Abstract
Age-dependent alterations of the vessel wall may predispose older individuals to increased cardiovascular pathology. Aging is associated with an impaired bioactivity of nitric oxide (NO). Plasma nitrite reflects NO-synthase activity under fasting conditions and is an important storage pool of NO. To test the hypothesis that aging is associated with an impaired capacity of the vasculature to increase plasma nitrite during exercise, 29 young and 28 old healthy individuals (25 ± 1 years and 58 ± 2 years; P < 0.001) without major cardiovascular risk factors were enrolled. Exercise stress was similar in both groups. Baseline nitrite did not differ (107 ± 8 vs. 82 ± 10 nmol/l, young vs. old; n.s.) although a trend toward higher nitrite levels in young individuals was seen. In young subjects, exercise increased plasma nitrite by 38 ± 7% (P < 0.001) compared to only 13 ± 8% (P = n.s.) in older subjects. L-NMMA blocked increases of nitrite. Endothelial function, as defined by flow–mediated-dilation (FMD) of the brachial artery via ultrasound, was impaired in older subjects (5.4 ± 0.4% vs. 6.7 ± 0.3%; P < 0.01). Multivariate analysis showed that age (P = 0.007), BMI (P = 0.010), and LDL (P = 0.021) were independent predictors of nitrite increase. The fact that aging is associated with an impaired capacity of the vasculature to adequately increase nitrite to physiological stimuli may contribute to attenuated maintenance and further deterioration of vascular homeostasis with aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Endothelial dysfunction is an early state of atherosclerosis [21, 23]. Disruption of endothelial homeostasis predisposes blood vessels to vasoconstriction, inflammation, leukocyte adhesion, thrombosis, and proliferation of vascular smooth muscle cells [26, 39]. Among the risk factors for atherosclerosis, aging is a significant predictor of impairment of endothelium-dependent vasodilation [5, 18, 40, 42]. Experimental and clinical research has shown an increased risk of atherosclerotic disease and poor outcomes in older patients as compared with their younger counterparts. It has been suggested that age-dependent changes in the cardiovascular system may predispose older individuals to increased cardiovascular pathology [6, 13]. This suggests that aging is associated with an endogenous alteration of the vessel wall promoting vascular dysfunction and progression of atherosclerosis. Although the mechanisms underlying age-related endothelial dysfunction are not well understood, along with other risk factors for atherosclerosis, aging is associated with an impaired bioactivity of nitric oxide (NO) [14] and an increase in oxidative stress [10]. Nitrite is the main oxidation product of NO in plasma and sensitively reflects acute and chronic changes in endothelial nitric oxide synthase (eNOS) activity in healthy fasting volunteers [20, 22]. In dependence of cardiovascular risk factors [19] concentrations in plasma nitrite are reduced. Nitrite has been discovered to be an important storage pool of NO [8]. In the cardiovascular system, nitrite can be reduced under hypoxic conditions to NO via its reaction with deoxyhemoglobin and deoxymyoglobin, regulating vascular tone and myocardial function [16, 31]. Along these lines, our recent study suggests a role of endothelium-derived plasma nitrite in the adaptation of hemodynamics during exercise, a condition with high oxygen consumption, and that an impaired increase in plasma nitrite levels may limit exercise capacity [34]. Thus, ability of the vasculature to regulate nitrite homeostasis may be crucial for vascular function. In the current study, we focused on the question whether aging is associated with an impaired capacity of the vasculature to increase plasma nitrite after physiological stimuli such as exercise, leading to disturbance of vascular function with age.
2 Methods
2.1 Study subjects
We studied 29 young individuals (25 ± 1 years) and 28 old (58 ± 2 years) healthy subjects without clinical evidence of other cardiovascular risk factors. All subjects were asymptomatic, normotensive as defined by systolic blood pressure (BP) <140 mmHg and diastolic BP <90 mmHg), nondiabetic (as defined by fasting glucose levels <126 mg/dl), normocholesteremic (as defined by total cholesterol levels <240 mg/dl and low-density-lipoproteins (LDL) cholesterol levels < 160 mg/dl), and did not smoke [1, 7, 28]. Subjects had no significant medical history and were not taking regular or incidental medication. The clinical characteristics are summarized in Table 1. Informed consent was obtained from all study subjects before enrolment. The study protocol was approved by the local ethics committee.
2.2 Determination of endothelium-dependent and endothelium-independent vasodilation
Endothelium-dependent dilation of the brachial artery (BA) was measured non-invasively by high-resolution ultrasound (SONOS 5500, Agilent, with a 15 MHz linear-array transducer) using standard techniques as previously described [32]. Briefly, baseline data for diameter and blood-flow velocity of the BA were quantified after 10 min of supine rest in an air-conditioned room (21°C) 1–2 cm above the elbow. Then a blood-pressure cuff was placed around the forearm distal to the cubital fossa and inflated to 200 mmHg for 5 min. Blood-flow velocity and diameter were measured immediately after deflation of the cuff as well as 60, 75, 90, and 120 s thereafter. Maximal BA diameter observed during this time period was used to calculate FMD. Endothelium-independent dilation of BA was quantified 4 min after sublingual administration of 400 µg glycerol trinitrate (GTN; Nitrolingual mite, Pohl, Germany). All ultrasound scans were performed by the same operator using the same equipment. An automated analysis system was used to measure diameters (Brachial Analyzer, Medical Imaging Applications, Iowa City, IO) yielding low variabilities of our methodology described elsewhere [34]. Internal quality control was performed by an independent investigator blinded to the protocol (96% approved). FMD and endothelium-independent dilation were expressed as percent change from baseline.
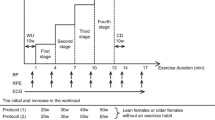
2.3 Ergometric exercise test
All subjects underwent an ergometric exercise test with a stepwise increase in force. Five millilitre blood draws were taken before (basal) and 10 min after exercise termination (peak) over a 20 g iv catheter from the cephalic vein. Time point of peak was determined in a subset of 15 subjects, where blood was drawn consecutively for 60 min as described before [34].
Exercise was ceased when subjects were not able to continue, demonstrated clinical symptoms which met test termination criteria, or reached maximum heart rate (heart rate 220-age (years)) [15, 27]. Prior to, during and following the ET, heart rate and 12 lead ECG were recorded continuously. Blood-pressure measurements were obtained every minute throughout and until 5 min after the test.
We further examined the effect of L-NMMA (NG-monomethyl-L-arginine; Clinalfa, Schwalbach, Germany), a competitive NOS-inhibitor, on changes in nitrite levels after exercise in a subset of five old (53 ± 3 years, three females) and five young subjects (23 ± 2 years, two females). L-NMMA was administered intravenously via the right antecubital vein at a dosage of 1 mg kg−1 min−1 for 3 min followed by a continuous infusion of 0.2 mg kg−1 min−1 until the end of the experiment [35]. To ensure complete NOS inhibition, L-NMMA was continuously infused for 60 min prior to exercise and FMD suppression confirmed [22, 35]. Venous blood was collected from the left antecubital vein at baseline, after 60 min of L-NMMA infusion (before ET), and at 10 min after cessation of exercise.
2.4 Measurement of nitrite and nitrate in plasma
All measurements were performed under fasting conditions between 7:00 and 9:00 am. Plasma levels of nitrite were determined using a triiodide/ozone-based chemiluminescence assay, essentially as described [30]. Nitrate was quantified after enzymatic reduction to nitrite by nitrate reductase using flow-injection analysis based on the Griess reaction [33, 36].
2.5 Statistical analysis
Differences were assessed by repeated measurements ANOVA, with P values for multiple comparisons adjusted by the Bonferroni criterion. Comparisons between two groups were performed with unpaired t-test. Univariate correlations were Pearson’s correlations. A multivariate regression analysis was performed to determine whether nitrite increase is an independent predictor of endothelial function. Standardized coefficients were calculated as a measure for the relative predictive value. Statistical significance was assumed if a null hypothesis could be rejected at P = 0.05. All analyses were performed with SPSS 11.0.1 (SPSS Inc., Chicago, IL).
3 Results
3.1 Study subjects
The baseline characteristics of young and old subjects are listed in Table 1. Endothelial vasodilator function measured by FMD was impaired in old compared with young subjects (Fig. 1). Baseline diameters of the brachial artery increased from 3.91 ± 0.12 cm to 4.17 ± 0.01 (P = 0.01) in young subjects and from 4.25 ± 0.13 cm to 4.45 ± 0.02 cm (P = 0.01) in old subjects with baseline values being not significant different between the two groups. No significant differences were seen in blood flow at baseline and during hyperemia (114 ± 21 ml/min vs. 111 ± 12 ml/min and 653 ± 43 ml/min vs. 689 ± 75 ml/min; n.s.).
3.2 Effect of exercise on baseline plasma nitrite and nitrate levels
Under baseline conditions there were no differences in plasma nitrite (107 ± 8 vs. 82 ± 10 nmol/l; n.s., young vs. old) and nitrate (24.6 ± 1.4 vs. 23.5 ± 1.8 µmol/l; n.s.) between the two groups, although there appears to be a tendency for plasma nitrite levels to decline with age. Ergometric exercise significantly increased plasma nitrite levels (107 ± 8 to 143 ± 11 nmol/l; P < 0.001) in young subjects, compared to only a slight increase (82 ± 10 to 101 ± 14 nmol/l; n.s.) in old subjects (Fig. 1). No changes were observed in nitrate (1% vs. 2%, young vs. old, n.s.). The point of bodily exhaustion as assessed by increases in heart rate was comparable (224 ± 7 % vs. 205 ± 7% increase; n.s., young vs. old) in both groups. Ergometric induced stress, quantified as pressure-rate-product, did not differ between the two groups (17,371 ± 703 vs. 16,621 ± 710 mmHg bpm; young vs. old, n.s.) (Table 2).
3.3 Effect of NOS-inhibition on nitrite levels after exercise
To determine whether the observed increase in nitrite levels after exercise was NOS-dependent, we studied the effect of NOS-inhibition on nitrite levels after ET in a subgroup of five young and five old subjects. Exercise increased plasma nitrite from 135 ± 12 to 163 ± 8 in young and from 107 ± 5 to 113 ± 11 in old subjects. The increases in nitrite levels were abolished during NOS-inhibition (from 125 ± 8 to 110 ± 9 in young and from 98 ± 8 to 95 ± 6 in old subjects, respectively).
3.4 Increase in plasma nitrite as an independent predictor of endothelial dysfunction
The increase in nitrite after exercise (r = 0.355, P = 0.010) univariately correlated with FMD and inversely with age (r = −0.39, P = 0.01). In a multivariate regression model age (P = 0.007), BMI, and LDL-cholesterol but not glucose and rate-pressure product were independent predictors of the nitrite increase (Table 3).
4 Discussion
The key findings of the present study are that (A) exercise stress increases endothelium-derived plasma nitrite levels in young subjects whereas in old subjects there is a reduced capacity of the vasculature to adequately increase nitrite levels to exercise. (B) This increase in plasma nitrite is not the result of secretion by skeletal muscle but the increase appeared to be NOS dependent as it was abolished and even reversed by L-NMMA infusion. (C) Age, BMI, and LDL-cholesterol were independent predictors of increases in plasma nitrite after exercise.
4.1 Origin of plasma nitrite
Nitrite is an oxidation product of endothelium-derived NO. It has been shown that under fasting conditions up to 70%–90% of circulating plasma nitrite is derived from eNOS activity in humans and other mammals [20]. Recent studies have shed light on ceruloplasmin as a NO oxidase [38] that converts NO to nitrite and is involved in maintaining endocrine NO homeostasis. Furthermore, nitrite is present in food, especially in processed meat [24]. Ingestion of large amounts of inorganic nitrate also increases plasma nitrite. This increase is mainly due to enterosalivary circulation of nitrate (as much as 25% is actively taken up by the salivary glands) and reduction to nitrite by commensal bacteria [24]. This nitrite enters the gastrointestinal tract when saliva is swallowed.
4.2 The role of nitrite as an endocrine modulator
During the last years, nitrite has emerged as a bioactive endocrine form of NO [8, 9]. Several mechanism have been described in vivo for the conversion of nitrite to NO such as acidic conditions [2, 25, 43], conversion via xanthine oxidoreductase [41], and reduction by deoxyhemoglobin [11] and deoxymyoglobin [31, 37]. It is suggested that these mechanisms ensure an autoregulated NO generation in regions of poor oxygenation where deoxyhemoglobin predominates. Under physiological and pathophysiological conditions, nitrite seems to be crucial for hypoxic vasodilation, signal transduction, and modulation of mitochondrial respiration, thus being cytoprotective [12, 29], regulating gene expression [3], and modifying nitrosation reactions [4]. Taken together nitrite is a potent regulator of cardiovascular functions and may mediate protective effects under hypoxic and acidic conditions.
4.3 Age-related endothelial dysfunction and the failure to increase nitrite after exercise
We have recently shown that basal levels of plasma nitrite are reduced with increasing numbers of cardiovascular risk factors [19]. In this study we focused on the question of whether the sole phenotype aging without apparent cardiovascular disease is associated with a reduction of circulating nitrite levels at rest and after exercise. In the current study, we observed an impairment in endothelial vasodilator function in old subjects. In a further step, we applied an ergometric exercise test as a physiological stimulus to increase blood flow. Despite similar ergometric stress with similar RPP, older subjects failed to exhibit a nitrite increase after exercise. This suggests an impaired capacity of the aging vasculature to produce NO as both FMD and changes in plasma nitrite were shown to be sensitive markers of NOS activity. This notion was further confirmed by a lack of nitrite increase following systemic NOS inhibition using L-NMMA. Therefore, older subjects appear to have a limited capacity to NOS-dependently increase their nitrite pool during exercise. The lower levels of vasoactive nitrite may contribute to age-dependent impairment of exercise capacity.
4.4 Study limitations
Further studies are needed to clarify whether eNOS activity is impaired with aging or whether aging and exercise alter nitrite production as well as degradation. Aging and exercise may also alter a variety of determinants of microcirculatory flow that affect conversion of NO to nitrite without a change in NO production. Measurements of pO2, pCO2, lactate, and arterial-venous-gradients of nitrite are needed to identify the mechanism of nitrite increase. Furthermore, in our study, we did not investigate the role of oxidative stress in age-dependent endothelial dysfunction. It is known that endothelial function is at least in part impaired due to increases in superoxide anion production [17]. To further elucidate the impact of ROS on age-dependent endothelial dysfunction, the influence of vitamin C on FMD in old compared to young people should be investigated under consideration of, e.g., isoprostane levels in plasma and urine, respectively. Moreover, age-matched studies with patients suffering from cardiovascular disease have to follow in order to elucidate the impact of, e.g., medication, coronary artery disease, and the respective cardiovascular risk factors on nitrite changes.
5 Conclusion
We have shown that aging is associated with an impaired capacity of the vasculature to adequately increase circulating nitrite to physiological stimuli. Due to its bioactivation to NO, nitrite may play a crucial role in the modulation of vascular tone and maintenance of vascular homeostasis during exercise.
References
Adult Treatment Panel III (2001) Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. JAMA 285:2486–2497
Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H (1994) Stomach NO synthesis. Nature 368:502
Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M (2005) Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1:290–297
Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M (2004) Cellular targets and mechanisms of nitros(yl)ation: An insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA 101:4308–4313
Castelli WP (1984) Epidemiology of coronary heart disease: the Framingham study. Am J Med 76:4–12
Chen X, Niroomand F, Liu Z, Zankl A, Katus HA, Jahn L, Tiefenbacher CP (2006) Expression of nitric oxide related enzymes in coronary heart disease. Basic Res Cardiol 101:346–353
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ (2003) Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of hight blood pressure. Hypertension 42:1206–1252
Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon III RO, Gladwin MT (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9:1498–1505
Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD Jr., Kraus D, Ho C, Gladwin MT, Patel RP (2006) Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107:566–574
Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR (2007) Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100:1659–1666
Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D (1981) Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem 256:12393–12398
Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ (2005) Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 115:1232–1240
Finkel T (2005) Opinion: Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol 6:971–976
Fisher ND, Hollenberg NK (2006) Aging and vascular responses to flavanol-rich cocoa. J Hypertens 24:1575–1580
Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD Jr., Winters WL, Yanowitz FG, Ritchie JL, Gibbons RJ, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr., Lewis RP, O’Rourke RA, Ryan TJ (1997) ACC/AHA guidelines for exercise testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol 30:260–311
Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel R, Hogg N, Shiva S, Cannon III RO, Kelm M, Wink D, Espey MG, Oldfield E, Pluta RM, Freeman BA, Lancaster Jr. JR, Feelisch M, Lundberg JO (2005) The emerging role of nitrite. Nat Chem Biol 1:308–314
Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF (2001) Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37:529–534
Huang X, Yang P, Du Y, Zhang J, Ma A (2007) Age-related down-regulation of HCN channels in rat sinoatrial node. Basic Res Cardiol 102:429–435
Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M (2006) Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 40:295–302
Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M (2003) Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35:790–796
Lauer T, Kleinbongard P, Preik M, Rauch BH, Deussen A, Feelisch M, Strauer BE, Kelm M (2003) Direct biochemical evidence for eNOS stimulation by bradykinin in the human forearm vasculature. Basic Res Cardiol 98:84–89
Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M (2001) Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98:12814–12819
Loscalzo J (1995) Nitric oxide and vascular disease. N Engl J Med 333:251–253
Lundberg JO, Govoni M (2004) Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37:395–400
Lundberg JO, Weitzberg E, Lundberg JM, Alving K (1994) Intragastric nitric oxide production in humans: measurements in expelled air. Gut 35:1543–1546
Mujynya-Ludunge K, Viswambharan H, Driscoll R, Ming XF, von Segesser LK, Kappenberger L, Yang Z, Vassalli G (2005) Endothelial nitric oxide synthase gene transfer restores endothelium-dependent relaxations and attenuates lesion formation in carotid arteries in apolipoprotein E-deficient mice. Basic Res Cardiol 100:102–111
Noakes TD (2006) The limits of endurance exercise. Basic Res Cardiol 101:408–417
Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF Jr., Smith SC Jr., Stone NJ, Taubert KA (2002) AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 106:388–391
Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, Losano G, Pagliaro P (2006) Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol 101:168–179
Rassaf T, Feelisch M, Kelm M (2004) Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Radic Biol Med 36:413–422
Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J (2007) Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res 100:1749–1754
Rassaf T, Heiss C, Hendgen-Cotta U, Balzer J, Matern S, Kleinbongard P, Lee A, Lauer T, Kelm M (2006) Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic Biol Med 41:295–301
Rassaf T, Kleinbongard P, Preik M, Dejam A, Gharini P, Lauer T, Erckenbrecht J, Duschin A, Schulz R, Heusch G, Feelisch M, Kelm M (2002) Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO: Experimental and clinical study on the fate of NO in human blood. Circ Res 91:470–477
Rassaf T, Lauer T, Heiss C, Balzer J, Mangold S, Leyendecker T, Rottler J, Drexhage C, Meyer C, Kelm M (2007) Nitric oxide synthase derived plasma nitrite predicts exercise capacity. Br J Sports Med 41:669–673
Rassaf T, Poll LW, Brouzos P, Lauer T, Totzeck M, Kleinbongard P, Gharini P, Andersen K, Schulz R, Heusch G, Modder U, Kelm M (2006) Positive effects of nitric oxide on left ventricular function in humans. Eur Heart J 27:1699–1705
Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiß C, Strauer BE, Feelisch M, Kelm M (2002) Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest 109:1241–1248
Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, Macarthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT (2007) Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res
Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT (2006) Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol 2:486–493
Tanner FC, van der LB, Shaw S, Greutert H, Bachschmid MM, Berrozpe M, Rozenberg I, Blau N, Siebenmann R, Schmidli J, Meyer P, Luscher TF (2007) Inactivity of nitric oxide synthase gene in the atherosclerotic human carotid artery. Basic Res Cardiol 102:308–317
Turcato S, Turnbull L, Wang GY, Honbo N, Simpson PC, Karliner JS, Baker AJ (2006) Ischemic preconditioning depends on age and gender. Basic Res Cardiol 101:235–243
Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A (2004) Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA 101:13683–13688
Weinsaft JW, Edelberg JM (2001) Aging-associated changes in vascular activity: a potential link to geriatric cardiovascular disease. Am J Geriatr Cardiol 10:348–354
Zweier JL, Wang P, Samouilov A, Kuppusamy P (1995) Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1:804–809
Acknowledgments
This work was in part sponsored by the DFG (RA 969/4-1 to TR and Ke 405/5-1 to MK and Ne 1821/3-1 to MWM) and by the Hans-und Gertie Fischer-Stiftung (to TL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lauer, T., Heiss, C., Balzer, J. et al. Age-dependent endothelial dysfunction is associated with failure to increase plasma nitrite in response to exercise. Basic Res Cardiol 103, 291–297 (2008). https://doi.org/10.1007/s00395-008-0714-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-008-0714-3