Abstract
To determine the effect of intracoronary transfer of superparamagnetic iron oxide (SPIO) labeled heme oxygenase-1 (HO-1) overexpressed bone marrow stromal cells (BMSCs) in a porcine myocardial ischemia/reperfusion model. Cell apoptosis was assayed and supernatant cytokine concentrations were measured in BMSCs that underwent hypoxia/reoxygen in vitro. Female mini-swines that underwent 1 h LAD occlusion followed by 1 h reperfusion were randomly allocated to receive intracoronary saline (control), 1 × 107 SPIO-labeled BMSCs transfected with pcDNA3.1-Lacz plasmid (Lacz-BMSCs), pcDNA3.1-human HO-1 (HO-1-BMSCs), pcDNA3.1-hHO-1 pretreated with a HO inhibitor, tin protoporphyrin (SnPP, n = 10 each). MRI and postmortem histological analysis were made at 1 week or 3 months thereafter. Post hypoxia/reoxygen in vitro, apoptosis was significantly reduced, supernatant VEGF significantly increased while TNF-α and IL-6 significantly reduced in HO-1-BMSCs group compared with Lacz-BMSCs group (all p < 0.05). Myocardial expression of VEGF was significantly higher in HO-1-BMSCs than in Lacz-BMSCs group at 1 week post transplantation (all p < 0.05). Signal voids induced by the SPIO were detected in the peri-infarction region in all BMSC groups at 1 week but not at 3 months post transplantation and the extent of the hypointense signal was the highest in HO-1-BMSCs group, and histological analysis showed that signal voids represented cardiac macrophages that engulfed the SPIO-labeled BMSCs. Pretreatment with SnPP significantly attenuated the beneficial effects of HO-1-BMSCs. Transplantation of HO-1-overexpressed BMSCs significantly enhanced the beneficial effects of BMSCs on improving cardiac function in this model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellular cardiomyoplasty (CCM) has emerged as a promising approach for the treatment of acute myocardial infarction (AMI) [20, 30, 31]. Various animal studies and clinical trials have demonstrated that grafted bone marrow stromal cells (BMSCs; also known as mesenchymal stem cells, MSCs) could improve left ventricular (LV) function post AMI [1, 12, 32, 39]. However, most of the transplanted cells could not survive post transplantation, the underlying mechanisms by which transplantation of BMSCs improves LV function remain largely unknown and speculative now [26].
Heme oxygenase-1 (HO-1) is an inducible enzyme with potent anti-apoptosis and anti-inflammatory activities due to its ability to degrade heme into biliverdin/bilirubin, carbon monoxide (CO), and free iron. Overexpression of HO-1 in MSCs improved cell viability after transplantation [37, 45]. However, still there were no reports on the effects of HO-1-overexpressed BMSCs transfer on porcine ischemia/reperfusion model. Due to morphological and physiological similarities to human being, porcine serves as a valuable model for preclinical studies.
There is also some discrepancy between basic and clinical trials for the effects after stem cell therapy [6]. A possible explanation could be that cardiac function was evaluated by MRI in the majority of clinical trials but by non-MRI procedure in basic experiments.
To evaluate cell-based therapy, it is essential to track the retention and migration of donor cells in vivo. Superparamagnetic iron oxide (SPIO) labeled cells could be identified in vivo by MRI due to their property of superparamagnetism [2, 13, 14]. However, similar magnetic signal voids derived from the micro hemorrhage within reperfused myocardial infarcts [40]. Moreover, enhanced MRI signals might arise not only from the injected SPIO-labeled MSCs but also from the cardiac macrophages that engulfed the SPIO nanoparticles [3]. Therefore, it is necessary to take all these factors in mind on interpreting the MRI cell tracking results. In this study, we compared the effects of intracoronary transfer of SPIO-labeled BMSCs with or without HO-1 overexpression in a porcine ischemia/reperfusion model and tested the feasibility of tracking these cells by MRI.
Materials and methods
In vitro experiments
BMSCs isolation
Bone marrow was aspirated from the iliac crest of male donor swine and purified by density gradient centrifugation (Ficoll-Paque, GE Healthcare Bio-sciences AB, Sweden; 1.077 g/ml) using a standardized protocol as previously described [27].
Transfection
pcDNA3.1-human HO-1 was constructed by using the mammalian expression vector pcDNA3.1/Zeo and a 1.0 kb EcoRI/XbaI fragment containing the entire protein-coding region of the HO-1 gene; the correctness of pcDNA3.1-hHO-1 plasmid was verified by DNA sequencing and digestion with restriction enzyme. Then pcDNA3.1-hHO-1 plasmid was transfected to BMSCs(HO-1-BMSCs) was transfected to BMSCs, after BMSCs were expanded to three passages at 70% confluence, using FuGene 6 transfection reagent (Roche, Mannheim, Germany) according to commercial protocol, and then under G418 selection. The pcDNA3.1-Lacz plasmid pcDNA3.1-Lacz plasmid was transfected to BMSCs (Lacz-BMSCs) was transfected to BMSCs using the same procedure.
Cell labeling
At 70–80% confluence, BMSCs and HO-1-BMSCs were incubated with a mixture of SPIO (Laboratory of Molecular and Boimolecular Electronics, Southeast University, China) for 48 h at 37°C in 5% CO2. The labeling efficiency was determined by Prussian blue staining. Briefly, cells were fixed with 4% formaldehyde, incubated for 30 min with 5% potassium ferrocyanide (Perls agent) in 10% hydrochloric acid, washed, and cytoplasm stained with eosin red.
Tetrazolium salt measurement (MTT assay)
BMSCs, BMSCs labeled by SPIO (BMSCs + SPIO), HO-1-BMSCs, and HO-1-BMSCs labeled by SPIO (HO-1-BMSCs + SPIO) were cultured with DMEM for 48 h in 96-well flat-bottomed tissue culture trays; the supernatant in each well was discarded and washed with phosphate-buffered saline (PBS) twice. Then 20 μl of MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] solution (5 mg/ml, Fluka Co., Switzerland) was added into each well and cultured at 37°C for 4 h. After removing the MTT solution, 150 μl dimethylsulfoxide (DMSO, Shanghai Bioengineering Co., Shanghai, China) was added; the optical density (OD) was quantified at 570 nm in spectrophotometer (Sepctra MAX 250, Molecular Devices Corp, Sunnyvale, CA, USA). The experiment was repeated thrice.
Trypan blue staining
After removing the culture medium, BMSCs, BMSCs + SPIO, HO-1-BMSCs, and HO-1-BMSCs + SPIO were stained with trypan blue (Sigma) [23]. The ratio of viable cells was counted using a light microscopy (10× magnification). Two independent observers performed this experiment thrice.
HO-1 expression determined by Western blot analysis
Homogenates of BMSCs, Lacz-BMSCs, HO-1-BMSCs, and HO-1-BMSCs + SnPP were centrifuged at 10,000 × g for 10 min at 4°C, and 70 μg supernatant was lysed in electrophoresis buffer and boiled for 10 min, subjected to electrophoresis in a SDS–polyacrylamide gel according to the methods of Laemmli [21]. The separated blots were transferred to nitrocellulose membranes and blocked for 1 h in TTBS buffer containing 5% non-fat milk. The membranes were incubated overnight with primary anti-HO-1 polyclonal antibody (Santa Cruz Biotechnology, Inc., 1:1000 dilution), and incubated with a 1:1000 dilution of horseradish peroxidase (HRP) conjugated rabbit anti-goat IgG antibody for 2 h at 37°C. Blots were detected by chemiluminesence and relative protein expression was quantified by scanning densitometry.
Assay for heme oxygenase activity
Heme oxygenase activity assay was performed following a standard protocol as described [9] in BMSCs after various treatments and post SPIO labeling. Briefly, microsomes from harvested cells were added to a reaction mixture containing magnesium chloride (2 mmol/l), NADPH (0.8 mmol/l), glucose-6-phosphate (2 mmol/l), glucose-6-phosphate dehydrogenase (0.2 units), rat liver cytosol as a source of biliverdin reductase, and the substrate hemin (20 μmol/l). The reaction was conducted at 37°C in the dark for 1 h and terminated by the addition of 1 ml chloroform, and then the extracted bilirubin was calculated by measuring the ΔD (D464–D530) and using an absorption coefficient of 40 mmol/l−1 cm−1.
Hypoxia/reoxygenation
BMSCs, Lacz-BMSCs, HO-1-BMSCs, and HO-1-BMSCs + SnPP (20 μmol/l) were cultured in an airtight incubation bottle for 24 h with <1% oxygen concentration, followed by 12 h of reoxygenation. Thereafter, cell apoptosis was evaluated by flowcytometry. Briefly, cells (1 × 106) were washed twice with cold PBS and labeled with titrated FITC-conjugated annexin-V in dark for 10 min at room temperature. Adding propidine iodide (PI) solution, the percentages of dead cells and cells undergoing apoptosis were then evaluated 5 min later by using a flowcytometer (Becton–Dickinson, San Jose, CA, USA). The annexin+/PI+ was judged as late apoptosis or death and annexin+/PI− as early apoptosis. VEGF, TNF-α, and IL-6 concentrations in supernatant were determined by ELISA.
In vivo experiments
Animals
Handling of the animals was approved by the Care of Experimental Animals Committee of Southeast University, Nanjing, China, and in compliance with the National Research Council’s guidelines. Forty Chinese female minipigs were randomly allocated to the following treatment groups (n = 10 each): control group (saline), BMSCs transfected with pcDNA3.1-Lacz-BMSCs, BMSCs transfected with pcDNA3.1-hHO-1-BMSCs, and HO-1-BMSCs plus pretreatment with a HO inhibitor, tin protoporphyrin (SnPP, Sigma) (HO-1-BMSCs + SnPP).
Ischemia/reperfusion and cell transfer
Animals were sedated with ketamine (15 mg/kg) and anesthesia was maintained using pentobarbital sodium (intermittent 10–15 mg/kg intravenous injection). Baseline LVEF was evaluated by LV ventriculography, left anterior descending coronary artery just distal to the second largest diagonal branch was occluded for 1 h, followed by reperfusion for 1 h, and then left ventriculography was performed again to assess the LVEF post ischemia/reperfusion. Aspirin (325 mg/day) and clopidogrel (75 mg/day) were fed per gavage 3 days before operation and daily thereafter till the end of study. Amiodarone was administered as one intravenous bolus injection of 150 mg before operation and maintained at a dose of 1 mg/min during operation. Heparin (300 units/kg, intravenous injection) was used when needed to maintain an activated clotting time >300 s during the surgical procedure. ECG, blood pressure, and oximetry were monitored throughout the perioperative period, and life-threatening ventricular fibrillation was treated with semi-automatic defibrillator (Philips Heartstart XL, USA). After LVEF determination by left ventriculography 1 h post LAD occlusion, 1 × 107 allogeneic Lacz-BMSCs, HO-1-BMSCs, or equal volume saline were infused into the coronary through the over-the-wire (OTW) balloon angioplasty catheter for over 5 min. In HO-1-BMSCs + SnPP group, SnPP (2 mg/kg, i.v.) was administered immediately before coronary occlusion. Successful occlusion was confirmed by coronary angiography. ST segment elevation and TIMI grade were measured before and immediately post infusion to observe possible BMSCs infusion induced minor myocardial injury.
MR imaging
Cardiac MRI was performed at 1 week or 3 months after ischemia/reperfusion using a 3.0 T MR scanner (Magnetom Trio, Simens Medical Systems) with a phase array radiofrequency receiver coil. Tracking of implanted BMSCs was acquired by multislice ECG- and respiratory-gated T2*-weighted gradient echo image (T2*WI); the DE-MRI images at the same location were also acquired; left ventricular ejection fraction (LVEF), end-diastolic volume (EDV), end-systolic volume (ESV), and left ventricular mass index (LV mass index) were measured by cine-MRI and assessed by ARGUS auto-quantization program.
Postmortem analysis
Post MRI, animals (1 week, n = 3 each group or 3 months, n = 5–7) were sacrificed under anesthesia, the hearts were immediately excised, left ventricle including septum was separated from right ventricle, left ventricle was sectioned into 1 cm thick short-axis slices from apex to base and 5–6 sections were obtained. The first section from apex was used for VEGF, IL-6, TNF-α, Bax, and Bcl-2 expressions by Western blot analysis; the second section for detecting the myocardial Y-chromosome-specific SRY DNA by qRT–PCR method; and the third section was halved and used to determine the localization of transplanted BMSCs by Prussian blue staining (first portion) and for analyzing the capillary density and arteriole density by immunochemistry method (second portion).
Prussian blue staining method was used to determine the localization of transplanted BMSCs at 1 week and 3 months post BMSCs infusion. The first part of the third section from apex was cut into consecutive 4 μm sections, stained with hematoxylin-eosin (HE) for infarction zone identification, and the neighboring section stained by Prussian blue reagent. Sections with positive iron staining were costained with a rabbit polyclonal antibody raised against CD68 (a marker for macrophages). Single Prussian blue-positive cells were deemed as SPIO-labeled BMSCs, whereas double-stained cells were defined as cardiac macrophages containing iron.
Angiogenesis was observed in the second part of the third section from apex of hearts 3 months post BMSCs transplantation. Tissue was fixated for 24 h in 10% neutral buffered formalin at room temperature, paraffin-embedded, and consecutive 4 μm paraffin sections were then obtained and numbered. HE staining was made in one 4 μm section to identify the peri-infarct area and the neighboring five sections were then immunohistochemically stained by primary antibody against vWF (1:200, DAKO) and SM α-actin (1:200, Abcam), then detected with appropriate secondary antibodies. DAB (Zymed, Laboratories Inc.) was used as a chromogenic substrate and the color reaction was carried out for 3–10 min and the slides were washed before covering with Crystal/Mount. Slides from each group were used as negative controls by omitting the primary antibody. The number of capillary and arteriole in peri-infarct area was counted in five random high power fields (HPF, ×400) from each section by investigators blindly to the experiment design. The results from 25 randomly chosen HPFs were averaged and expressed as vascular density per HPF.
Western blot analysis
The first section from apex at 1 week post BMSCs transplantation was homogenized and equal amounts of protein were used for measuring the VEGF, TNF-α, IL-6, Bax, and Bcl-2 expressions by Western blot analysis.
Quantitative real-time PCR
The second section from apex was used to identify exogenous BMSCs at 1 week and 3 months post BMSCs transplantation, genomic DNA was isolated from the entire frozen sections and was used for real-time PCR for swine male-specific Sry gene as previously described [37]. A standard curve was generated by serially diluting swine male genomic DNA prepared from male BMSCs. In Sry gene, forward primer was 5′-AAAGCGGACGATTACAGC-3′ and reverse primer was 5′-TTTGCATTTGAGGGTTCT-3′ [46].
The RT–PCR protocol consisted of starting with 20 min at 50°C, 10 min at 95°C for activation of polymerase, then followed by 45 cycles of denaturation at 94°C for 15 s, annealing at 59°C for 20 s, and elongation at 72°C for 30 s. Real-time PCR assay of Sry gene quantified the number of survival male BMSCs in female hearts. The survival ratio is equal to survival number of BMSCs divided by total implanted cells.
Statistical analysis
Data are presented as mean ± standard deviation. Analysis was performed using the SPSS Software (version 11.5). Unpaired Student t test or one-way ANOVA was used to detect differences between groups as indicated. Values of p < 0.05 were considered statistically significant.
Results
In vitro experiments
The effect of SPIO on BMSCs characteristics
Prussian blue staining results showed that the labeling efficiency of SPIO on BMSCs or HO-1-BMSCs was satisfactory (approximately 100%). MTT assay results demonstrated that SPIO labeling did not affect cell growth in either BMSCs or HO-1-BMSCs (BMSCs, 0.46 ± 0.09; BMSCs + SPIO, 0.49 ± 0.08, p > 0.05; HO-1-BMSCs, 0.75 ± 0.11; HO-1-BMSCs + SPIO, 0.74 ± 0.11, p > 0.05). More than 97% of cells were alive as shown by Trypan Blue staining.
HO-1 expression in BMSCs and heme oxygenase activity assay
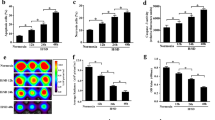
The cellular expression of HO-1 was detected by Western blot analysis. There was a pronounced increase in HO-1 protein expression in HO-1-BMSCs, an effect that was associated with a significant increase in heme oxygenase activity, and these were significantly downregulated by pretreatment with SnPP. Heme oxygenase activity remained unchanged post labeling with SPIO (Fig. 1).
HO-1 protein expression and heme oxygenase activity in cultured BMSCs. The expression of HO-1 protein increased significantly in HO-1-BMSCs, an effect that was associated with a pronounced increase in heme oxygenase activity; these were down-regulated obviously by pretreatment with SnPP. Heme oxygenase activity remained unchanged post labeling with SPIO. *p < 0.05 versus BMSCs; † p < 0.05 versus Lacz-BMSCs; ‡ p < 0.05 versus HO-1-BMSCs
Effect of hypoxia/reoxygen on cytokine expressions and apoptosis of various BMSCs
ELISA was used to measure VEGF, TNF-α, and IL-6 concentrations in supernatant of BMSCs that underwent hypoxia/reoxygen (Fig. 2a–c). VEGF was significantly higher while TNF-α and IL-6 were significantly lower in supernatant of HO-1-BMSCs compared with BMSCs and Lacz-BMSCs. Apoptotic cells identified by flowcytometry after double-staining with annexin-V/PI (Fig. 3a, b) were significantly reduced in HO-1-BMSCs than in BMSCs, Lacz-BMSCs, and HO-1-BMSCs pretreated with SnPP.
Supernatant VEGF, TNF-α, and IL-6 concentrations measured by ELISA in cells underwent hypoxia/reoxygen. VEGF was significantly higher (a) while TNF-α (b) and IL-6 (c) were significantly lower in HO-1-BMSCs compared with BMSCs and Lacz-BMSCs. *p < 0.05 versus BMSCs; † p < 0.05 versus Lacz-BMSCs; ‡ p < 0.05 versus HO-1-BMSCs
Apoptosis in cells underwent hypoxia/reoxygen. (a) Apoptotic cells identified by flowcytometry after double-staining with annexin-V/PI. (b) Quantitative analysis of apoptosis and necrotic in vitro. Cell apoptosis and necrotic rate was significantly reduced in HO-1-BMSCs compared with that in BMSCs and Lacz-BMSCs. *p < 0.05 versus BMSCs; † p < 0.05 versus Lacz-BMSCs; ‡ p < 0.05 versus HO-1-BMSCs
In vivo studies
Ischemia/reperfusion model
Five pigs died of ventricular fibrillation during the experiment despite the use of defibrillator (one in control group, two in Lacz-BMSCs group, and two in HO-1-BMSCs + SnPP group). Thirty-five pigs survived the experimental procedures and no malignant ventricular arrhythmias were observed in these animals.
Left ventricular function and geometry determined by MRI
Cardiac function was determined by MRI at 1 week and 3 months after ischemia/reperfusion operation. One week post operation, LVEF was significantly increased in HO-1-BMSCs group than that in saline group (Table 1). At 3 months post operation, LVEF was significantly higher in HO-1-BMSCs and Lacz-BMSCs groups than that in saline and HO-1-BMSCs + SnPP groups. EDV was similar among groups. ESV and LV mass index were significantly lower in HO-1-BMSCs group than that in saline group and these effects could be abolished by pretreatment with SnPP (Table 1).
Western blot analysis
One week post transplantation, myocardial expression of VEGF was significantly enhanced and myocardial expressions of TNF-α and IL-6 were significantly reduced in HO-1-BMSCs group than those in Lacz-BMSCs group (Fig. 4a–c), while myocardial Bax expression was similar among the groups (Fig. 4d). Bcl-2, which may prevent the activation of downstream apoptotic signaling, was also significantly increased in HO-1-BMSCs group than that in Lacz-BMSCs group (Fig. 4e). HO-1-overexpression induced effects were significantly abolished by pretreatment with SnPP.
Myocardial VEGF, TNF-α, IL-6 (a–c), Bax, and Bcl-2 (d–e) expressions detected by Western blot analysis. One week post transplantation, enhanced VEGF, Bcl-2, and reduced TNF-α, IL-6 myocardial expressions were found in HO-1-BMSCs group compared to Lacz-BMSCs group. *p < 0.05 versus saline group; † p < 0.05 versus Lacz-BMSCs group; ‡ p < 0.05 versus HO-1-BMSCs group
SRY gene detection by real-time PCR
One week after cell transplantation, the survival ratio of male, donor-derived MSCs (SRY gene) was significantly higher in HO-1-BMSCs group (1.79 ± 0.28%) than that in Lacz-BMSCs group (1.01 ± 0.19%, p < 0.001); this effect could be blocked by pretreatment with SnPP (0.75 ± 0.14%, p < 0.001 vs. HO-1-BMSCs group; p = 0.017 vs. Lacz-BMSCs group). SRY gene was not detectable in all groups at 3 months after cell transplantations.
SPIO-labeled MSCs detection by MRI
Signal voids induced by the SPIO were detectable in all SPIO-labeled BMSCs groups but not in the saline group based on T2*WI or using DE-MRI at 1 week post transplantation; signal voids were concentrated in the peri-infarction region. The extent of the hypointense signal was significantly higher in HO-1-BMSCs group than that in Lacz-BMSCs group and HO-1-BMSCs + SnPP group (Fig. 5). Hypointense signals were not detectable in all groups at 3 months after cell transplantation in vivo by MRI.
Prussian blue staining and costaining with CD68
Positive iron staining was evidenced mostly in the border zone of the infarction at 1 week post BMSCs transplantation, scarce iron-positive cells could also be found in saline group in the border zone of the infarction, probably due to hemosiderin from infarction hemorrhages (Fig. 6a). Most of the iron-positive cells were also positively costained for CD68 (Fig. 6b). SPIO-labeled cells were not detectable in the cardiac tissue at 3 months after transplantation.
Positive iron staining at 1 week post transplantation in Prussian blue stained sections (×400). a Example of positive Prussian blue staining. b CD68 costaining for iron-positive cells indicating most of the iron-positive cells were engulfed by cardiac macrophages (DAB was used as a chromogenic substrate)
Angiogenesis in the peri-infarct region
Capillary density was determined by immunohistochemical staining for vWF in the peri-infarct area 3 months after cell transplantation. Capillary and arteriolar densities were significantly higher in HO-1-BMSCs group than in Lacz-BMSCs group and saline group, and these effects could be blocked by SnPP (Fig. 7a, b).
Neovascularization post BMSCs transplantation (×200; DAB was used as a chromogenic substrate). Capillary density (a) and arteriolar density (b) were higher in HO-1-BMSCs group compared with that in Lacz-BMSCs group. These effects were blocked by pretreatment with SnPP. *p < 0.05 versus saline group; † p < 0.05 versus Lacz-BMSCs group; ‡ p < 0.05 versus HO-1-BMSCs group
Discussion
The main findings of the present study are as follows: (1) HO-1-overexpressed BMSCs could be efficiently labeled with SPIO nanoparticles without altering their viability. (2) HO-1-overexpressed BMSCs significantly enhanced the efficacy of BMSCs on improving cardiac function in this porcine model of ischemia/reperfusion, associated with reduced inflammatory cytokine levels and apoptosis and enhanced neovascularization. However, all these beneficial effects induced by HO-1 overexpression in BMSCs could be significantly attenuated by pretreatment with a HO inhibitor, SnPP, indicating the specific effects of HO-1 overexpression on BMSCs. (3) Most of the iron-positive cells in myocardium detected by MRI at 1 week but not at 3 months post transplantation were stained positive for both iron and CD68, therefore, could be classified as cardiac macrophages; this finding suggested that most transplanted BMSCs were engulfed by macrophages 1 week post transplantation in this model.
Route of BMSCs transplantation
Intracoronary BMSCs administration was mostly performed by interventional cardiologists during PCI [38] and direct intramyocardial injection of BMSCs by cardiac surgeons during and after open-chest thoracotomy [4]. Intracoronary administration was applied in this study to follow the clinical delivery method preferred by interventional cardiologists. It is well established that BMSCs might be trapped in the coronary microcirculation causing minor myocardial injury [24, 41]. In our experiments, TIMI grade 3 was evidenced post reperfusion and remained unchanged post various intracoronary BMSCs or saline infusions, moreover, microinfarcts were not observed in HE stained section at remote regions at 1 week and 3 months post procedure. Therefore, intracoronary BMSCs transfusion might not result in significant additional ischemia in our study protocol, if there was any. The possible reason might be that a relative lower BMSC numbers (1 × 107) were infused in a relative long duration (>5 min) in our study as compared to others. Moelker et al. [24] injected ~5 × 107 USSC into the LAD of normal hearts in four swines, 4 days after injection, extensive micro infarctions were observed in the injected area, and each swine received intracoronary injection of ~108 human USSC in the LCX in a porcine model of myocardial infarction and reperfusion; however, this increased infarct size compared to medium group. Perin et al. [28] explored the safety of IC delivery of 1 × 108 BMSCs at three different velocities (1 × 106; 1.5 × 106; and 3 × 106 cells/min); post-procedural transient TIMI-2 flow was seen in the higher velocity groups (1.5 × 106 and 3 × 106 cells/min). In line with our results, Valina et al. [39] showed that the maximal number of cells that could be safely administered was around 1 × 107. It is to note that all animals also received oral aspirin (325 mg/day) and clopidogrel (75 mg/day) 3 days before operation and daily thereafter till the end of study, and anti-coagulation regimen in this study might also be an important factor to minimize BMSCs infusion induced coronary microcirculation and minor myocardial injury [15].
Effects of HO-1 in post-MI model
Cumulative evidences have shown that implanted BMSCs could improve the cardiac function after AMI [1, 12, 32, 39]. The precise mechanism of the beneficial effects of transplanted BMSCs is still unknown. Several possible explanations, such as differentiation into myogenic-like cells [12, 33], cell protective cytokine secretions [10, 25], were suggested. However, the majority of transplanted cells died shortly after transplantation [42, 47] which might prevent a more significant effect of BMSCs transplantation on improving myocardial regeneration. Consequently, numerous studies have focused on how to improve stem cell homing or survival after myocardial transplantation [16, 34, 37, 48]. Heme oxygenase-1 (HO-1), a rate-limiting enzyme for heme metabolism, received most attention from researchers, and previous studies in small animal model showed that HO-1 overexpression is a valuable strategy to amplify the beneficial effects of MSCs transplantation on improving cardiac function and post transplantation MSCs survival [37, 45]. Our study confirmed the previously reported effects of HO-1 overexpression in small animal models in this porcine ischemia/reperfusion model which moved one step forward for possible application of this strategy in future clinical practice on patients with AMI.
Following mechanisms might be related to our observed effects. First, reduced inflammatory cytokine (IL-6 and TNF-α) secretion as well as myocardial expression as seen in supernatant of BMSCs post hypoxia/reoxygen in vitro and at 1 week post BMSCs transplantation in vivo. It is well known that cytokines may be important modulators in the ventricular remodeling process after AMI [7, 35]. Reperfusion of the ischemic myocardium is associated with increased TNF-α and IL-6 secretion which could exert negative inotropic effects on heart muscle [17]. Similar to our observations, Guo et al. [11] reported decreased myocardial protein production and gene expression of TNF-α and IL-6 post myocardial infarction, and improved left ventricular function induced by MSC transplantation. Second, overexpression of HO-1 in BMSCs reduced the BMSCs apoptosis under hypoxic conditions in vitro, and upregulated myocardial expression of Bcl-2, a cardiac protection mediator under various stress conditions [18] in vivo 1 week after transplantation. Simultaneously we also observed enhanced hypointense signal in HO-1-BMSCs group, and all these observations suggested increased homing of transferred HO-1-BMSCs. Our results are in line with previous report in the transplantation of MSCs overexpressing Bcl-2 which significantly reduced apoptosis and improved heart function [22]. Third, overexpression of HO-1 in BMSCs significantly enhanced serum VEGF production in hypoxic conditions in vitro and promoted angiogenesis in vivo compared to BMSCs alone. VEGF is a proangiogenic cytokine, which can be secreted by MSCs [43], and previous study suggested that, apart from stimulating angiogenesis capacities, VEGF itself could also prevent cells from apoptosis [8]. Therefore, increased myocardial VEGF production and angiogenesis could be one of the important mechanisms responsible for observed enhanced beneficial effects of HO-1 overexpression of BMSCs. Pretreatment with SnPP, a HO-1 inhibitor, abolished the HO-1 overexpression induced effects which indicated the specific effects of HO-1 overexpression in our model.
Though the presence of donor cell was significantly increased at 1 week post transplantation in HO-1-BMSCs, evidenced by the enhanced hypointense signal observed by MRI and the increased Y-chromosome-specific SRY DNA from male donors, histology analysis showed that BMSCs were presented mostly in the form of BMSCs engulfed by cardiac macrophages. It seems there were less macrophages accumulated in the myocardium in HO-1-BMSCs + SnPP group compared with that in HO-1-BMSCs group, and we postulated that the ischemic local milieu in SnPP treated HO-1-BMSCs was responsible for reduced transplanted BMSCs survival and less macrophages in this group [44]. It remains unknown now if the engulfed BMSCs still behave regenerative capacity and if the SPIO labeling increased the BMSCs engulfment by cardiac macrophages and further studies are warranted to answer these questions. Despite the fact that the BMSCs were not detectable at 3 months post transplantation, transplantation of both Lacz-BMSCs and HO-1-BMSCs still provided beneficial effect on attenuating cardiac remodeling and improving cardiac function after ischemia/reperfusion in this model at this late stage of transplantation, and again the beneficial effects by BMSCs were amplified by HO-1 overexpression. Similar findings on the dissociation between observed beneficial therapeutic effects and presence of transplanted cells at late stage were also reported in previous studies [3, 5] and the reasons are largely unclear. The paracrine effect induced by transplanted MSCs [3, 37] was postulated as an important determinant responsible for cardiac function improvement at this stage.
Feasibility of tracking SPIO-labeled BMSCs in vivo
SPIO particles were used as the sensitive existing labeling markers for labeling transplanted cells for the purpose of in vivo MRI detection [19]. Though cell growth and survival were not affected by SPIO labeling in vitro, in vivo MRI detected BMSCs were mostly presented in the form of engulfed by cardiac macrophages. We knew that the transplanted BMSCs homing was increased in HO-1-BMSCs at 1 week post transplantation but did not know if these BMSCs were alive or not and the functionality of these cells also could not be shown by MRI. It is also unknown if the SPIO labeling increased the engulfment of BMSCs by cardiac macrophages or the myocardial microenvironment post ischemia (inflammation) was not favorable for the donor cells survival, thus the apoptotic and dead BMSCs were engulfed by cardiac macrophages [3]. Another reason might be that the allogeneic BMSCs were not totally immunoprivileged and the donor cells were treated as foreign body and eliminated via cellular rejection mechanism in vivo [29, 36]. Further studies using an MRI marker, which did not increase the engulfment by cardiac macrophages or autologous stem cells or allogeneic stem cells in an immunocompromised host, would be helpful to evaluate the survival issue of post donor cells.
In conclusion, our study demonstrates that transplantation of BMSCs overexpressing HO-1 enhanced the beneficial effects on cardiac function improvement by BMSCs via reducing myocardial inflammatory cytokine expression, apoptosis and promoting VEGF expression and angiogenesis in the hypoxic condition in vitro, and in ischemia/reperfusion porcine model in vivo. These data suggest that BMSCs HO-1 overexpression might be a promising strategy for future CCM in the clinical practice. Though BMSCs transfected with HO-1 gene could be efficiently labeled with SPIO nanoparticles without altering viability, MRI could successfully detect SPIO-labeled BMSCs in vivo at 1 week post transplantation. Acquired information from MRI was limited and only showed the homing status at this stage, and this technique supplies no information on cell viability information in vivo, since most SPIO positive BMSCs in vivo were engulfed by cardiac macrophages. Further studies are warranted to clarify if SPIO labeling increased engulfment of BMSCs by cardiac macrophages in vivo.
References
Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM (2005) Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 102:11474–11479
Arai T, Kofidis T, Bulte JW, de Bruin J, Venook RD, Berry GJ, Mcconnell MV, Quertermous T, Robbins RC, Yang PC (2006) Dual in vivo magnetic resonance evaluation of magnetically labeled mouse embryonic stem cells and cardiac function at 1.5 t. Magn Reson Med 55:203–209
Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, Ocherashvilli A, Holbova R, Yosef O, Barbash IM, Leor J (2007) Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 116:I38–I45
Ben-Dor I, Fuchs S, Kornowski R (2006) Potential hazards and technical considerations associated with myocardial cell transplantation protocols for ischemic myocardial syndrome. J Am Coll Cardiol 48:1519–1526
Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC (2004) Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 428:668–673
Carr CA, Stuckey DJ, Tatton L, Tyler DJ, Hale SJ, Sweeney D, Schneider JE, Martin-Rendon E, Radda GK, Harding SE, Watt SM, Clarke K (2008) Bone marrow-derived stromal cells home to and remain in the infarcted rat heart but fail to improve function: an in vivo cine-MRI study. Am J Physiol Heart Circ Physiol 295:H533–H542
Dörge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G (2002) Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol 34:51–62
Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M (2007) HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol 42:1036–1044
Foresti R, Sarathchandra P, Clark JE, Green CJ, Motterlini R (1999) Peroxynitrite induces haem oxygenase-1 in vascular endothelial cells: a link to apoptosis. Biochem J 339:729–736
Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ (2005) Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11:367–368
Guo J, Lin GS, Bao CY, Hu ZM, Hu MY (2007) Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation 30:97–104
Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J (2008) Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol 103:525–536
Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, Pessanha BS, Guttman MA, Varney TR, Martin BJ, Dunbar CE, McVeigh ER, Lederman RJ (2003) Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation 108:1009–1014
He G, Zhang H, Wei H, Wang Y, Zhang X, Tang Y, Wei Y, Hu S (2007) In vivo imaging of bone marrow mesenchymal stem cells transplanted into myocardium using magnetic resonance imaging: a novel method to trace the transplanted cells. Int J Cardiol 114:4–10
Herbert JM (2004) Effects of ADP-receptor antagonism beyond traditional inhibition of platelet aggregation. Expert Opin Investig Drugs 13:457–460
Koch KC, Schaefer WM, Liehn EA, Rammos C, Mueller D, Schroeder J, Dimassi T, Stopinski T, Weber C (2006) Effect of catheter-based transendocardial delivery of stromal cell-derived factor 1 alpha on left ventricular function and perfusion in a porcine model of myocardial infarction. Basic Res Cardiol 101:69–77
Kosmala W, Przewlocka-Kosmala M, Mazurek W (2005) Proinflammatory cytokines and myocardial viability in patients after acute myocardial infarction. Int J Cardiol 101:449–456
Kirshenbaum LA, de Moissac D (1997) The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation 96:1580–1585
Kraitchman DL, Bulte JW (2008) Imaging of stem cells using MRI. Basic Res Cardiol 103:105–113
Lyngbaek S, Schneider M, Hansen JL, Sheikh SP (2007) Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res Cardiol 102:101–114
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li W, Ma N, Ong LL, Nesselmann C, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lützow K, Lendlein A, Stamm C, Li RK, Steinhoff G (2007) Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25:2118–2127
Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP (1995) Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol 154:363–374
Moelker AD, Baks T, Wever KM, Spitskovsky D, Wielopolski PA, van Beusekom HM, van Geuns RJ, Wnendt S, Duncker DJ, van der Giessen WJ (2007) Intracoronary delivery of umbilical cord blood derived unrestricted somatic stem cells is not suitable to improve LV function after myocardial infarction in swine. J Mol Cell Cardiol 42:735–745
Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S (2005) Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 112:1128–1135
Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S (2008) Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26:2201–2210
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT (2008) Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol 44:486–495
Poncelet AJ, Vercruysse J, Saliez A, Gianello P (2007) Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 83:783–790
Schächinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM (2004) Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol 44:1690–1699
Schuh A, Liehn EA, Sasse A, Schneider R, Neuss S, Weber C, Kelm M, Merx MW (2009) Improved left ventricular function after transplantation of microspheres and fibroblasts in a rat model of myocardial infarction. Basic Res Cardiol 104:403–411
Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, Hatzistergos KE, Oskouei BN, Zimmet JM, Young RG, Heldman AW, Lardo AC, Hare JM (2008) Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol 294:H2002–H2011
Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC (2005) Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111:150–156
Shujia J, Haider HK, Idris NM, Lu G, Ashraf M (2008) Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res 77:525–533
Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G (2007) Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res 100:140–146
Sadek HA, Garry DJ (2008) Letter by Sadek and Garry regarding article, iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 117:e306
Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI (2005) Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol 46:1339–1350
Templin C, Kotlarz D, Marquart F, Faulhaber J, Brendecke V, Schaefer A, Tsikas D, Bonda T, Hilfiker-Kleiner D, Ohl L, Naim HY, Foerster R, Drexler H, Limbourg FP (2006) Transcoronary delivery of bone marrow cells to the infarcted murine myocardium: feasibility, cellular kinetics, and improvement in cardiac function. Basic Res Cardiol 101:301–310
Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E (2007) Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J 28:2667–2677
van den Bos EJ, Baks T, Moelker AD, Kerver W, van Geuns RJ, van der Giessen WJ, Duncker DJ, Wielopolski PA (2006) Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: possible interference with iron oxide-labelled cell tracking? Eur Heart J 27:1620–1626
Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD (2004) Intracoronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 363:783–784
Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, Fishbein MC, Gambhir SS (2003) Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation 108:1302–1305
Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M (2007) In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol 42:441–448
Yeh CH, Chen TP, Wang YC, Lin YM, Lin PJ (2009) HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. J Surg Res 155:147–156
Zeng B, Chen H, Zhu C, Ren X, Lin G, Cao F (2008) Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur J Cardiothorac Surg 34:850–856
Zhang H, Song P, Tang Y, Zhang XL, Zhao SH, Wei YJ, Hu SS (2007) Injection of bone marrow mesenchymal stem cells in the borderline area of infarcted myocardium: heart status and cell distribution. J Thorac Cardiovasc Surg 134:1234–1240
Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE (2001) Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 33:907–921
Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS (2007) SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 21:3197–3207
Acknowledgments
This work was supported by National Basic Research Program in China (no. 30670853).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jiang, Y., Chen, L., Tang, Y. et al. HO-1 gene overexpression enhances the beneficial effects of superparamagnetic iron oxide labeled bone marrow stromal cells transplantation in swine hearts underwent ischemia/reperfusion: an MRI study. Basic Res Cardiol 105, 431–442 (2010). https://doi.org/10.1007/s00395-009-0079-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0079-2