id1 promoted the migration and proliferation of EPCs in vitro [41]. Thus, to further verify the decreased expression of Id1 we used quantitative RT-PCR and western blot analysis. As shown in Fig. 5a, an 80% decrease in mRNA level and a 70% decrease in protein level of Id1 were detected in response to Ad-CCN1. Although the exact mechanism of how Id1 expression is down-regulated remains to be fully understood, we sought to investigate whether and what impact Id1 may play on the EPC differentiation induction effect of CCN1.
Impact of Id1 on CCN1 induced EPC-EC differentiation. a CCN1 inhibited the expression of transcription factor Id1. b, c PCR and flow cytometry analysis showed that the differentially expression of cell markers (CD34, CD133, VE-cadherin, eNOS, and vWF) induced by Ad-CCN1 was reversed by cotransfection with Ad-Id1. Ad-Id1 alone effectively increased the expression of CD34 and CD133, and decreased VE-cadherin, eNOS, and vWF expression. d Effect of Ad-Id1 on the number of DiI-acLDL- and lectin-positive cells per field (n = 9). *p < 0.05 versus Ad-GFP; #p < 0.05 versus Ad-CCN1
Then, EPCs were either transfected with Ad-Id1 alone or cotransfected with Ad-CCN1. Interestingly, we found that the induction of EC marker eNOS, vWF, and VE-cadherin expression by Ad-CCN1 was remarkably reduced by cotransfection with Ad-Id1. In addition, the reduced expression of progenitor maker CD133 and CD34 was significantly reversed by Ad-Id1 cotransfection. Moreover, interestingly, we found that Ad-Id1 transfection alone also decreased the EPC-EC differentiation events when compared with the control, as depicted in Fig. 5b–d (n = 3 or 9; p < 0.05).
Taken together, these data demonstrated that Id1 suppression induced by CCN1 contributed to EPC differentiation and that, potently, Id1 mediated the effect of CCN1 on EPC differentiation.
Discussion
The major findings of the present study are as follows: (1) CCN1 is dynamically expressed in vascular lesions and promotes reendothelialization in the early phase after vascular injury; (2) EPCs are recruited, differentiate and contribute, at least partly, to the CCN1-induced vascular regeneration; (3) CCN1 stimulates the EPC-EC differentiation in vitro and is associated with the transcriptional repressor Id1; (4) Upon Ad-CCN1 stimulation, 8 genes were up-regulated and 12 down-regulated in EPCs, including Vegf-b, Vegf-c, Kdr, Igf-1, Ereg, Tgf, Mdk, and Ptn, suggesting their potential involvement in the effects of CCN1 on EPC differentiation. These findings imply that matricellular protein CCN1, at the site of vascular injury, promotes the differentiation of EPCs and thus provides a novel mechanism underlying the process of vascular regeneration.
The maintenance of endothelial integrity is crucial for preventing atherosclerosis and restenosis [31]. EPCs, regardless of its origin, can differentiate into mature ECs and participate prominently in vascular repair process [19, 39, 40]. However, the recruitment and differentiation of EPCs are documented to be tightly regulated by the vascular microenvironment [7, 9, 22, 42], in particular, local noncellular elements and especially secreted factors play important roles in the regulation of EPCs and thus in reendothelialization.
CCN1, belonging to the emerging CCN family, is a secreted, extracellular matrix associated proangiogenic factor. CCN1 is expressed by all types of vascular cells in response to a variety of physical and chemical stimuli such as growth factors, proteases, ischemia, hypoxia, and shear stress [16, 18, 23, 32]. In addition, aberrant expression of CCN1 is associated with several vascular diseases such as atherosclerosis [17], mechanical injury [11], and hypertension [38]. In accordance with other observations, we identified that CCN1 effectively induce the adhesion, proliferation, and tube-formation of EPCs [44]. Therefore, it is tempting to speculate that CCN1 may play an important role in EPCs-involved vascular regeneration.
Here, we focused on the effect of CCN1 on EPCs differentiation. We demonstrated that CCN1 is sufficient for EPC-EC maturation, as evidenced by the following observations after Ad-CCN1 transduction into EPCs. First, Ad-CCN1 induced differentiated EPCs displayed typical “cobblestone” EC morphology in culture. Second, those cells expressed a high level of EC marker VE-cadherin, vWF, and eNOS, whereas the expression of progenitor marker CD34 and CD133 were strikingly decreased. Third, the number of DiI-acLDL- and lectin-positive cells was significantly increased.
However, the precise mechanisms by which CCN1 affects EPCs differentiation remain largely unknown. Although a number of integrins and proteoglycans have been proposed to interact with CCN1, none have been shown to be the ‘CCN1 receptor’ [4]. Actually, CCN1 appears to rely upon neither specific receptors nor post-receptor signaling pathways. Herein, we performed cDNA microarray analysis to identify the downstream molecular targets that may account for the effect of CCN1 on EPC differentiation. The array data displayed 20 genes differentially expressed in EPCs upon CCN1 stimulation, including components of the plasminogen/plasmin system (u-PA), members of metalloproteinase family putatively involved in angiogenesis (Timp-2 and Timp-3), the most important known growth factors and their receptors (Vegf-b, Vegf-c, Kdr, Ereg, Igf-1, Tgfα, Tgfβ, Mdk, and Ptn), and so on. Though we cannot provide evidence at this time to describe the precise interactions between those genes and CCN1, neither can we exclude the possibility that genes responsible for the effects of CCN1 in EPCs are not represented on this array, our observations strongly support the opinion that CCN1 acts as a co-factor for cytokines, growth factors, and matrix proteins, which has been proposed initially by Kireeva et al. [21] and Leask et al. [25]. However, more detailed functional studies are needed to verify this point.
Besides, noticeably, the transcriptional regulator Id1 was identified among the most significantly down-regulated genes in array analysis. It is of particular interest because Id1, as a critical transcriptional regulator of vasculature development, has been demonstrated to play an essential role in vascular regeneration and EPC mobilization [5, 10, 28]. Therefore, in the present study, we investigated whether Id1 has a role in the effect of CCN1 on EPC differentiation. Interestingly, we found that Id1 expression in EPCs was strikingly down-regulated by Ad-CCN1, and importantly, overexpression of Id1 significantly inhibited the EPC-EC differentiation induced by Ad-CCN1. In addition, Ad-Id1 alone also decreased EPC-EC differentiation event significantly. Our results implied a negative role of Id1 in the regulation of EPC differentiation, which is concordant with that of other cell types, such as erythrocytes [2] and premature neurons [27].
The present study provides the first evidence that CCN1, as a matricellular protein from the microenvironment of injured vessels, promotes reendothelialization via a direct action on EPC differentiation. EPCs have been identified as important source of “repair” cells in endothelial regeneration, and the rate of reendothelialization is critical in limiting neointimal formation after vascular injury [19, 20, 39]. In our study, accelerated reendothelialization by CCN1 effectively inhibited neointimal formation on day 14 but appeared to have no significant effect on day 21. The lack of an effect on day 21 seems difficult to understand. However, previous studies indicated that CCN1 also exerts effects on vascular smooth muscle cells (VSMCs), a major component of the neointima. Grzeszkiewicz et al. [13] reported that CCN1 supported VSMC adhesion and stimulated chemotaxis in vitro. In addition, Lee et al. [26] demonstrated that CCN1 might be an independent and critical regulator of VSMC proliferation and growth. Thus, CCN1-induced VSMC adhesion, migration, and proliferation may help explain our observations: CCN1 overexpressed injured media which contains lots of VSMCs will be able to develop neointimal formation even though endothelial recovery is fast in early phase following injury.
Of note, Unoki et al. [38] proved that overexpression of the ccn1 gene in rat VSMCs caused rather inhibitory effects on the proliferation and DNA and protein synthesis. Thus, CCN1 was concluded to act as a growth inhibitor of VSMCs rather than mitogenic growth factors. As well as CCN1, the other CCN family members CCN2, CCN3, and CCN5 have been shown to exert opposite effects on VSMC migration, proliferation and neointimal formation [6, 8, 24, 26, 29]. To date, the exact role of CCN1 in VSMCs remains elusive and further studies are needed to make it clear.
Recently, Matsumae and co-workers [31] reported that knockdown of CCN1 by lentiviral delivery of siRNA significantly suppresses neointimal hyperplasia in a rat carotid artery balloon-injury model at days 14. Our observations seem contrary to those of Matsumae et al. However, here are two points we are considering: First, aging dependent functional deficits and depletion of EPCs in experiment animals. The male SD rats used in our study were all aged 1–2 months, weighing 180–220 g, whereas in studies by Matsumae et al. the adult male SD rats weighed 400–450 g. Therefore, the CCN1-induced EPC contribution to neointimal inhibition could be reduced in those older animals. Second, progenitor cells are able to differentiate into not only ECs but also VSMCs [33]. Considering that balloon manipulation may be variable in different experiments, we presume that CCN1 may stimulate the immature VSMCs to augment vascular healing under certain circumstances, such as sever media destruction. Whether such changes lead to the differentiation of immature VSMCs and the durable benefit of EPCs in healing the vascular injury requires further investigation.
However, the present study has two limitations: (1) adenovirus-mediated ccn1 transgene expression remained elevated for only 7–14 days in rat arteries and our observations were limited by the decreased efficiency of gene transfection; (2) our observations were based on an animal model without atherosclerosis, and thus, the conclusions drawn might be limited to non-atherosclerotic arteries.
In summary, we demonstrate for the first time that CCN1 functions as an Id1-associated matricellular protein to promote reendothelialization and inhibit neointimal formation through its effect on EPCs in the early phase after vascular injury. These results provide a novel mechanism underlying the process of vascular regeneration.
References
Babic AM, Kireeva ML, Kolesnikova TV, Lau LF (1998) CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA 95:6355–6360
Cammenga J, Mulloy JC, Berguido FJ, MacGrogan D, Viale A, Nimer SD (2003) Induction of C/EBPalpha activity alters gene expression and differentiation of human CD34+ cells. Blood 101:2206–2214
Chen CC, Mo FE, Lau LF (2001) The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem 276:47329–47337
Chen CC, Lau LF (2009) Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol 41:771–783
Ciarrocchi A, Jankovic V, Shaked Y, Nolan DJ, Mittal V, Kerbel RS, Nimer SD, Benezra R (2007) Id1 restrains p21 expression to control endothelial progenitor cell formation. PLoS One 2:e1338–e1340
Delmolino LM, Stearns NA, Castellot JJ (2001) COP-1, a member of the CCN family, is a heparin-induced growth arrest specific gene in vascular smooth muscle cells. J Cell Physiol 188:45–55
Dernbach E, Randriamboavonjy V, Fleming I, Zeiher AM, Dimmeler S, Urbich C (2008) Impaired interaction of platelets with endothelial progenitor cells in patients with cardiovascular risk factors. Basic Res Cardiol 103:572–581
Ellis PD, Chen Q, Barker PJ, Metcalfe JC, Kemp PR (2000) Nov gene encodes adhesion factor for vascular smooth muscle cells and is dynamically regulated in response to vascular injury. Arterioscler Thromb Vasc Biol 20:1912–1919
Friedrich EB, Werner C, Walenta K, Böhm M, Scheller B (2009) Role of extracellular signal-regulated kinase for endothelial progenitor cell dysfunction in coronary artery disease. Basic Res Cardiol 104:613–620
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V (2008) Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 319:195–198
Grote K, Bavendiek U, Grothusen C, Flach I, Hilfiker KD, Drexler H, Schieffer B (2004) Stretch-inducible expression of the angiogenic factor CCN1 in vascular smooth muscle cells is mediated by Egr-1. J Biol Chem 279:55675–55681
Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B (2007) The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood 110:877–885
Grzeszkiewicz TM, Lindner V, Chen NY, Lan S, Lau LF (2002) The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin α6β1 and cell surface heparan sulfate proteoglycans. Endocrinology 143:1441–1450
Guo RW, Wang H, Gao P, Li MQ, Zeng CY, Yu Y, Chen JF, Song MB, Shi YK, Huang L (2009) An essential role for STIM1 in neointima formation following arterial injury. Cardiovasc Res 81:660–668
Hadjiargyrou M, Ahrens W, Rubin CT (2000) Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J Bone Miner Res 15:1014–1023
Han JS, Macarak E, Rosenbloom J, Chung KC, Chaqour B (2003) Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. Eur J Biochem 270:3408–3421
Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, Rothkotter HJ, Schieffer B, Drexler H (2002) Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation 106:254–260
Hilfiker KD, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC, Hilfiker A (2004) Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation 109:2227–2233
Hutter R, Carrick FE, Valdiviezo C, Wolinsky C, Rudge JS, Wiegand SJ, Fuster V, Badimon JJ, Sauter BV (2004) Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation 110:2430–2435
Kasprzak EM, Jagodziski PP (2007) Endothelial progenitor cells as a new agent contributing to vascular repair. Arch Immunol Ther Exp 55:247–259
Kireeva ML, Mo FE, Yang GP, Lau LF (1996) Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol 16:1326–1334
Koyanagi M, Urbich C, Chavakis E, Hoffmann J, Rupp S, Badorff C, Zeiher AM, Starzinski PA, Haendeler J, Dimmeler S (2005) Differentiation of circulating endothelial progenitor cells to a cardiomyogenic phenotype depends on E-cadherin. FEBS Lett 579:6060–6066
Kunz M, Moeller S, Koczan D, Lorenz P, Wenger RH, Glocker MO, Thiesen HJ, Gross G, Ibrahim SM (2003) Mechanisms of hypoxic gene regulation of angiogenesis factor Cyr61 in melanoma cells. J Biol Chem 278:45651–45660
Lake AC, Bialik A, Walsh K, Castellot JJ (2003) CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am J Pathol 162:219–231
Leask A, Abraham DJ (2006) All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 119:4803–4810
Lee HY, Chung JW, Youn SW, Kim JY, Park KW, Koo BK, Oh BH, Park YB, Chaqour B, Walsh K, Kim HS (2007) Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 100:372–380
Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R (1999) Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401:670–677
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201
Matsumae H, Yoshida Y, Ono K, Togi K, Inoue K, Furukawa Y, Nakashima Y, Kojima Y, Nobuyoshi M, Kita T, Tanaka M (2008) CCN1 knockdown suppresses neointimal hyperplasia in a rat artery balloon injury model. Arterioscler Thromb Vasc Biol 28:1077–1083
Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF (2002) CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol 22:8709–8720
Ong AT, McFadden EP, Regar E, de-Jaegere PP, van-Domburg RT, Serruys PW (2005) Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol 45:2088–2092
Pendurthi UR, Tran TT, Post M, Rao LV (2005) Proteolysis of CCN1 by plasmin: functional implications. Cancer Res 65:9705–9711
Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R (2002) Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 8:403–409
Schuppan D, Ocker M (2003) Integrin-mediated control of cell growth. Hepatology 38:289–291
Schütze N, Schenk R, Fiedler J, Mattes T, Jakob F, Brenner RE (2007) CYR61/CCN1 and WISP3/CCN6 are chemoattractive ligands for human multipotent mesenchymal stroma cells. BMC Cell Biol 8:45–52
Seeger FH, Sedding D, Langheinrich AC, Haendeler J, Zeiher AM, Dimmeler S (2010) Inhibition of the p38 MAP kinase in vivo improves number and functional activity of vasculogenic cells and reduces atherosclerotic disease progression. Basic Res Cardiol 105:389–397
Tsuzuki M (2009) Bone marrow-derived cells are not involved in reendothelialized endothelium as endothelial cells after simple endothelial denudation in mice. Basic Res Cardiol 104:601–611
Unoki H, Furukawa K, Yonekura H, Ueda Y, Katsuda S, Mori M, Nakagawara K, Mabuchi H, Yamamoto H (2003) Up-regulation of cyr61 in vascular smooth muscle cells of spontaneously hypertensive rats. Lab Invest 3:973–982
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353
Van Craenenbroeck EM, Hoymans VY, Beckers PJ, Possemiers NM, Wuyts K, Paelinck BP, Vrints CJ, Conraads VM (2010) Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res Cardiol 105:665–676
Wang H, Yu Y, Guo RW, Shi YK, Song MB, Chen JF, Yu SY, Yin YG, Gao P, Huang L (2010) Inhibitor of DNA binding-1 promotes the migration and proliferation of endothelial progenitor cells in vitro. Mol Cell Biochem 335:19–27
Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC, Pratt RE, Dzau VJ (2006) Essential role of ICAM-1/CD18 in mediating epc recruitment, angiogenesis, and repair to the infracted myocardium. Circ Res 99:315–322
Yin Y, Huang L, Zhao X, Fang Y, Yu S, Zhao J, Cui B (2007) AMD3100 mobilizes endothelial progenitor cells in mice, but inhibits its biological functions by blocking an autocrine/paracrine regulatory loop of stromal cell derived factor-1 in vitro. J Cardiovasc Pharmacol 50:61–67
Yu Y, Gao Y, Wang H, Huang L, Qin J, Guo R, Song M, Yu S, Chen J, Cui B, Gao P (2008) The matrix protein CCN1(CYR61) promotes proliferation, migration and tube formation of endothelial progenitor cells. Exp Cell Res 314:3198–3208
Acknowledgments
Appreciation goes to Dr. Jun Wang for his encouragement and kind help in this work. These studies were supported in part by the National Natural Science Foundation of China (grant 30770852, 81000070) and Chongqing Municipal Natural Science Foundation (grant 2007BB5028).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Y., Gao, Y., Qin, J. et al. CCN1 promotes the differentiation of endothelial progenitor cells and reendothelialization in the early phase after vascular injury. Basic Res Cardiol 105, 713–724 (2010). https://doi.org/10.1007/s00395-010-0117-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-010-0117-0