Abstract
The cardioprotective effects of ischemic preconditioning (IPC) can be mimicked or blocked by pharmacologic agents, which modulate the mitochondrial ATP-sensitive potassium (mKATP) channel, thereby implicating this channel in the mechanism of IPC. Cardioprotection can also be achieved via inhibition of mitochondrial respiratory complex II, and significant pharmacologic overlap exists between complex II inhibitors and mKATP channel agonists. However, the relationship between complex II and the mKATP channel remains unclear. Atpenin A5 (AA5) is a potent and specific complex II inhibitor, and herein we report that AA5 (1 nM) also activates the mKATP channel and protects against simulated ischemia-reperfusion (IR) injury in isolated cardiomyocytes. Similar to known mKATP agonists, AA5-mediated protection was sensitive to the mKATP antagonists 5-hydroxydecanoate (5HD) and glyburide. Notably, the optimal mKATP opening and protective concentration of AA5 had no effect on complex II enzymatic activity, suggesting an interaction of AA5 with complex II, but not inhibition of the complex per se, is necessary for protection. A cardioprotective effect of AA5 was also observed in isolated perfused hearts, wherein AA5 increased post-IR contractile function and decreased infarct size, in a 5HD-sensitive manner. In conclusion, the specific complex II inhibitor AA5 is the most potent mKATP activator discovered to date, and provides a novel method of activating mKATP channels and protecting the heart from IR injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heart is highly dependent on mitochondrial ATP synthesis for contraction, but cardiac mitochondria are also a major site of damage caused by ischemia-reperfusion (IR) injury. Post-ischemic recovery of mitochondrial function is an important predictor of cardiac contractile recovery [2, 9], and thus the preservation of mitochondrial function has received much attention as a potential strategy for cardioprotection [23].
Ischemic preconditioning (IPC) is a widely studied endogenous cardioprotective mechanism, triggered by several short non-lethal periods of IR prior to a prolonged ischemic insult [34]. The mechanisms underlying IPC signaling remain under debate [17], but interestingly IPC is known to inhibit mitochondrial electron transport at complexes I and II [11, 38, 45], and consistent with this, several cardioprotective pharmacologic agents are also mitochondrial respiratory inhibitors [10, 12, 42]. In addition to respiratory inhibition, extensive pharmacological evidence has implicated a mitochondrial ATP-sensitive potassium (mKATP) channel [4, 5, 21, 33] as a central player in IPC [5, 27]. Activators of this channel mimic IPC [20, 33] in a connexin 43-dependent manner [24], while inhibitors of the channel prevent the protection afforded by IPC [5, 21, 25].
Despite much attention, the molecular identity of the mKATP channel remains unclear. While it has been suggested that the mKATP may be comprised of surface KATP channel-related KIR and SUR subunits [31, 39], the specificity of antibodies used to indentify KATP subunits has been questioned [18, 40], and others have proposed that mKATP is comprised of mitochondrial proteins such as complex II [3]. A significant pharmacological overlap exists between complex II and the mKATP channel. For example the commonly used mKATP agonist diazoxide (DZX) is an effective complex II inhibitor [14, 42], while known complex II inhibitors such as 3-nitropropionic acid [3, 36] nitroxyl [37, 43], and malonate [45] are able to activate the mKATP channel, and protect against IR injury.
The lack of molecular identity for mKATP, and the poor specificity (1–300 μM) of pharmacologic agents used to probe both mKATP channel and complex II activity, creates a need for novel tools to investigate the channel. In this regard we hypothesized that the novel, potent, and highly specific complex II inhibitor atpenin A5 (AA5, IC50 ~ 10 nM) [32], may be an effective mKATP channel agonist and cardioprotective agent.
Materials and methods
Eighty male Sprague-Dawley rats, 200–225 g, were purchased from Harlan (Indianapolis, IN) and housed on a 12-h light/dark cycle with food and water available ad libitum. All procedures were performed in accordance with the US National Institutes of Health “Guide for the care and use of laboratory animals”, and were approved by the University of Rochester’s Committee on Animal Resources (protocol # 2007-087). Atpenin A5 (AA5) was from Axxora LLC (San Diego, CA), and was added from stock solutions in DMSO such that the final concentration of vehicle was <0.1%. Unless otherwise stated, all other chemicals were of the highest grade obtainable from Sigma (St. Louis, MO). In all experiments, each “N” was an independent heart perfusion or mitochondria/cardiomyocyte isolation. Statistical differences between groups were determined using ANOVA, with significance obtained when P < 0.05.
Cardiac mitochondria and SMPs
Mitochondria were isolated by differential centrifugation in sucrose-based buffer as previously described [45]. Rapid isolation was critical to ensure mKATP channel activity. Protein concentration was determined by the Folin-phenol method [30]. Submitochondrial particles (SMPs) were prepared from stored frozen mitochondria as previously described [45].
Mitochondrial assays
The enzymatic activity of complex II was measured as previously described [45], as the thenoyltrifluoroacetone-sensitive rate of succinate-driven, co-enzyme Q2-linked, reduction of dichlorophenolindophenol (DCPIP). The activity of mKATP was monitored spectrophotometrically at 520 nm, as the light scatter (absorbance) changes due to K+ uptake and swelling, as previously described [15, 45], within 1.5 h of mitochondrial isolation. The swelling buffer contained 1 μg/ml oligomycin to stop hydrolysis of ATP into ADP (via reverse mode ATP synthase), thus preventing mitochondria from entering state 3 respiration. This is important because complex II exerts greater control over mitochondrial respiration in state 3, and thus the effects of inhibitors (such as AA5) are somewhat respiratory state-dependent. Where indicated, K+ in the buffer was replaced with Na+ as a control. Also where indicated 2 mM succinate was replaced either by 2 mM glutamate plus 2 mM malate, or 2.5 mM ascorbate plus 0.25 mM NNN′N′-tetramethyl-p-phenylene diamine (TMPD).
Cardiomyocyte isolation and incubations
Ventricular myocytes were isolated by perfusion with endotoxin-stripped collagenase as previously described [13, 35], yielding ~4 × 106 cells/heart, >85% rod-shaped and excluding Trypan blue. Incubations comprised 5 × 105 cells in 5 ml Krebs Henseleit (KH) buffer plus 2% BSA, in 50 ml round-bottomed tubes in a shaking water bath (80 cycles/min) at 37°C. Cells were divided into the following groups (Fig. 4a): (1) Control, gassed with 95%O2/5%CO2, pH 7.4; (2) Simulated ischemia-reperfusion (SIR) comprising 1 h anoxia (95%N2/5%CO2, glucose-free KH buffer, pH 6.5), followed by 30 min. reoxygenation (95%O2/5%CO2, glucose-replete KH buffer, pH 7.4); (3) IPC + SIR, comprising 1 × 20 min anoxia plus 20 min reoxygenation, followed by SIR as above; (4) Drug + SIR, comprising treatment with compounds 20 min prior to SIR; (5) Drug + IPC + SIR, comprising treatment with compounds 20 min prior to IPC then SIR. Compounds tested (final concentrations in parentheses) were DZX (10 μM), malonate (100 μM), AA5 (0.1–100 nM), glyburide (2 μM), and 5HD (300 μM), with vehicles (DMSO or KH buffer) comprising <0.1% of total incubation volume, and appropriate vehicle controls included. At the end of all protocols, viability was determined by Trypan blue exclusion.
Perfused hearts
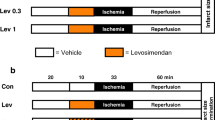
Isolated rat hearts were retrograde (Langendorff) perfused with KH buffer in constant flow mode, essentially as described [35, 45]. After 20 min equilibration, hearts were subjected to one of the following protocols (Fig. 5a): (1) Control, comprising 10 min perfusion, 20 min DMSO (vehicle) infusion, 30 s wash-out, then 145 min normoxic perfusion; (2) IR alone, comprising 10 min perfusion, 20 min DMSO (vehicle) infusion, 30 s wash-out, then 25 min global ischemia followed by 120 min reperfusion; (3) IPC + IR, comprising 3 × 5 min global ischemia plus 5 min reperfusion, then IR as above; (5) AA5 + IR, comprising 10 min perfusion, 20 min AA5 infusion (50 nM), 30 s. wash-out, then IR as above; (5) 5HD + AA5 + IR, comprising the AA5 + IR protocol as above, with additional infusion of 5HD (300 μM) during 0–30 min.; (6) 5HD + IR, comprising the IR alone protocol as above, with additional infusion of 5HD (300 μM) during 0–30 min. Pharmacologic agents (AA5, 5HD or DMSO vehicle) were infused into the perfusion cannula just above the aorta, with the final DMSO level being 0.017%, which did not affect cardiac function. Following all protocols, hearts were sliced transversely into 2 mm slices and stained in 1% (w/v) tetrazolium chloride (TTC) in phosphate buffer at 37°C for 20 min. Slices were fixed in 10% neutral buffered formalin for 24 h then placed between glass gel-plates, scanned, and infarct (white) versus live (red) tissue area quantified using public domain ImageJ software. Area at risk was 100% in this global ischemia model.
Results
The specificity and potency of mitochondrial complex II inhibition by AA5 was confirmed in a variety of systems. Figure 1 shows the inhibition profile for SMPs, mitochondria, and cardiomyocytes, with IC50 values of 8.3, 9.3, and 8.5 nM, respectively, obtained. These values agree with the published data on AA5 inhibition of complex II [32], and indicate that AA5 is specific for complex II even in complex systems such as the cardiomyocyte, which may contain competing AA5 binding sites.
Inhibition of complex II activity by AA5 in SMPs, isolated mitochondria, and cardiomyocytes. Complex II activity was measured as detailed in the “Materials and methods”. Data are mean ± SEM (N = 4–6) and are expressed as a percentage of the appropriate control values: 162 ± 4 nmol/(min mg) protein in SMPs, 98 ± 10 nmol/(min mg) protein in mitochondria, and 439 ± 49 nmol/(min 104) cardiomyocytes. Inset: structure of atpenin A5
As discussed in the introduction, pharmacological overlap exists between complex II and the mKATP channel, and thus we hypothesized that AA5 may activate mKATP. This hypothesis was tested using the popular mitochondrial swelling-based assay of mKATP activity [15, 28, 45]. The assay is based on a five-way pharmacologic profile, in which: (a) mitochondria rapidly swell in K+ based media, (b) swelling is inhibited by ATP, (c) a KATP channel agonist (e.g. DZX) overrides the inhibitory effect of ATP, (d) a KATP channel antagonist (e.g. 5HD) overrides the activating effect of the agonist, and (e) no effect of ATP, agonists or antagonists is observed in Na+ based media. The results (Fig. 2) show that AA5 (1 nM) fit this pharmacologic profile; it opened the mKATP channel in a 5HD- and glyburide-sensitive manner, and this activity was lost in Na+ media. An effect of AA5 on mKATP channel opening was also observed in mitochondria respiring on complex I-linked substrates (see below), despite the fact that AA5 is a complex II inhibitor.
Effects of AA5 on mKATP channel activity in isolated rat heart mitochondria. mKATP activity was determined by osmotic swelling assay as described in the Materials and methods. a Representative swelling traces of mitochondria (~0.25 mg/ml) in K+ media supplied with succinate (2 mM) as respiratory substrate. Optical density (OD) at 520 nm is on the y axis. Where indicated, 1 mM ATP (black squares), 1 nM AA5 plus ATP (gray squares), or 300 μM 5HD plus AA5 plus ATP (open triangles) were present in the media. b Magnitude of swelling relative to controls, determined by the decrease in OD after 0.2 min in either K+ media (black bars) or Na+ media (gray bars). Delta OD520 in controls was 0.020 ± 0.003 in K+ media and 0.015 ± 0.003 in Na+ media. Experimental conditions are listed below the x axis. Data are mean ± SEM, N > 6. * P < 0.05 versus ATP alone, # P < 0.05 versus ATP + AA5
Full dose responses of mKATP channel opening to AA5 in the presence of different respiratory substrates (Fig. 3a) revealed an interesting effect: with glutamate/malate as substrates, driving complex I-linked respiration, all concentrations of AA5 >1 nM opened the mKATP channel. However, with succinate as substrate, driving complex II-linked respiration, 1 nM AA5 opened the channel, but this effect was diminished at higher AA5 concentrations; i.e., concentrations which inhibit complex II. This inhibitory effect of high AA5 (>1 nM) on mKATP channel activity was likely due to complex II inhibition by AA5, limiting membrane-potential driven K+ uptake. Even though complex II was not completely inhibited under such conditions, it is known that cation uptake into mitochondria is exquisitely sensitive to small changes in ΔΨ [22]. With complex I-linked respiration, no such inhibition of complex II activity occurred [32], so maximal channel activity was maintained at high AA5 concentrations.
Dose responses of mKATP channel opening to AA5. a mKATP activity was determined as shown in Fig. 2, in mitochondria respiring on the complex I linked substrates glutamate plus malate (black bars), or complex II linked substrate succinate (gray bars). Varying concentrations of AA5 were added, as indicated below the x axis. Data are mean ± SEM, N > 4. * P < 0.05 versus ATP alone. b Comparison of the dose response to AA5 of mKATP channel opening (black circles, left y axis) versus complex II inhibition (gray triangles, right y axis). Data are from Figs. 1 and 3a
Furthermore, AA5 (1 nM) was able to induce mKATP channel opening and swelling in mitochondria respiring on ascorbate plus TMPD (swelling as % of control: ATP = 49 ± 1; ATP + DZX = 88 ± 1; ATP + AA5 = 104 ± 7; ATP + AA5 + Gly = 53 ± 1; ATP + AA5 + 5HD = 53 ± 9). Since ascorbate plus TMPD feed electrons directly into complex IV, bypassing the TCA cycle and upstream complexes I and II, this result precludes the possibility that the effect of AA5 on mitochondria respiring on complex I substrates (see above), was due to side effects of complex II inhibition on TCA cycle metabolism of glutamate plus malate. In further support of this, the respiration of mitochondria in the presence of glutamate plus malate was not inhibited by a very high concentration of AA5 (respiration rate with 1 μM AA5 = 98 ± 2% of control).
Interestingly, superimposing the mKATP channel opening and complex II inhibition dose responses to AA5 (Fig. 3b) reveals that the concentration of AA5 which optimally opened the mKATP channel (1 nM) was an order of magnitude below that which inhibited complex II (10 nM). This result suggests that complex II enzymatic inhibition is not the mechanism by which AA5 opens the mKATP channel.
Building on the knowledge that mKATP channel agonists can mimic the cardioprotective effects of IPC [4, 5, 14, 19, 21, 33], we next tested the cardioprotective efficacy of AA5 in an isolated cardiomyocyte model of simulated IR injury (protocols in Fig. 4a). As shown in Fig. 4b, cardiomyocytes were protected in a dose-dependent manner by AA5, with optimal protection at 1 nM (i.e. the same AA5 concentration which maximally opened the mKATP channel, Fig. 2). Furthermore, as shown in Fig. 4c, AA5-induced protection was blocked by the mKATP antagonists 5HD and glyburide. In this regard, AA5-induced protection was similar to that afforded by IPC, DZX, or the complex II inhibitor malonate, all of which were also protective in this model system, in a 5HD- and glyburide-sensitive manner (Fig. 4c). This suggests a similar mechanism of action for IPC, DZX, malonate, and AA5, i.e., opening of the mKATP channel.
Effect of mKATP openers and blockers on the response of cardiomyocytes to simulated IR injury. An isolated adult rat cardiomyocyte model of SIR injury was used as detailed in the methods. Cell viability was measured via trypan blue exclusion at the end of reoxygenation. a Experimental design scheme; “Drug” refers to either DZX (10 μM), malonate (100 μM), AA5 (1nM), 5HD (300 μM), or glyburide (2 μM). b Dose response of post-SIR cell viability to AA5 pre-treatment. Concentrations of AA5 are listed below the x axis. data are mean ± SEM, N ≥ 5. * P < 0.05 versis SIR alone. c Response of post-SIR cell viability to mKATP activators alone (IPC, DZX, malonate, AA5), or in the presence of mKATP antagonists (5HD or glyburide). Experimental conditions are listed below the x axis. All data are mean ± SEM, N ≥ 5. * P < 0.05 versus SIR alone, # P < 0.05 versus the effect of each mKATP activator (IPC, DZX, malonate, AA5) alone
The use of AA5 as a cardioprotective agent was next translated into a whole organ system, the Langendorff-perfused rat heart (protocols in Fig. 5a). Initial dose response investigations (not shown) revealed cardioprotection at 50 nM AA5, i.e., far higher than the protective dose in cardiomyocytes (1 nM). We hypothesize this difference may be due to drug diffusion or binding kinetics: In cardiomyocyte experiments AA5 was present in a fixed volume of incubation media, thus slow equilibration would still result in complex II inhibition. In contrast, the transit-time of buffer through the coronary circulation in the non-recirculating perfused heart system is very short (~1 s.) and thus a higher concentration in the perfusion buffer may be required to achieve an adequate intracellular AA5 concentration, to drive the equilibrium toward inhibition.
Effect of AA5 on the response of perfused rat hearts to IR injury. Rat hearts were perfused in Langendorff mode and subjected to IR injury as detailed in the methods. a Experimental design scheme. Left ventricular balloon pressure was monitored throughout, and TTC staining for infarct performed after 2 h reperfusion. b Typical left ventricular pressure traces from each of the six IR protocols, with the time (x) axis compressed. The upper and lower boundaries of the black shaded area represent systolic and diastolic pressure, respectively. The onset of ischemia and reperfusion are indicated by arrows “Isch.” and “Rep.”, respectively. Labels to the right indicate the protocol followed, per panel A. c Typical images of cross-sectional slices from TTC-stained hearts in each experimental group. White indicates dead tissue (infarct) while red indicates live one. d Rate pressure product (RPP) values calculated from traces of the type shown in panel B, for hearts subjected to each protocol. Data are split into two panels for clarity, and are mean ± SEM, N ≥ 5. * P < 0.05 versus IR alone. # P < 0.05 versus AA5 + IR group. e Quantitation of infarct size from images of the type shown in panel C, for hearts subjected to each protocol. Infarct is expressed as percentage of the area at risk, which was 100% in this global ischemia model. Data are mean ± SEM, N ≥ 5. * P < 0.05 versus IR alone. # P < 0.05 versus AA5 + IR group
As the data in Fig. 5b and d show, pre-administration of AA5 afforded a significant improvement in post-IR recovery of left ventricular function (rate pressure product), with the degree of protection rivaling that of IPC. Furthermore, AA5-induced cardioprotection was blocked by the mKATP antagonist 5HD, suggesting that it was not mediated by inhibition of complex II (since the complex II inhibiting effects of AA5 are not 5HD sensitive). 5HD alone was without effect on IR injury.
There was a significant drop in RPP immediately following AA5 administration (Fig. 5b, d), although this was not significantly different from that seen with administration of IPC. In addition, consistent with the known vasoactive effects of AA5 [1], a 36 ± 4% drop in coronary perfusion pressure (85 ± 10 → 53 ± 3 mmHg) was observed immediately following AA5 administration. However, a similar decrease (32 ± 4%, i.e. 103 ± 9 → 68 ± 3 mmHg) was also observed in the AA5 plus 5-HD group, which was not protected against IR injury. Thus, we consider it unlikely that changes in coronary pressure were responsible for cardioprotection by AA5.
The functional data were corroborated by measurements of infarct size (Fig. 5c, e), in which AA5-treated hearts exhibited significantly smaller infarcts than controls (IR alone), and 5HD blocked the infarct-sparing effects of AA5. Similar to the functional data, 5HD alone did not affect infarct size.
Discussion
The major findings of this study are that the potent and specific complex II inhibitor AA5 both opens the mKATP channel and protects cardiomyocytes from simulated IR injury, at concentrations well below those at which it inhibits complex II. Furthermore, AA5 protects the intact perfused heart from IR injury, and this protection is blocked by a mKATP channel antagonist.
Previously we reported that the complex II inhibitor malonate both opens the mKATP and inhibits complex II enzymatic activity, at 25–100 μM [45]. This is consistent with the proposal that complex II and mKATP may be related at the molecular level [3]. However, we noted that the ability of malonate to open the mKATP was also seen in mitochondria respiring on complex I-linked substrates, thereby suggesting that the effects of malonate on the mKATP channel may not be directly related to the effects on complex II enzymatic activity. Owing to the relatively high concentrations of malonate required to observe these effects in isolated mitochondria, and the nature of malonate as a metabolic acid with numerous roles in the cell, further development of these studies to investigate the role of mKATP/complex II interplay in cardioprotection was precluded. Thus, a more potent and specific inhibitor of complex II was sought, yielding AA5 which is structurally related to ubiquinone and inhibits complex II by blocking electron transfer at its ubiquinone binding site [26, 32].
The results in Figs. 2 and 3 show that AA5 is the most potent mKATP channel opener discovered to date, opening the channel at only 1 nM, which is 10,000-fold more potent than the archetypal mKATP agonist DZX [20]. Interestingly, DZX is also known to inhibit complex II [14, 42], but this occurs at higher concentrations (<30 μM) than those which maximally open the mKATP channel (10 μM) [14, 45]. In a similar manner, the effect of AA5 on the mKATP channel occurred at a concentration one order of magnitude below the IC50 for complex II inhibition (Fig. 3b). On the basis of these data, showing that inhibition of complex II enzymatic activity per se is not required for channel opening, we propose that the processes of mKATP channel opening and complex II enzymatic inhibition may be mechanistically unrelated. Nevertheless, there are several compelling reasons to believe that the complex II protein may play a structural role in the channel itself, or its regulation. First, significant pharmacological overlap exists between complex II and the channel (including AA5 as described herein). Second, genetic sequence overlap exists between subunit C of complex II and the sulfonylurea receptor (SUR) subunit of surface KATP channels [44]. While this subunit alone is not the binding site for AA5, it is possible that AA5 binding to the ubiquinol site in complex II may bring about structural changes in the complex which facilitate its recruitment or interaction with bona fide mKATP channel proteins (KIR or SUR subunits).
It should be noted that our data do not preclude the possibility that the mKATP channel is a protein unrelated to complex II, which coincidentally happens to contain a high affinity AA5-binding site. However, AA5 is effective at very low concentrations (2–4 orders of magnitude lower than other complex II inhibitors and mKATP channel openers), and we consider it unlikely that such a specific reagent would bind to structurally unrelated proteins. Furthermore, mitochondria contain a lot of complex II, which any other AA5 binding proteins would have to compete with. In addition, inhibitors which bind to distinct sites on complex II (i.e. the succinate-binding site and the Q-binding site, the latter of which straddles several complex II subunits) both activate the mKATP channel. If the channel was a distinct molecule unrelated to complex II, it would be a highly unlikely coincidence that it would possess both types of inhibitor binding site within its structure. Thus, Occam’s razor leads us to conclude that complex II plays an important regulatory or structural role in the mKATP channel itself.
Whether the mKATP comprises similar structural components to surface KATP channels (KIR/SUR) is unclear, and this is confounded by the pharmacologic overlap between surface and mitochondrial KATP channels [16]. A recent study [1] reported that arteries from SUR2−/− mice dilated less in response to the general KATP opener pinacidil. However, vasodilatation in response to the mKATP opener DZX was not affected by SUR2 ablation. Notably, vasodilatation was also observed in response to the complex II inhibitor AA5 (albeit at 1 μM), and was also unaffected by SUR2 ablation. These differential results suggest that pinacidil-induced vasodilatation depends on both surface and mitochondrial KATP channels, but that DZX- and AA5-induced vasodilatation are SUR2-independent and presumably require mKATP channels or complex II. Thus, complex II may substitute for SURs in the assembly of the mKATP channel. The fact that complex II activity is allosterically activated by ATP [45] (the endogenous ligand of the KATP channels), also suggests a functional overlap between these two proteins. Another recent study found that several truncated splice variants of SUR are found in cardiomyocytes and it was hypothesized that these short forms of SUR2 may be targeted to mitochondria [40]. Thus, the precise molecular nature of the relationship between complex II, SURs and KIR, in assembling the mKATP channel remains to be elucidated. AA5, identified herein as a potent (1 nM) mKATP agonist, may prove to be an important tool in the future elucidation of a complete molecular identity for mKATP.
Regardless the nature of the mKATP channel and the role of complex II in its make-up, the results of the current investigation suggest that AA5 may be a potent therapeutic for cardioprotection. Similar to DZX, IPC and malonate, AA5 protected cardiomyocytes from simulated IR injury in a 5HD- and glyburide-sensitive manner. This cardioprotection translated to a whole organ model of IR injury, in which AA5 afforded both improved post-IR contractile function and lessened infarct size. The mechanism by which AA5 protected the heart appears to be independent of its inhibition of complex II, since protection was blocked by the mKATP channel antagonist 5HD. This is in agreement with findings that AA5 opened the mKATP channel at a concentration which did not inhibit complex II. Thus, while reversible inhibition of the mitochondrial respiratory chain is emerging as an important cardioprotective paradigm, with several inhibitors of complexes I, II, and IV exhibiting cardioprotective efficacy [11, 12, 35, 36, 41], AA5 does not protect via this mechanism.
Future studies will be aimed at investigating the in vivo efficacy of AA5 as a cardioprotectant, but clearly the usefulness of AA5 in vivo may be limited by its effects on other organ systems. For example, while the complex II inhibitor 3-NP is known to protect hearts in vitro [36], and malonate protects cardiomyocytes in vitro (Fig. 4c), prolonged administration of 3-NP or malonate in vivo leads to striatal lesions which mimic Huntington’s disease [6, 8]. In a similar manner, the complex I inhibitor rotenone can be cardioprotective [29], but prolonged administration of rotenone and other complex I inhibitors such as MPTP in vivo leads to a Parkinson’s disease like syndrome [7]. Thus, the potential clinical use of AA5 will have to be carefully balanced against any potential long term neurodegenerative side effects. The development of cardiac-specific or non-blood-brain-barrier-penetrating complex II inhibitors or mKATP channel agonists may provide a mechanism to bypass such effects.
In summary, we have shown herein that the potent and specific complex II inhibitor AA5 protects the heart from IR injury through a mKATP channel dependent mechanism. This finding suggests that complex II plays a role in the composition or regulation of the mKATP channel, which may be determined using AA5, a useful tool.
References
Adebiyi A, McNally EM, Jaggar JH (2008) Sulfonylurea receptor-dependent and -independent pathways mediate vasodilation induced by ATP-sensitive K+ channel openers. Mol Pharmacol 74:736–743
Akar FG, Aon MA, Tomaselli GF, O’Rourke B (2005) The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115:3527–3535
Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E (2004) Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci USA 101:11880–11885
Armstrong SC, Liu GS, Downey JM, Ganote CE (1995) Potassium channels and preconditioning of isolated rabbit cardiomyocytes: effects of glyburide and pinacidil. J Mol Cell Cardiol 27:1765–1774
Auchampach JA, Grover GJ, Gross GJ (1992) Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res 26:1054–1062
Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT (1993) Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem 61:1147–1150
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Borlongan CV, Koutouzis TK, Sanberg PR (1997) 3-Nitropropionic acid animal model and Huntington’s disease. Neurosci Biobehav Rev 21:289–293
Brookes PS, Digerness SB, Parks DA, Darley-Usmar VM (2002) Mitochondrial function in response to cardiac ischemia-reperfusion after oral treatment with quercetin. Free Radic Biol Med 32:1220–1228
Burwell LS, Brookes PS (2008) Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal 10:579–600
Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS (2006) Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394:627–634
Chen Q, Hoppel CL, Lesnefsky EJ (2006) Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther 316:200–207
Dai L, Brookes PS, Darley-Usmar VM, Anderson PG (2001) Bioenergetics in cardiac hypertrophy: mitochondrial respiration as a pathological target of NO•. Am J Physiol Heart Circ Physiol 281:H2261–H2269
Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, Terzic A (2003) Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol 284:H1048–H1056
Facundo HT, de Paula JG, Kowaltowski AJ (2007) Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med 42:1039–1048
Facundo HT, Fornazari M, Kowaltowski AJ (2006) Tissue protection mediated by mitochondrial K + channels. Biochim Biophys Acta 1762:202–212
Ferdinandy P, Schulz R, Baxter GF (2007) Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59:418–458
Foster DB, Rucker JJ, Marban E (2008) Is Kir6.1 a subunit of mitoKATP? Biochem Biophys Res Commun 366:649–656
Garlid KD, Dos SP, Xie ZJ, Costa AD, Paucek P (2003) Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K+ channel in cardiac function and cardioprotection. Biochim Biophys Acta 1606:1–21
Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ (1997) Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res 81:1072–1082
Gross GJ, Auchampach JA (1992) Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res 70:223–233
Gunter TE, Pfeiffer DR (1990) Mechanisms by which mitochondria transport calcium. Am J Physiol 258:C755–C786
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res 61:372–385
Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, Garcia-Dorado D, Di LF, Schulz R, Heusch G (2005) Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res 97:583–586
Hide EJ, Thiemermann C (1996) Limitation of myocardial infarct size in the rabbit by ischaemic preconditioning is abolished by sodium 5-hydroxydecanoate. Cardiovasc Res 31:941–946
Horsefield R, Yankovskaya V, Sexton G, Whittingham W, Shiomi K, Omura S, Byrne B, Cecchini G, Iwata S (2006) Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction. J Biol Chem 281:7309–7316
Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD (1998) State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem 273:13578–13582
Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD (2001) Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol Heart Circ Physiol 280:H649–H657
Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL (2004) Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem 279:47961–47967
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mironova GD, Negoda AE, Marinov BS, Paucek P, Costa AD, Grigoriev SM, Skarga YY, Garlid KD (2004) Functional distinctions between the mitochondrial ATP-dependent K+ channel (mitoKATP) and its inward rectifier subunit (mitoKIR). J Biol Chem 279:32562–32568
Miyadera H, Shiomi K, Ui H, Yamaguchi Y, Masuma R, Tomoda H, Miyoshi H, Osanai A, Kita K, Omura S (2003) Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase). Proc Natl Acad Sci USA 100:473–477
Mizumura T, Nithipatikom K, Gross GJ (1995) Bimakalim, an ATP-sensitive potassium channel opener, mimics the effects of ischemic preconditioning to reduce infarct size, adenosine release, and neutrophil function in dogs. Circulation 92:1236–1245
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Nadtochiy SM, Burwell LS, Brookes PS (2007) Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 42:812–825
Ockaili RA, Bhargava P, Kukreja RC (2001) Chemical preconditioning with 3-nitropropionic acid in hearts: role of mitochondrial KATP channel. Am J Physiol Heart Circ Physiol 280:H2406–H2411
Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA, Paolocci N (2003) Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic Biol Med 34:33–43
Pasdois P, Beauvoit B, Tariosse L, Vinassa B, Bonoron-Adele S, Santos PD (2006) MitoKATP-dependent changes in mitochondrial volume and in complex II activity during ischemic and pharmacological preconditioning of Langendorff-perfused rat heart. J Bioenerg Biomembr 38:101–112
Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD (1992) Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem 267:26062–26069
Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ (2008) Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J Mol Cell Cardiol 44:188–200
Riess ML, Stowe DF, Warltier DC (2004) Cardiac pharmacological preconditioning with volatile anesthetics: from bench to bedside? Am J Physiol Heart Circ Physiol 286:H1603–H1607
Schafer G, Wegener C, Portenhauser R, Bojanovski D (1969) Diazoxide, an inhibitor of succinate oxidation. Biochem Pharmacol 18:2678–2681
Shiva S, Crawford JH, Ramachandran A, Ceaser EK, Hillson T, Brookes PS, Patel RP, Darley-Usmar VM (2004) Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J 379:359–366
Wohllk N, Thomas PM, Huang E, Cote GJ (1998) A human succinate-ubiquinone oxidoreductase CII-3 subunit gene ending in a polymorphic dinucleotide repeat is located within the sulfonylurea receptor (SUR) gene. Mol Genet Metab 65:187–190
Wojtovich AP, Brookes PS (2008) The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: Implications for ischemic preconditioning. Biochim Biophys Acta 1777:882–889
Acknowledgments
This work was funded by an American Heart Association pre-doctoral fellowship to APW and a grant to PSB from the National Institutes of Health (RO1-HL071158).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wojtovich, A.P., Brookes, P.S. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol 104, 121–129 (2009). https://doi.org/10.1007/s00395-009-0001-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0001-y