Abstract
Aim
During atrial fibrillation, arterial hypertension and systolic or diastolic heart failure, atrial myocytes are exposed to increased baseline stretch. Atrial stretch has been shown to induce cellular hypertrophy and extracellular matrix remodeling (ECM) via angiotensin-II dependent pathways and the matrix metalloproteinases system (MMPs). We hypothesized that atrial myocytes exposed to static stretch may increase their ECM remodeling activity via up-regulation of MMP-2/-9. We then tested the hypothesis that the membrane bound angiotensin-II type 1 (AT1) receptor and the intracellular calcineurin (Cn)-NFAT signaling pathway are potential mediators of stretch-induced MMP alterations, since Cn-NFAT is one important contributor to myocyte hypertrophy.
Methods and results
Neonatal rat atrial myocytes (NRAM) were cultured under conditions of static stretch by 21%. The differential effects of selective AT1 receptor blockade by losartan, Cn blockade by Cyclosporine-A (CsA) or NFAT inhibition by 11R-VIVIT (VIV), were analyzed. Stretch resulted in a significant up-regulation of active-MMP-2/-9 protein amount (active-MMP-2 ng/µg: control 8.95 ± 0.64 vs. stretch 13.11 ± 0.74 / active-MMP-9 ng/µg: control 1.45 ± 0.18 vs. stretch 1.94 ± 0.21, all n = 5) and enzyme activity (MMP-2 in %: control 1 ± 0.0 vs. stretch 1.87 ± 0.25, n = 7) associated with a significant increase of the membrane-type-1-MMP (MT1-MMP) protein expression (MT1-MMP in %: control 1 ± 0.0 vs. stretch 2.17 ± 0.21, n = 8). These observations were accompanied by an activation of the Cn-NFAT pathway (Cn-activity in nmol PO4 release/20 µg protein/30 min: control 0.37 ± 0.08 vs. stretch 0.65 ± 0.09, n = 3 / NFATc1-DNA binding activity in %: control 1 ± 0.0 vs. stretch 1.53 ± 0.17, n = 3). Losartan, CsA or VIV abolished stretch-induced alterations in MMP-2/-9 and MT1-MMP expression and enzyme activity by normalizing the Cn-activity and the DNA binding activity of NFATc1.
Conclusion
Our results present new insights in molecular mechanisms of ECM remodeling activity of atrial myocytes exposed to static stretch. The AT1-Cn-NFAT pathway is a potential mediator of MMP activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most frequent arrhythmia encountered in clinical practice [9]. There is a huge body of evidence of various structural and functional atrial-remodeling processes associated with AF once established. Less, however, is known about atrial-remodeling processes during conditions, which favor the occurrence of AF. Among these, increasing age, arterial hypertension, congestive heart failure (CHF) and recurring AF itself are the most prominent ones [11, 25, 37]. The common denominator of these risk factors is mechanical load. It is conceivable that the concomitant left atrial pressure due to mechanical load may cause stretch of the individual myocyte and may contribute to atrial electrical and structural remodeling processes before AF develops. In line with this hypothesis, it has recently been shown that stretch-induced changes of atrial ionic currents occur and may promote AF [33]. An elevated left atrial pressure may also cause left atrial enlargement, which is another common characteristic of the above mentioned conditions. Chronic left atrial enlargement in turn is accompanied by profound interstitial remodeling processes involving the renin–angiotensin–aldosterone system (RAAS) and the matrix metalloproteinases family (MMP), especially MMP-2/-9 and TIMP-1 [6, 24, 32]. Accordingly, numerous reports suggest a decrease in the development or recurrence of AF in patients with hypertension or heart failure treated with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blocking (ARB) agents [34, 35].
ARBs seem to counteract several stretch-induced changes of cardiac tissue including electrical and structural remodeling [12]. Recently our group could show that inhibition of the AT1 receptor with losartan attenuated stretch-induced electrical remodeling in NRAM [30]. In addition, ARBs abolished stretch-induced cardiac hypertrophy, which is mainly mediated by the Ca2+/calmodulin-activated cytoplasmic serine/threonine phosphatase calcineurin (Cn) [19]. Cn dephosphorylates nuclear factor of activated T cells (NFAT) leading to nuclear translocation and transcriptional activation of numerous genes [17]. The Cn-NFAT pathway is up-regulated in clinical conditions associated with cellular stretch and hypertrophy like arterial hypertension, heart failure, and AF itself [13, 19, 31]. In particular, Goette and co-workers [3] recently demonstrated an increase of the Cn-NFAT cascade in atrial tissue of patients with AF, which contributed to atrial myocyte hypertrophy.
Of note, in neonatal rat ventricular myocytes (NRVM) Wang and co-workers [36] recently demonstrated that angiotensin-II is also a potential mediator in stretch-induced activation of MMPs, indicating an essential link of angiotensin-II and MMPs in the pathogenesis of ventricular structural remodeling.
We therefore hypothesized that atrial myocytes exposed to mechanical stretch may increase their ECM remodeling activity due to up-regulation of active-MMP-2/-9. We then tested the hypothesis that the membrane bound AT1 receptor and the intracellular Cn-NFAT signaling pathway are potential mediators of stretch-induced MMP activation.
Materials and methods
Cell culture and application of homogeneous equibiaxial static stretch
All animal experiments were approved by the local and state Ethics in Animal Research Committee. NRAM from 1 to 3-day-old Sprague–Dawley rats were isolated and cultured as described previously [30]. Cardiomyocytes were purified from fibroblasts through gradient centrifugation. Regularly the purity of cell culture was 95% assessed by specific immunofluorescence staining for troponin T-C. Twenty-four hours after cell isolation the medium was replaced with a complete serum free medium. After medium change, homogeneous equibiaxial static stretch by 21% was introduced to the attached cells for 24 h as described previously [26]. To assess the integrity of the culture, the numbers of cells of identical microscopic windows were counted before and after exposure to stretch. If the cell number per view deviated by more than 10% the culture was discarded.
Preparation of conditioned medium for ELISA
Depending on the drug protocol 1 × 10−7 mol/l of BQ123 or BQ788 [22], 1 × 10−6 mol/l of losartan or Cyclosporin A (CsA) [15, 40, 41] or 1 × 10−5 mol/l of 11R-VIVIT (VIV) (a cell permeable version of NFAT inhibitor) was added at the initiation of stretch [2]. Losartan, CsA, BQ123, BQ788 were purchased from Sigma–Aldrich (Germany, Steinheim), 11R-VIVIT was purchased from Calbiochem (Germany, Darmstadt).
Before and after 24 h of static stretch the conditioned medium was collected and concentrated using the Viva Spin Columns (Sartorius, Germany). Normalized protein amounts were used for ELISA. Protein concentration was determined with the Bradford method using the DC Protein Assay (Bio-Rad) as described previously [26].
ELISA for MMP quantity
During AF associated atrial-remodeling alterations of MMP-2/-9 and TIMP-1 have been described [24]. To measure the MMP-2/-9 quantity in its pro- and active-forms and the amount of MMP-2/-9 bound to TIMP-1 the sandwich ELISA technique with specific capture- and biotinalyted detection antibodies was performed (R&D Systems). All antibodies were raised against respective rat proteins. Representative standards (R&D Systems) were added to the precoated wells containing the antibody of interest. The resultant reaction of the streptavidin-horseradish-peroxidase was read at a wavelength of 450 nm on a microplate reader (Spectrafluor Plus from Tecan, Switzerland). In order to measure the bound complexes with TIMP-1 the first antibody captured the MMP of interest (pro- or active domain) and the second antibody detected TIMP-1. MMPs are present in pro- and active-forms, which are both able to form complexes with TIMP-1, which in turn inactivates MMPs. To determine the amount of active-MMPs the amount of MMP pro-forms and the amount of MMPs in complex with TIMP-1 was subtracted from the total-MMPs.
Zymography for MMP enzyme activity
MMP enzyme activity was measured by gelatine zymography as described by Kleiner et al. [10]. Samples normalized for protein concentration (50 µg) were mixed in zymogram sample buffer (0.4 M Tris–HCl pH 6.8, 10% SDS, 20% glycerol and 0.1% bromphenol blue) and were subjected to electrophoresis carried out at 20 mA for 1.5 h, without boiling or reduction, through a 10% polyacrylamide gel co-polymerized with 0.1% gelatine at 4°C. After electrophoresis, gels were incubated for 30 minutes at RT in renaturing buffer (2.5% Triton X-100) on a rotary shaker, then washed two times, 30 min each, with developing buffer (50 mM Tris–HCl pH 7.5, 200 mM NaCl, 5 mM CaCl, 0.02% Brij 35) and then incubated overnight at 37°C in the same developing buffer. Staining and destaining were carried out at RT on a rotary shaker. Each gel was fixed with 100 ml of 40% methanol and 7% acetic acid, stained with 0.1% Coomassie brilliant blue R250 for 3 h and then destained with 10% methanol and 7% acetic acid for 1 h. Standards for the active forms of recombinant human MMP-2 and MMP-9 were included on the gels for comparison and identification. Gelatinolytic activity was quantified by densitometric analysis.
Immunocytofluorescence for MT1-MMP
Cells were fixed in 4% paraformaldehyde, permeabilized with Triton X-100 and blocked with 1% BSA. Cells were incubated with the primary antibody, (1:150) rabbit anti-MT1-MMP (Santa Cruz), (1:150) mouse anti-Trop T-C (Santa Cruz) overnight at 4°C and then with a goat anti-rabbit (1:100) and anti-mouse (1:100) secondary antibody for 2 h coupled to Alexa Fluor 488 (MT1-MMP) or Alexa Fluor 647 (Trop T-C) (Invitrogen). Nuclei were stained with 4′,6-diamidino-2-phenylindoldihydrochlorid (DAPI). To visualize fluorescence signals the Axiovert 200 M microscope and the AxioVision Rel. 4.5 software from Zeiss was used.
Western blot for calcineurin and MT1-MMP quantification
Cells were homogenized in lysis buffer containing 128 mM Tris–HCl (pH 7.6), 4.6% SDS and 10% glycerol. Cell lysates were cleared by centrifugation at 17.000g for 20 min. Extracts (30 µg) were subjected to electrophoretic separation through a 10% SDS-polyacrylamide gel and subsequently transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% bovine serum albumin in phosphate-buffered saline containing 0.1% Tween 20 (PBST). Primary rabbit anti-calcineurin A polyclonal antibody (1:100, Calbiochem, Merck) was used for calcineurin A detection. Primary rabbit anti-MT1-MMP (1:100, Santa Cruz) was used for MT1-MMP detection. Blots were washed in PBST and incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated antibody (GE Healthcare, UK). Finally, the ECL Advance Western Blotting Detection Kit (GE Healthcare Life Sciences, Germany) was used to visualize the bands.
Calcineurin activity
Calcineurin activity was measured with the Biomol Green Cellular Calcineurin Assay Kit Plus (AK816), following the manufactures instructions as described previously [41].
DNA binding activity of NFATc1
To determine the amount of nuclear bound NFAT the DNA binding activity of dephosphorylated NFATc1 was measured. NFATc1 was chosen as a representative member of the NFAT family (NFATc1-c4), which are all expressed in cardiac tissue [38]. Nuclear protein was extracted from cultured NRAM 24 h after static stretch using the nuclear extract kit from Active Motif (Rixensart, Belgium), according to the manufacturer’s instructions. The DNA binding activity of activated NFATc1 was measured by ELISA using the TransAm Transcription Factor Assay Kit (Active Motif, Belgium), following the manufacturer’s instructions.
RNA preparation and first-strand cDNA synthesis
Total RNA was extracted from NRAM using the Qiagen RNeasy Mini Kit. A total of 1 µg of RNA was reverse transcribed using random hexamers from the Fermentas first-strand cDNA synthesis kit (#K1622).
Quantitative real-time reverse transcription-PCR
Real-time PCR was performed in 96-well plates on the ABI Prism 7000 sequence detection system (ABI) as described previously [30]. For data analysis the comparative 2−ΔΔCT method was used [14]. Data were collected with instrument spectral compensation by Applied Biosystems SDS 1.2.3 software.
Primers and probes for quantitative real-time reverse transcription-PCR
PCR primers and fluorogenic probes for the target genes and the endogenous control were purchased as assays-on-demand (Applied Biosystems, Foster City, CA, USA). The assay numbers for the endogenous control and target genes were as follows: Rn00560865_m1 (beta-2 microglobulin), Rn01538177_m1 (MMP-2), Rn00675895_g1 (MMP-9), Rn00579172_m1 (MT1-MMP) and Rn01430875_g1 (TIMP-1).
Statistical analysis
All values are expressed as mean ± SEM. Comparisons of two groups were made by the student’s t test and multiple groups were made by one-way ANOVA followed by LSD post hoc test. Values of P < 0.05 were considered statistically significant.
Results
Static stretch increases MMP-2/-9 protein amount and enzyme activity in atrial myocytes
NRAM were subjected to static stretch for 24 h in a complete serum free condition as described in the method section. Static stretch by 21% resulted in a significant increase of unbound active-MMP-2/-9 protein levels in the stretch-induced cell-conditioned medium (active-MMP-2: control 8.95 ± 0.64 vs. stretch 13.11 ± 0.74 ng/µg protein / active-MMP-9: control 1.45 ± 0.18 vs. stretch 1.94 ± 0.21 ng/µg protein, all n = 5), whereas the pro-forms of both MMP-2/-9 were significantly down-regulated (proMMP-2: control 5.13 ± 0.55 vs. stretch 2.89 ± 0.77 ng/µg protein / proMMP-9: control 0.84 ± 0.18 vs. stretch 0.46 ± 0.15 ng/µg protein, all n = 5), indicating an increase of proteolytic conversion (Fig. 1a, b). These observations were accompanied by a significant down-regulation of the amount of MMP-2/-9 + TIMP-1 bound complexes (MMP-2 + TIMP-1: control 1.73 ± 0.27 vs. stretch 1.03 ± 0.21 ng/µg protein / MMP-9 + TIMP-1: control 0.77 ± 0.09 vs. stretch 0.49 ± 0.11 ng/µg protein, all n = 5), suggesting a net increase in the gelatinolytic activity of MMP-2/-9 caused by static stretch (Fig. 1c, d).
Static stretch increases MMP-2/-9 protein amount and enzyme activity in atrial myocytes: atrial myocytes were stretched for 24 h by 21% and MMP protein levels in the cell-conditioned medium were quantified with the ELISA technique as described in the method section. a, b Pro- and active-MMP-2/-9 protein levels of stretched and un-stretched control cells. Static stretch by 21% caused a significant up-regulation of unbound active-MMP-2/-9 whereas the pro-forms of MMP-2/-9 decreased in a significant manner (n = 5, with three samples each). c, d Measurements of MMP-2/-9 protein levels in complex with TIMP-1 revealed a significant down-regulation of MMP-2/-9 + TIMP-1 complexes (n = 5, with three samples each). e Gelatine zymography for MMP-2 enzyme activity. MMP-2 enzyme activity increased by static stretch nearly twofold (n = 7, with 3 samples each). f Real-time PCR experiments were performed to scan mRNA levels of MMP 2/-9 and TIMP-1. Stretch caused a significant up-regulation of MMP 2/-9 and TIMP-1 mRNA (n = 3, with 3 samples each). *P < 0.05 versus control
In line with the increased expression level of mature MMP-2/-9, functional data on enzyme activity revealed a significant increase in MMP-2 enzyme activity measured with gelatine zymography (MMP-2 in %: control 1 ± 0.0 vs. stretch 1.87 ± 0.25, n = 7) (Fig. 1e). MMP-9 enzyme activity, however, could not be detected in the gelatine zymography experiments, possibly by a nearly 6.5-fold lower amount of MMP-9 in the conditioned medium. Therefore, in our model of cultured NRAM, gelatinolytic activity is predominantly mediated by MMP-2. To analyze whether these observations were regulated on transcriptional level, we performed real-time PCR experiments to scan mRNA levels of MMP-2/-9 and TIMP-1. The increase of active-MMP-2 and -9 protein amount and MMP-2 enzyme activity was paralleled by a significant up-regulation of MMP-2/-9 mRNA (relative mRNA expression: control 1 ± 0.0 vs. stretch for MMP-2: 1.83 ± 0.17 and MMP-9: 1.55 ± 0.29, n = 3). In addition, TIMP-1 mRNA also increased within 24 h of static stretch by 21% as compared to control cells (control 1 ± 0.0 vs. stretch for TIMP-1: 1.97 ± 0.19, n = 3) (Fig. 1f).
Static stretch-induced MMP activation is mediated by angiotensin-II type 1 receptors
Myocytes respond to stretch with a release of intracellular stored angiotensin-II that subsequently binds to AT1 receptors in an autocrine/paracrine manner [20, 29]. To investigate whether stretch-mediated activation of MMPs in atrial myocytes depends on AT1 receptors, we first examined if the AT1 receptor blocker losartan prevents MMP-2/-9 and TIMP-1 mRNA up-regulation caused by static stretch. In fact, losartan prevented the stretch-induced elevation of MMP-2/-9 and TIMP-1 mRNA in a significant manner (relative mRNA expression: MMP-2 stretch 1.83 ± 0.15 vs. stretch + losartan 1.23 ± 0.21 / MMP-9 stretch 1.55 ± 0.14 vs. stretch + losartan 1.11 ± 0.14/TIMP-1 stretch 1.97 ± 0.17 vs. stretch + losartan 1.27 ± 0.19, all n = 3) (Fig. 2h). Moreover, losartan abolished the stretch-induced increase of unbound active-MMP-2/-9 protein levels (active-MMP-2: stretch 13.11 ± 1.24 vs. stretch + losartan 9.54 ± 0.74 ng/µg protein / active-MMP-9: stretch 1.94 ± 0.19 vs. stretch + losartan 1.55 ± 0.13 ng/µg protein, all n = 5) (Fig. 2a, b) as well as the down-regulation of the MMP-2/-9 pro-forms (proMMP-2: stretch 2.89 ± 0.35 vs. stretch + losartan 5.07 ± 0.37 ng/µg protein / proMMP-9: stretch 0.46 ± 0.09 vs. stretch + losartan 0.79 ± 0.11 ng/µg protein, n = 5), indicating an inhibition of the proteolytic conversion (Fig. 2c, d). Pretreatment with losartan also prevented the stretch-induced decrease of MMP-2/-9 bound TIMP-1 complexes (MMP-2 + TIMP-1: stretch 1.03 ± 0.2 vs. stretch + losartan 1.61 ± 0.13 ng/µg protein / MMP-9 + TIMP-1: stretch 0.49 ± 0.07 vs. stretch + losartan 0.69 ± 0.05 ng/µg protein, all n = 5) (Fig. 2e, f) and normalized the MMP-2 enzyme activity measured with gelatine zymography (MMP-2 in %: stretch 1.71 ± 0.15 vs. stretch + losartan 1.15 ± 0.13, n = 5) (Fig. 2g).
Losartan prevents stretch-induced MMP activation. a–d Losartan reversed stretch-induced up-regulation of unbound active-MMP-2/-9 and prevented the stretch-induced down-regulation of pro MMP-2/-9 forms in a significant manner (n = 5, with 3 samples each). e, f Losartan abolished stretch-induced down-regulation of MMP-2/-9 bound TIMP-1 complexes (n = 5, with 3 samples each). g Gelatine zymography for MMP-2 enzyme activity revealed, pre-treatment with losartan abolished stretch-induced up-regulation of MMP-2 enzyme activity (n = 5, with 3 samples each). h On transcriptional level losartan was also able to prevent stretch-induced up-regulation of MMP-2/-9 and TIMP-1 mRNA (n = 3, with 3 samples each). *P < 0.05 versus control, #P < 0.05 versus stretch
It has been shown that angiotensin-II can release endothelin from non-myocytes, which may mediate various responses (e.g. trophic effects via inositol phosphate (IP)-formation, or regulation of cardiac gap junctions) probably via the ET (A) receptor [16, 21, 28].
Since the purity of our cell culture was 95%, this means 5% non-myocytes, which may be sufficient to release ET-1. On the other hand, Polontchouk et al. [22] showed that in a comparable cell culture system (with ≤5% fibroblasts) the angiotensin response was independent from endothelin. To exclude in the present study this possibility of an indirect ET-1 effect, we performed additional experiments using an ET(A) (BQ123, 100nM) or an ET(B) (BQ788, 100nM) antagonist with specific respect to MMP-2 protein expression and enzyme activity, since the major part of the gelatinolytic activity is predominantly mediated by MMP-2 in our model. The stretch-induced up-regulation of MMP-2 expression and activity remained unchanged in the presence of BQ123 or BQ788 (Fig. 3a, c).
To exclude the possibility of an indirect endothelin effect, maybe released from non-myocytes via angiotensin-II stimulus, the ET (A) or ET (B) receptor was blocked with BQ123 or BQ788. The stretch-induced up-regulation of MMP-2 protein expression and enzyme activity remained unchanged in the presence of BQ123 or BQ788 (A–C)
Inhibition of the calcineurin-NFAT signaling pathway prevents stretch-induced activation of MMP-2/-9 in atrial myocytes
Recently we have demonstrated that Cn, an important mediator of cardiomyocyte hypertrophy, is profoundly up-regulated in NRAM during static stretch by 21% [26]. To investigate a possible role of the Cn-NFAT signaling pathway on stretch-induced modifications in MMP enzyme activity and protein amount, the effect of specific Cn blockade by CsA or NFAT inhibition by VIV was analyzed.
CsA completely prevented the up-regulation of unbound active-MMP-2/-9 (active-MMP-2: stretch 13.11 ± 0.97 vs. stretch + CsA 9.31 ± 0.79 ng/µg protein / active-MMP-9: stretch 1.94 ± 0.21 vs. stretch + CsA 1.5 ± 0.17 ng/µg protein, all n = 5) (Fig. 4a, b) as well as the down-regulation of the pro-forms of MMP-2/ -9 mediated by static stretch (proMMP-2: stretch 2.89 ± 0.3 vs. stretch + CsA 4.97 ± 0.31 ng/µg protein / proMMP-9: stretch 0.46 ± 0.07 vs. stretch + CsA 0.77 ± 0.09 ng/µg protein, n = 5) (Fig. 4c, d). Similarly, complexes of MMP-2/-9 and TIMP-1 did not excess control levels in stretched myocytes treated with CsA (MMP-2 + TIMP-1: control 1.73 ± 0.27 vs. stretch 1.03 ± 0.21 vs. stretch + CsA 1.67 ± 0.17 ng/µg protein / MMP-9 + TIMP-1: control 0.77 ± 0.05 vs. stretch 0.49 ± 0.05 vs. stretch + CsA 0.7 ± 0.06 ng/µg protein, all n = 5) (Fig. 4e, f).
Prevention of MMP up-regulation by inhibition of the Cn-NFAT pathway. a–d CsA or 11R-VIVIT completely blunted the stretch-induced up-regulation of unbound active-MMP-2/-9 and prevented stretch-induced down-regulation of pro-MMP-2/-9 in a significant manner (n = 5, with 3 samples each). e, f CsA or 11R-VIVIT abolished stretch-induced down-regulation of MMP-2/-9 bound TIMP-1 complexes significantly (n = 5, with 3 samples each). g Gelatine zymography for MMP-2 enzyme activity revealed, pre-treatment with CsA or 11R-VIVIT normalized stretch-induced up-regulation of MMP-2 enzyme activity (n = 5, with 3 samples each). h On transcriptional level CsA or 11R-VIVIT was also able to prevent stretch-induced up-regulation of MMP-2/-9 and TIMP-1 mRNA (n = 3, with 3 samples each). i–k To analyze if losartan prevents stretch-induced MMP up-regulation by interfering with the Cn–NFAT pathway, we determined Cn protein amount and enzyme activity as well as the DNA binding activity of NFATc1 with and without losartan. Losartan normalized Cn protein amount and enzyme activity as well as the DNA binding activity of NFATc1 in stretched myocytes (all n = 3, with 3 samples each). l Dose dependent inhibition of NFAT–DNA binding activity by 11R-VIVIT. The stretch-induced up-regulation of NFAT–DNA binding activity was partly inhibited in the presence of 1 or 5 µM, whereas 10 and 100 µM of 11R-VIVIT showed a complete inhibition in our model. Thus we chose 10 µM for our cell culture experiments (n = 5, with 3 samples each). *P < 0.05 versus control, #P < 0.05 versus stretch, **P < 0.05 versus stretch + 1 or 5 µM 11R-VIVIT
Since, to our knowledge there are no available data on VIV concentrations in cardiomyocytes we first perfomed NFAT-DNA binding activity experiments with different concentrations of VIV (1, 5, 10 and 100 µM) to analyze its inhibitaroy efficiency of NFAT translocation into the nucleus induced by static stretch by 21%. We observed partly inhibition of NFAT translocation at 1 and 5 µM and a completely inhibition at 10 and 100 µM (DNA binding activity of NFATc1 in %: control 1 ± 0.0 vs. stretch 1.53 ± 0.17 vs. stretch + VIV 1 µM 1.43 ± 0.23 vs. stretch + VIV 5 µM 1,29 ± 0.13 vs. stretch + VIV 10 µM 0.87 ± 0.09, vs. stretch + VIV 100 µM 0.79 ± 0.17, all n = 5) (Fig. 4l). Thus we chose 10 µM for our cell culture experiments.
VIV abolished the stretch-induced up-regulation of unbound active-MMP-2/-9 (active-MMP-2: stretch 13.11 ± 0.97 vs. stretch + VIV 8.69 ± 0.81 ng/µg protein / active-MMP-9: stretch 1.94 ± 0.21 vs. stretch + VIV 1.39 ± 0.21 ng/µg protein, all n = 5) (Fig. 4a, b) and also prevented the down-regulation of both MMP pro-forms caused by static stretch (proMMP-2: stretch 2.89 ± 0.3 vs. stretch + VIV 5.09 ± 0.49 ng/µg protein / proMMP-9: stretch 0.46 ± 0.07 vs. stretch + VIV 0.83 ± 0.11 ng/µg protein, all n = 5) (Fig. 4c, d). Pretreatment of stretched myocytes with VIV also normalized MMP-2/-9 bound TIMP-1 complexes to almost control levels (MMP-2 + TIMP-1: control 1.73 ± 0.27 vs. stretch 1.03 ± 0.21 vs. stretch + VIV 1.87 ± 0.19 ng/µg protein / MMP-9 + TIMP-1: control 0.77 ± 0.05 vs. stretch 0.49 ± 0.05 vs. stretch + VIV 0.83 ± 0.04 ng/µg protein, all n = 5) (Fig. 4e, f).
Quantitative data were confirmed by activity measurements. In the presence of CsA or VIV gelatinolytic activity of MMP-2 normalized to control levels (MMP-2 in %: control 1 ± 0.0 vs. stretch 1.79 ± 0.17 vs. stretch + CsA 1.13 ± 0.19 vs. stretch + VIV 1.05 ± 0.13, all n = 5) (Fig. 4g). There were no significant differences between stretched myocytes treated with CsA or VIV regarding protein amount and enzyme activity. On transcriptional level, CsA or VIV completely prevented the up-regulation of MMP-2/-9 and TIMP-1 mRNA caused by static stretch (relative mRNA expression of MMP-2: stretch 1.83 ± 0.15 vs. stretch + CsA 1.15 ± 0.18 vs. stretch + VIV 1.07 ± 0.13/MMP-9: stretch 1.55 ± 0.14 vs. stretch + CsA 1.09 ± 0.11 vs. stretch + VIV 1.03 ± 0.22 / TIMP-1: stretch 1.97 ± 0.17 vs. stretch + CsA 1.29 ± 0.13 vs. stretch + VIV 1.11 ± 0.19, all n = 3) (Fig. 4h). Conclusively, we analyzed whether AT1 receptor blockade abolishes stretch-induced up-regulation of active-MMPs by interfering with the Cn-NFAT signaling pathway. Losartan completely prevented the static stretch-induced up-regulation of Cn enzyme activity and protein amount comparable to CsA (Cn-activity in nmol PO4 release/20 µg protein/30 min: control 0.37 ± 0.08 vs. stretch 0.65 ± 0.09 vs. stretch + losartan 0.41 ± 0.07 vs. stretch + CsA 0.33 ± 0.09, n = 3 / Cn protein expression in %: control 1 ± 0.0 vs. stretch 1.43 ± 0.15 vs. stretch + losartan 1.13 + 0.11 vs. stretch + CsA 1.07 ± 0.13, all n = 3) (Fig. 4i, j). No significant differences were observed between stretched myocytes treated with CsA or losartan. In addition, the stretch-induced up-regulation of the DNA binding activity of NFATc1 was also abolished in the presence of losartan (NFATc1 DNA binding activity in %: control 1 ± 0.0 vs. stretch 1,53 ± 0.17 vs. stretch + losartan 1.13 ± 0.07 vs. stretch + CsA 0.92 ± 0.13 vs. stretch + VIV 0.87 ± 0.09, all n = 3) (Fig. 4k). These data confirm that blockade of the AT1 receptor normalizes MMP activity via a Cn-NFAT signaling dependent pathway.
Increased proteolytic conversion due to mechanical stretch is linked to MT1-MMP up-regulation
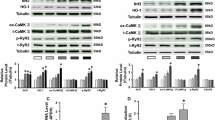
To analyze whether static stretch regulates the ECM remodeling activity of NRAM by modulating extracellular factors, we finally analyzed the expression of MT1-MMP. MT1-MMP has been shown to be involved in activation of pro-MMPs, especially pro-MMP-2 [18, 39]. Static stretch resulted in a significant increase of MT1-MMP mRNA and protein expression as shown in our real-time PCR, Western blotting and immunocytofluorescence experiments (relative mRNA expression: control 1 ± 0.0 vs. stretch 2.57 ± 0.31, n = 3 / protein expression in %: control 1 ± 0.0 vs. stretch 2.17 ± 0.21, n = 8) (Fig. 5a–c). Pre-treatment of stretched myocytes with losartan, CsA or VIV blunted this effect (relative mRNA expression: stretch 2.57 ± 0.31 vs. stretch + losartan 1.35 ± 0.23 vs. stretch + CsA 0.95 ± 0.27 vs. stretch + VIV 0.91 ± 0.31, all n = 3 / protein expression in %: stretch 2.17 ± 0.21 vs. stretch + losartan 0.98 ± 0.15 vs. stretch + CsA 0.89 ± 0.17 vs. stretch + VIV 0.73 ± 0.21, all n = 8) (Fig. 5a–c). These data demonstrate that static stretch increases the ECM remodeling activity of NRAM by an angiotensin-Cn-NFAT dependent pathway. Fig. 5d shows a proposed model for intracellular MMP signaling in NRAM.
Stretch-induced MMP activation is associated with increased MT1-MMP levels. a Representative immunofluorescence images for MT1-MMP (green), counterstained for Trop T-C (red) and nuclei (blue). A negative control was performed, with no unspecific MT1-MMP staining. Stretch resulted in a significant up-regulation of MT1-MMP. Pre-treatment with losartan, CsA or VIV abolished the stretch-induced MT1-MMP up-regulation. b mRNA expression of MT1-MMP (n = 3, with 3 samples each). c Western blotting for protein expression of MT1-MMP (n = 8, with 3 samples each). d Proposed model for intracellular MMP signaling in NRAM. *P < 0.05 versus control, #P < 0.05 versus stretch
Discussion
This study shows: (a) NRAM exposed to static stretch increase their ECM remodeling activity by up-regulation of unbound active-MMP-2/-9. (b) Increased MT1-MMP levels possibly mediate the proteolytic conversion of pro-MMPs to mature-MMPs. (c) The angiotensin-Cn-NFAT signaling pathway is a potential mediator of static stretch-induced MMP-2/-9 activation and MT1-MMP up-regulation.
Static stretch up-regulates MMP-2/-9 activity in NRAM
Active-MMP-2/-9 forms significantly increased within 24 h of static stretch. This was accompanied by a down-regulation of its pro-forms and a decreased binding activity of TIMP-1 resulting in reduced MMP-2/-9 + TIMP-1 complexes. This is a new approach, since the activation and inactivation of MMPs has to be considered in a dynamic nature. The observed elevation in TIMP-1 levels (e.g. in the failing human heart, in hypertensive patients or in patients with AF), which inhibits most of the known MMPs by forming complexes in a stoichiometric ratio of 1:1, may not necessarily translate into reduced MMP activity because the amount of MMP/TIMP complexes can be decreased while the ratio of free to bound TIMP-1 will increase [23]. Therefore we did not restrict our studies in quantifying the amount of TIMP-1 protein levels rather than analyzed MMP + TIMP-1 complexes, to earn functional information about TIMP-1 under conditions of increased baseline stretch.
As a further important novel finding and in addition to these results, MT1-MMP levels significantly increased through static stretch by 21%. Therefore, static stretch consequently resulted in a net increase in the gelatinolytic activity of MMP-2. Despite a significant increase of active-MMP-9 caused by mechanical stretch, enzyme activity of MMP-9, however, could not be determined in our zymography experiments, possibly due to a nearly 6.5-fold lower amount of MMP-9 compared to MMP-2. These data provide some evidence that static stretch exposed atrial myocytes regulate their own ECM remodeling activity via modulating MMP-2/-9 activity, the binding activity of TIMP-1 and MT1-MMP levels.
It is, however, up to now unknown to which extend the two components of stretch mainly contribute to ECM remodeling. For example, in CHF a static component (elevated enddiastolic pressure) is prevalent which is joined by cyclic stretch depending on heart rate and rhythm. In contrast, during AF, there may be an enhanced atrial static pressure depending on the underlying disease (mitral valve disease, arterial hypertension or CHF). Of note, Jaïs et al. [8] could show that even in patients without underlying heart disease (lone AF) the enddiastolic left ventricular pressure was increased. Taken together, during pathophysiological conditions like arterial hypertension, CHF and even in lone AF beside cyclic loading additional static stretch is imposed to cardiomyocytes resulting in cellular hypertrophy and cardiac dysfunction [5, 26, 41], in this study we used a model of static stretch. Another consideration to use static stretch was the fact that our primary cell cultures of NRAM showed inhomogeneous averaged spontaneous beating rates up to 3–5 Hz. An additional cyclic loading with heart rates up to 1 Hz or even more would not represent more physiological conditions, rather than result in un-physiological cellular environments or also in increased baseline stretch because of the discrepancy of frequencies. Up to now, there are only very scarce data on the influence of mechanical stretch on cardiac ECM remodeling processes [7, 12]. Our data and those of Wang and co-workers [36] provide some initial evidence for stretch modulated MMP activity in cardiac myocytes.
Stretch-induced activation of MMPs is mediated by angiotensin-II type 1 receptors
In cardiac ventricular myocytes mechanical stretch has been shown to evoke an increase of angiotensin-II leading to cellular hypertrophy which could be prevented by AT1 receptor blockade [20, 29, 36]. In the present study, AT1 receptor blockade completely abolished the static stretch-induced up-regulation of MMP-2/-9 in NRAM. Thus, stretch seems to evoke a dual cellular response of hypertrophy and induction of ECM remodeling via autocrine/paracrine AT1 receptor dependent mechanisms. This is remarkable since the hallmark of atrial and ventricular remodeling processes comprises loss of myocytes, cellular hypertrophy of the surviving myocytes and interstitial replacement fibrosis [1]. If stretch evokes such an early dual cellular response the hypothesis of an important pathophysiological role during the development of atrial and ventricular remodeling processes may be supported. This would also underline efforts to initiate early ARB treatment during heart failure, arterial hypertension and AF, all of them clinical conditions accompanied by an increase of atrial pressure.
Mechanical stretch increases proteolytic conversion of pro-MMPs via up-regulation MT1-MMP
There are some scarce data, that the proteolytic conversion of pro-MMPs are partly regulated by other membrane anchored MMPs. Monea and co-workers [18] previously demonstrated that pro-MMP-2 activation is coupled to a MT1-MMP dependent mechanism. In order to investigate whether stretch-induced up-regulation of mature MMP-2/-9 is coupled to increased MT1-MMP levels, finally we analyzed the expression of MT1-MMP. In stretched myocytes the amount of MT1-MMP was significantly elevated compared to un-stretched control cells, indicating an increase of the proteolytic conversion of pro-MMPs. Co-stimulation of stretched myocytes with losartan, CsA or VIV decreased the amount of MT1-MMP to control levels. This study demonstrates that stretch increases MMP-2/-9 activity in atrial myocytes via up-regulation of extracellular MT1-MMP levels. These results may partly explain the increase of proteolytic conversion of MMPs due to mechanical stretch, which may contribute to ECM remodeling activity.
The calcineurin–NFAT cascade is an intracellular signaling pathway for static stretch-induced and angiotensin-II-mediated MMP-2/-9 activation in NRAM
In this study static stretch within 24 h elicited an increase in Cn protein amount and enzyme activity. This increase was abolished by the ARB agent losartan. Specific inhibition of Cn with CsA or NFAT with 11R-VIVIT almost completely blunted the stretch-induced up-regulation of active-MMP-2/-9. Otherwise, pre-treatment of control cells with these drugs did not show any significant differences compared to un-stimulated control cells regarding the parameters examined in this study (data not shown). In conclusion, we could link stretch-induced activation of MMPs to an angiotensin-Cn-NFAT signaling dependent cascade. Of note, the Cn-NFAT signaling cascade seems to play a central role for different atrial-remodeling processes as it is also critically involved in the development of atrial cellular hypertrophy mediated by mechanical stretch [3, 26].
Although in our model of NRAM exposed to static stretch the angiotensin–Cn–NFAT cascade is the underlying pathway other cell lines or stretch conditions may use different signaling routes. For example, in ventricular myocytes exposed to 24 h of cyclic loading (20%) the JAK-STAT pathway was demonstrated to be responsible for the stretch-induced increase of MMP-2/-14 expression [36]. Comparable to our findings this rise of MMP activity was also abolished by ARB. A cell specific regulation of MMP transcription by different nuclear factors has been previously described [4, 27]. In addition, a differential utilization of diverse intracellular signaling pathways for unlike stretch qualities (e.g. static vs. cyclic) cannot be ruled out.
A potentially cell type specific intracellular signaling of stretch-mediated MMP activation theoretically opens the opportunity for a more substrate-specific therapeutical intervention depending on the targeted underlying pathophysiological condition. Irrespective of the employed intracellular signal transductions, however, ARB abolishes the MMP increase evoked by cyclic or static stretch.
Limitations
Our in vitro model of NRAM is far removed from the human patient. The genetic expression pattern of many proteins may differ substantially between these cells and human myocytes, therefore our isolated myocytes may not provide the full picture of activated signaling cascades involved in structural changes in pressure/volume overloaded atria. Furthermore we did not test whether other signaling cascades like MAPkinases were involved in MMP activation. Thus we cannot exclude a possible coexisting of other intracellular signaling pathways. This study investigated the short-time influence of stretch on MMP activation. Stretch in the clinical scenario usually develops gradually over long periods. However, there are clinical conditions during which atrial stretch occurs with a sudden onset like during hypertensive crisis or acute CHF or acute mitral regurgitation.
Conclusion
This study shows that NRAM exposed to static stretch respond with an up-regulation of extracellular unbound active-MMP-2/-9 via activation AT1 receptors. Static stretch also modulates MT1-MMP expression to increase the proteolytic conversion of pro-MMPs to mature-MMPs. The Calcineurin-NFAT cascade is a potential intracellular signaling pathway.
References
Allessie M, Ausma J, Schotten U (2002) Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 54:230–246
Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A (1999) Affinity-driven peptide selection of an NFAT inhibitor more selective than Cyclosporin A. Science 285:2129–2133
Bukowska A, Lendeckel U, Hirte D, Wolke C, Striggow F, Röhnert P, Huth C, Klein HU, Goette A (2006) Activation of the calcineurin signaling pathway induces atrial hypertrophy during atrial fibrillation. Cell Mol Life Sci 63:333–342
Fabunmi RP, Baker AH, Murray EJ, Booth RFG, Newby AC (1996) Divergent regulation by growth factors and cytokines of 95 kDa and 72 kDa gelatinases and tissue inhibitors of metalloproteinases-1, -2, and -3 in rabbit aortic smooth muscle cells. Biochem J 315:335-342
Gallagher G, Menzie S, Huang Y, Jackson C, Hunyor SN (2007) Regional cardiac dysfunction is associated with specific alterations in inflammatory cytokines and matrix metalloproteinases after acute myocardial infarction in sheep. Basic Res Cardiol 102:63–72
Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U (2000) Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol 35:1669–1677
Gupta V, Grande-Allen KJ (2006) Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res 72:375–383
Jaïs P, Peng JT, Shah DC, Garrigue S, Hocini M, Yamane T, Haïssaguerre M, Barold SS, Roudaut R, Clémenty J (2000) Left ventricular diastolic dysfunction in patients with so-called lone atrial fibrillation. J Cardiovasc Electrophysiol 11:623–625
Kannel WB, Abbott RD, Savage DD, McNamara PM (1982) Epidemiologic features of atrial fibrillation. N Engl J Med 306:1018–1022
Kleiner DE, Stetler-Stevenson WG (1994) Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 218:325–329
Li D, Fareh S, Leung TL, Nattel S (1999) Promotion of atrial fibrillation by heart failure in dogs. Atrial remodeling of a different sort. Circulation 100:87–95
Li Y, Li WM, Gong YT, Li BX, Liu W, Han W, Dong D, Sheng L, Xue JY, Zhang L, Chu S, Yang BF (2007) The effects of cilazapril and valsartan on the mRNA and protein expressions of atrial calpains and atrial structural remodeling in atrial fibrillation dogs. Basic Res Cardiol 102:245–256
Lin CC, Lin JL, Lin CS, Tsai MC, Su MJ, Lai LP, Huang SK (2004) Activation of the calcineurin-nuclear factor pathway of activated T-cell signal transduction in atrial fibrillation. Chest 126:1926–1932
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Malhotra R, Sadoshima J, Brosius FC, Izumo S (1999) Mechanical stretch and angiotensin-II differentially upregulate the renin-angiotensin system in cardiac myocytes in vitro. Circ Res 85:137–146
McEwan PE, Sherry L, Kenyon CJ, Webb DJ, Gray GA (2000) Regulation of the myocardial endothelin system by angiotensin-II and losartan. J Cardiovasc Pharmacol 36 (5 suppl 1):S144–S147
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228
Monea S, Lehti K, Keski-Oja J, Mignatti P (2002) Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol 192:160–170
Nagata K, Somura F, Obata K, Odashima M, Izawa H, Ichihara S, Nagasaka T, Iwase M, Yamada Y, Nakashima N, Yokota M (2002) AT1 receptor blockade reduces cardiac calcineurin activity in hypertensive rats. Hypertension 40:168–174
Pérez NG, de Hurtado MC, Cingolani HE (2001) Reverse mode of the Na+–Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res 88:376–382
Poenicke K, Heinroth-Hoffmann I, Becker K, Brodde OE (1997) Trophic effect of angiotensin-II in neonatal rat cardiomyocytes: role of endothelin-1 and non-myocyte cells. Br J Pharmacol 121:118–124
Polontchouk L, Ebelt B, Jackels M, Dhein S (2001) Chronic effects of endothelin 1 and angiotensin-II on gap junctions and intercellular communication in cardiac cells. FASEB J 16:87–89
Polyakova V, Hein S, Kostina S, Ziegelhoeffer T, Schaper J (2004) Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J Am Coll Cardiol 44:1609–1618
Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostina S (2008) Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med 12:189–208
Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM (1997) Incidence of and risk factors for atrial fibrillation in older adults. Circulation 96:2455–2461
Rana OR, Zobel C, Saygili E, Brixius K, Gramley F, Schimpf T, Mischke K, Frechen D, Knackstedt Ch, Schwinger RHG, Schauerte P, Saygili E (2008) A simple device to apply equibiaxial strain to cells cultured on flexible membranes. Am J Physiol Heart Circ Physiol 294:H532–H540
Ries C, Petrides PE (1995) Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem 376:345–355
Rossi GP, Sacchetto A, Cesari M, Pessina AC (1999) Interactions between endothelin-1 and the renin–angiotensin–aldosterone system. Cardiovasc Res 43:300–307
Sadoshima J, Xu Y, Slayter HS, Izumo S (1993) Autocrine release of angiotensin-II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75:977–984
Saygili E, Rana OR, Saygili E, Reuter H, Frank KF, Schwinger RH, Muller-Ehmsen J, Zobel C (2007) Losartan prevents stretch induced electrical remodeling in cultured atrial neonatal myocytes. Am J Physiol Heart Circ Physiol 292:H2898–H2905
Schott P, Asif AR, Gräf C, Toischer K, Hasenfuss G, Kögler H (2008) Myocardial adaptation of energy metabolism to elevated preload depends on calcineurin activity: a proteomic approach. Basic Res Cardiol 103:232–243
Seeland U, Selejan S, Engelhardt S, Müller P, Lohse MJ, Böhm M (2008) Interstitial remodeling in beta1-adrenergic receptor transgenic mice. Basic Res Cardiol 102:183–193
Verheule S, Wilson E, Everett T 4th, Shanbhag S, Golden C, Olgin J (2003) Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation 107:2615–2622
Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, Guerra PG, Ducharme A (2003) Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the studies of left ventricular dysfunction (SOLVD) trials. Circulation 107:2926–2931
Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB (2005) Angiotensin-II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol 45:712–719
Wang TL, Yang YH, Chang H, Hung CR (2004) Angiotensin-II signals mechanical stretch-induced cardiac matrix metalloproteinase expression via JAK-STAT pathway. J Mol Cell Cardiol 37:785–794
Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA (1995) Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92:1954–1968
Wilkins BJ, Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimbal TF, Molkentin JD (2002) Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin mediated cardiac hypertrophic growth. Mol Cell Biol 22:7603–7613
Will H, Atkinson SJ, Butler GS, Smith B, Murphy G (1996) The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiate autoproteolytic activation: regulation by TIMP 2 and TIMP 3. J Biol Chem 271:17119–17123
Yamamoto K, Dang QN, Kelly RA, Lee RT (1998) Mechanical strain suppresses inducible nitric-oxide synthase in cardiac myocytes. J Biol Chem 273:11862–11866
Zobel C, Rana OR, Saygili E, Bölk B, Saygili E, Diedrichs H, Reuter H, Frank K, Müller-Ehmsen J, Pfitzer G, Schwinger RHG (2007) Mechanisms of Ca2+-dependent calcineurin activation in mechanical stretch-induced hypertrophy. Cardiology 107:281–290
Acknowledgments
We thank the entire Institute of Laboratory Animal Science, University Hospital, RWTH Aachen in Germany for the helpful assistance in animal research. This work was supported in part by Deutsche Forschungsgemeinschaft (DFG) grant Lu 869/4-1 and IZKF Biomat. RWTH Aachen, Germany to A.L. and by the Network of Competence Atrial Fibrillation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Returned for 1. Revision: 15 August 2008 1. Revision received: 17 November 2008
E. Saygili and O. R. Rana contributed equally to this work.
Rights and permissions
About this article
Cite this article
Saygili, E., Rana, O.R., Meyer, C. et al. The angiotensin–calcineurin–NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/-9 in atrial myocytes. Basic Res Cardiol 104, 435–448 (2009). https://doi.org/10.1007/s00395-008-0772-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-008-0772-6