Abstract
Purpose
Nutrition plays a key role in supporting the human immune system and reducing the risk of infections. However, there is limited evidence exploring the relationship between diet and the risk of COVID-19. This study aimed to assess the associations between consumption of ultra-processed foods (UPF) and COVID-19 risk.
Methods
In total, 41,012 participants from the UK Biobank study with at least 2 of up to 5 times 24-h dietary assessments were included in this study. Dietary intakes were collected using an online 24-h dietary recall questionnaire and food items were categorized according to their degree of processing by the NOVA classification. COVID-19 infection was defined as individuals tested COVID-19 positive or dead of COVID-19. Association between average UPF consumption (% daily gram intake) and COVID-19 infection was assessed by multivariable logistic regression adjusted for potential confounders.
Results
Compared to participants in the lowest quartile of UPF proportion (% daily gram intake) in the diet, participants in the 2nd, 3rd, and highest quartiles were associated with a higher risk of COVID-19 with the odds ratio (OR) value of 1.03 (95% CI: 0.94–1.13), 1.24 (95% CI: 1.13–1.36), and 1.22 (95% CI: 1.12–1.34), respectively (P for trend < 0.001), after adjusting for potential confounders. The results were robust in a series of sensitivity analyses. No interaction effect was identified between the UPF proportions and age groups, education level, body mass index, and comorbidity status. BMI mediated 13.2% of this association.
Conclusion
Higher consumption of UPF was associated with an increased risk of COVID-19 infection. Further studies are needed to better understand the underlying mechanisms in such association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a big threat to public health and the economy worldwide [1]. Long COVID, defined by the persistence of symptoms beyond 3 months of SARS-CoV-2 infection, is expected to substantially alter millions of lives with a broad spectrum of manifestations since survivors of COVID-19 now exceed hundreds of millions globally [2, 3]. Although socioeconomic characteristics, lifestyle factors, poor metabolic health, and pre-existing chronic conditions were suggested to be risk factors for COVID-19, the whole impactors are still elusive [4,5,6]. While vaccines, effective treatments, and mitigation measures have been launched to deal with COVID-19, understanding the risk factors of COVID-19 would be of great importance in building up people’s health and preventing acute infectious disease in the future [7].
Nutrition is widely known to be an essential determinant of human health [8]. It provides a source of energy and substrates for immune system activity [9]. A balanced diet could promote a healthy gut microbiota, which plays a role in training and regulating the immune system [9]. Previous studies have shown that diet is associated with infectious diseases [10,11,12]. Recent evidence has shown that higher dietary intakes of fruit and vegetables and healthy plant-based foods are related to decreased risk of COVID-19 infection [13, 14], which shed a light on the relationships between nutrition and the risk of COVID-19.
The NOVA system classifies all foods, beverages, and food products into four groups based on the extent and purpose of the industrial processing they undergo, taking into consideration the physical, biological, and chemical methods used in their manufacture, including the use of additives [15]. Ultra-processed foods (UPFs), one of the four groups that make up NOVA, are industrial formulations of processed food substances (oils, fats, sugars, starch, protein isolates) that are submitted to hydrolysis, hydrogenation, or other chemical modifications by adding flavourings, colourings, emulsifiers, and other cosmetic additives [15] UPFs are often high in energy, added sugars, saturated fats, trans fats, and salt, and low in dietary fibre, protein, vitamins, and minerals [15]. Beyond nutritional composition, UPFs are also a major dietary source of contaminants, neo-formed compounds, which may impact several pathways, such as inflammation, and alter the gut microbiota composition [16]. Monotonous diets rich in UPFs may lead to vitamin and mineral deficiencies, impairing the immune system and increasing susceptibility to SARS-CoV-2[17]. And dietary imbalance caused by the consumption of UPFs might increase the inflammatory index of diet, which has been associated with respiratory infection of the airways [18,19,20]. Studies have shown that UPFs are associated with an increased risk of cardiovascular disease, inflammatory bowel disease, cancer, and all-cause mortality among general and high-risk populations [21,22,23,24,25,26]. However, to our best knowledge, there is limited evidence of the associations between UPF consumption and the risk of COVID-19. In this study, using data from the UK Biobank, a prospective cohort, we explored the association between UPF consumption and the risk of COVID-19 infection.
Methods
This study followed the Strengthening the reporting of observational studies in epidemiology (STROBE) reporting guideline for cohort studies.
Study design and participants
The UK Biobank is a prospective cohort with about half a million participants aged 40–69 years from one of the 22 assessment centres across England, Scotland, and Wales. The objective of this cohort is to explore the determinants of health. Details of this cohort were described elsewhere [25]. Briefly, the baseline information, including socio-demographic characteristics, lifestyle and environmental factors, physical measures, family history, and health and medical history, were collected from 2006 to 2010. Participants were followed-up to obtain their health outcomes with their informed consent. The ethical approvals of UK Biobank were obtained from the North West Multi-Centre Research Ethics Committee, as a Research Tissue Bank (RTB) approval. This study was approved under the UK Biobank application number 45676. Participants were excluded if: 1) withdrew consent or were lost to follow-up; 2) less than 2 online 24-h dietary recall completed; 3) missing COVID-19 testing results and COVID-19 death records; or 4) extreme mean energy intake (< 800 kcal/day or > 4200 kcal/day for males; and < 600 kcal/day or > 3500 kcal/day for females).
Dietary assessment
The Oxford WebQ dietary questionnaire on the quantities of up to 206 types of foods and 32 types of drinks consumed over the previous day was used to assess the detailed dietary intake over the previous 24-h in each UK biobank assessment centre [27]. The online 24-h dietary recall questionnaire was conducted five times, with the first assessment from April 2009 to September 2010, and then repeated in February 2011 to April 2011, June 2011 to September 2011, October 2011 to December 2011, and April 2012 to June 2012. The quantity of each food item consumed was obtained by multiplying the consumed portions times the corresponding portion size [28]. The averaged mean dietary intakes from all available dietary records of each participant were considered as baseline usual dietary intakes.
All food and drink items in the online 24-h dietary recall questionnaire were categorized into four groups (unprocessed or minimally processed foods, processed culinary ingredients, processed foods, and UPFs) according to their degree of processing by the NOVA system of food classification [29, 30]. (1) Unprocessed or minimally processed foods are unprocessed foods or foods altered by processes such as removal of inedible or unwanted parts, drying, crushing, et al. No process including salt, sugar, oils or fats, or other food substances are used. (2) Processed culinary ingredients, are obtained directly from unprocessed or minimally processed foods or nature, like oils and fats, sugar and salt, created by industrial processes such as pressing, centrifuging, refining, extracting, or mining. (3) Processed foods are industrial products made by adding salt, sugar, or other substance found in foods in the first two categories, using preservation methods such as canning and bottling, and non-alcoholic fermentation (in the case of bread and cheese). (4) UPFs are formulations of ingredients, most of the exclusive industrial use (e.g. protein isolates, hydrogenated oils, and modified starches), that result from a series of industrial processes. UPFs are the focus of this study. A detailed description of the NOVA classification was published elsewhere [15]. In addition, examples of foods in each category were listed in Supplemental Table 2.
The total weight (g/day) of UPF or all foods was calculated as the sum of the weight of each corresponding food item and the average weight of each food item per participant across all dietary recalls was obtained. The proportion of UPF intake in the weight of all food items was calculated for each participant. Such an approach is more advantageous in capturing the adverse effect of UPF than the energy ratio since some food components do not provide energy [24].
Outcomes
Data of COVID-19 testing results of UK Biobank for England, Scotland, and Wales are provided by Public Health England (PHE), Public Health Scotland (PHS), and Secure anonymized information linkage (SAIL), respectively, since 16 March 2020 [31]. Individuals with the death diagnosis of “U07.1” and “U07.2” according to International Classification of Diseases versions 10 (ICD-10) were also deemed as COVID-19 positive as a supplement to test records. Individuals who tested positive for COVID-19 or died because of COVID-19 were identified as COVID-19 infection cases. All outcome data were assessed on 11 December 2021. The date of the last update of COVID-19 tests results and death data are listed in Supplemental Table 1.
Covariates
Socio-demographic, lifestyle characteristics, and health conditions were collected at baseline. Covariates were adjusted in the multivariable model as potential confounding factors, including gender, age (year of birth), ethnicity (white, mixed, Asian or Asian British, Black or Black British, Chinese, and other ethnic groups), Townsend deprivation index at recruitment (continuous), educational level (college or university degree, A levels/AS levels or equivalent, O levels/GCSEs or equivalent, CSEs or equivalent, NVQ or HND or HNC or equivalent, and other professional qualifications), body mass index (BMI, kg/m2, continuous), physical activity (summed MET minutes per week for all activity, continuous), smoking status (never, previous, and current), alcohol intake frequency (three times a week or more, at least once a month, never or special occasions only), comorbidity status, energy intake (kcal/d, continuous), and healthy diet score. Smoking status was derived from a touchscreen questionnaire. Current smokers were defined as smoking on most or all days or only occasionally according to their response to the question, “Do you smoke tobacco now?”. Non-smokers were defined as those who have never smoked according to their response to the question above and those who just tried once or twice according to their answer to “In the past, how often have you smoked tobacco?”. The resource of disease history was from the datasets of the National Health Service (NHS) Information Centre (England and Wales) and the NHS Central Register Scotland (Scotland). The comorbidities in this study were defined with the ICD-10 code, including diabetes (E11-E14), chronic kidney disease (N03-N05, N07, N11-N15, N18-N19), chronic obstructive pulmonary disease (J41-J44), asthma (J45-J46), coronary heart disease (I20–I25), hypertension (I10-I15), atrial fibrillation (I48), stroke (G45-G46, I61, I63), dementia (F00-F03, G30) and cancer (C00-C97). And diseases that occurred before COVID-19 infection (before the latest update date for population tested COVID-19 negative) were considered comorbidities. The healthy diet score was adopted from previously published studies by estimating adherence to the main items of the Mediterranean diet [32, 33]. It was a 0 to 5 scale calculated by scoring five items each as one point, including vegetable intake above or equal to the median (four tablespoons each day), fruits intake above or equal to the median (3 pieces/day), fish intake above or equal to the median (once a week), unprocessed red meat intake less than the median (once a week), processed meat intake less than the median (once a week) [32, 33]. A higher score indicates a much healthier diet. The resource for healthy diet score assessment was from the food frequency questionnaire with the referral period of one year in the initial assessment visit at assessment centres from 2006 to 2010. The directed acyclic graph illustrating the rationale for the selection of confounders was provided in Supplemental Fig. 1.
Statistical analysis
Baseline characteristics of participants included in this study were described as means (standard deviation), median (Interquartile Range (IQR)), or percentages according to the gender-specific quartiles of UPF weight ratio. The analysis of variance (ANOVA), Kruskal–Wallis test, or χ2 tests was used to examine the differences in baseline factors according to quartiles of UPF consumption.
Logistic regression was used to detect the relationship between quartiles of UPF weight ratio and risk of COVID-19 with an estimated odds ratio (OR) and 95% confidential interval (CI). The potential confounding factors listed above were adjusted for in these models. Model 1 was adjusted for gender, age (year of birth), ethnicity, and Townsend deprivation index at recruitment and education levels. Besides covariates in model 1, model 2 was adjusted for BMI, physical activity, smoke status, alcohol intake frequency, and comorbidity status (defined as having at least one of the following: diabetes, chronic kidney disease, chronic obstructive pulmonary disease, asthma, coronary heart disease, hypertension, atrial fibrillation, stroke, dementia, or cancer. Model 3 was further adjusted for total energy intake. Model 4 was further adjusted for a healthy diet score. P for trend was calculated to investigate linear trends of quarters of UPF weight ratio by considering the quarters as an ordinal variable (1, 2, 3, 4). The restricted cubic spline (RCS) model was used to evaluate the non-linear relationship between UPF weight ratio and risk of COVID-19, adjusted for potential confounding factors. The RCS model with 3 knots (10th, 50th, and 90th) was used by comparing the value of the Akaike information criterion of models (with 3 to 7 knots, respectively). Tests for nonlinearity used the likelihood ratio test to compare the model that comprised the linear term with the model that comprised both the linear and the cubic spline terms.
Sensitivity analysis was conducted under five scenarios where basic assumptions were changed. Firstly, the multiple imputation method was used to fill the missing values of covariates (max missing rate: 14%. Details of missing proportions of covariates were listed in Supplemental Table 3), while only complete cases were included in each model in the main analyses. Secondly, the energy contribution of UPF (% of total energy intake) was used as the proxy of individual UPF exposure instead of relative weight proportion. The energy values (kcals) of each food item are derived from McCance and Widdowson’s The Composition of Foods Integrated Dataset 2021 [34]. Thirdly, the absolute weight of UPF was used as the proxy for individual UPF exposure. Fourth, we defined the 5th and 95th centiles as the plausible energy-intake limits. Last, the study population included participants who completed all five dietary recalls only.
In subgroup analysis, we explored the interaction between quarters of UPF weight ratio and different strata factors, including (age: < 65, or ≥ 65 years old; educational level: college or university, or other lower qualifications; BMI: < 25, or ≥ 25 kg/m2; comorbidity status: without comorbidities, or with at least one disease). P for interaction was calculated. And the OR and 95% CI were estimated in each stratum.
Further, mediation analysis was used to explore the mediation effect of BMI on the relationship between UPF weight ratio and the risk of COVID-19. In this analysis, BMI and UPF weight ratio were used as continuous variables. There were two models used. First, a multivariate linear regression model estimating the effect of UPF weight ratio (expose) on BMI (mediator) after adjusting confounders. Then, a multivariate logistic regression model estimates the effect of UPF weight ratio (expose) on COVID-19 incidence (outcome) after adjusting BMI (mediator) and confounders. Average causal mediation effect (ACME) was used to estimate the effect of UPF weight ratio on COVID-19 incidence that could be explained by BMI level. And average direct effect (ADE) represented the effect of UPF weight ratio on COVID-19 incidence independent of BMI. The mediation proportion was calculated describing the proportion of the association that goes through BMI. The nonparametric bootstrap method with 500 repeats was used for confidence intervals of parameters.
All analyses were conducted using R software, V.4.1.2 (R Foundation). All P values for the tests were two-sided, and P values < 0.05 were deemed statistically significant.
Results
In total, 41,012 (18,101 males and 22,911 females) UK Biobank participants were included in the final analysis (Fig. 1). The mean age of participants in the year 2020 (the year of the COVID-19 pandemic) was 56.47 (SD 7.98) years. The average UPF intake weight was 1721.50 g (IQR: 822.50, 1621.54). The weight contribution and energy contribution of UPF among all participants were 26.58% (IQR: 19.98, 34.19) and 52.31% (IQR: 43.62, 60.74). Overall, 6358 (15.5%) participants were diagnosed as COVID-19 positive or dead of COVID-19. Baseline characteristics of participants according to gender-specific quarters of UPF weight ratio were listed in Table 1. Participants in the fourth quarter (high consumption proportion of UPF) were more likely than participants in the lowest quartile to be younger, white, less educated, non-smokers, and to have lower socioeconomic deprivation index, less physical activity, lower alcohol intake, lower healthy diet score, higher BMI, higher energy intake. They were more likely to have a higher intake of carbohydrates, fat (saturated fat and polyunsaturated fat), and a lower intake of protein. And people in the highest quarter were more likely to have a clinical history of hypertension, diabetes, chronic kidney disease, or lung disease. Baseline characteristics of COVID-19 positive and negative participants were listed in Supplemental Table 4.
The relative contribution of each food group to the total weight of UPF was shown in Fig. 2. The food groups with greater contribution were drinks (26.86%), dairy products (20.25%), ultra-processed breads and pastries (19.42%), ready-to-eat meals (12.75%), and ultra-processed fruits and vegetables (10.43%). The cumulative contribution of these five groups of UPF is 89.71%.
Compared to individuals in the lowest quarter of UPF weight ratio, participants in the 2nd, 3rd, and highest quarter were associated with increased odds of COVID-19 incidence, with the ORs of 1.08 (95% CI: 1.00–1.18), 1.33 (95% CI: 1.23–1.44), and 1.57 (95% CI: 1.46–1.70), respectively. The association between UPFs and the risk of COVID-19 was consistent but attenuated, after adjusting for potential confounders. The ORs for 2nd, 3rd, and highest quarters were 1.03 (95% CI: 0.94–1.13), 1.24 (95% CI: 1.13–1.36), and 1.22 (95% CI: 1.12–1.34), respectively. (Table 2).
According to the restricted cubic spline analysis, there was a non-linear association between the proportion of UPF weight and the risk of COVID-19. It is suggested in Fig. 3 that the risk of COVID-19 increased rapidly and then was flat after around 30% of the predicted proportion of UPF consumption.
The main results were robust in sensitivity analyses (Table 2). However, the effect sizes decreased when the energy contribution of UPF (% of total energy intake) or absolute UPF weight (g/day) was used as exposure. Individuals in the highest quarter of UPF energy contribution and the highest quarter of UPF consumption weight were related to increased odds of COVID-19 incidence with the ORs of 1.09 (95% CI: 1.00–1.19) and 1.16 (95% CI: 1.06–1.27), compared with the lowest quarter. When only participants who attended all 5 times of dietary recall questionnaires were included, the proportion of UPF weight was positively associated with increased risk of COVID-19, although not significantly.
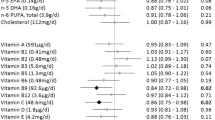
The subgroup analysis showed that there was no interaction effect between the quarters UPF weight ratio and age group, educational level, BMI, and comorbidity status. The association between UPF weight ratio (quarters) and the risk of COVID-19 did not change in most subgroups, but the relationship was not significant among individuals with BMI lower than 25 kg/m2 (Fig. 4).
Subgroup analysis of the proportion of ultra-processed food consumption and risk of COVID-19. Adjustment factors were the same as model 4 listed in Table 2
In a mediation analysis, the results showed that BMI mediated 13.2% (95% CI 8.0% to 23.5%; P < 0.001) of the effect of UPF weight ratio on the risk of COVID-19. The ADE of UPF weight ratio on COVID-19 susceptibility was also significant with an OR of 1.007 (95% CI: 1.004–1.013) per 10% increase in UPF weight contribution. Details are listed in Supplemental Table 5.
Discussion
Using data from UK Biobank, we found that UPF consumption was associated with an increased risk of COVID-19 infection. This association was similar in different subgroups defined by age, educational level, and comorbidity status. In addition, we observed that such association was partly (13.2%) mediated by BMI. However, there was still a direct effect of UPF weight ratio on the risk of COVID-19.
To our knowledge, no study explored the relationship between UPF intake and the risk of COVID-19. Previous studies have shown that nutrition was associated with infectious disease [9]. Most studies had investigated the favourable effect of nutritional factors on the risk of COVID-19. Evidence derived from the NutriNet-Santé cohort showed that higher dietary intakes of fruit and vegetables, vitamin C, folate, vitamin K and fibres were associated with a lower risk of SARS-CoV-2 infection [14]. Merino, J. et al. reported that healthy plant-based foods were related to a lower risk of COVID-19 in the UK Biobank [13]. Some studies showed that a higher intake of vitamins C, B9, K, and dietary fibre were associated with to lower risk of COVID-19 [14]. Consistent with these studies, our study revealed that UPF, which usually represented a lower diet quality, was related to an increased risk of COVID-19.
UPFs, which are frequently present in Western diets, are closely related to the functioning of the immune system [35]. Some potential mechanisms for the association between UPF intake and the risk of COVID-19 are as follow. First, UPFs with excess simple sugars and saturated fats would exert pro-inflammatory effects [36], which could affect the production of immune cells and, directly, the functions of these cells [36]. Second, high saturated fat and low fibres in UPFs could lead to chronic activation of the innate immune system and an inhibition of the adaptive immune system [37]. This is especially relevant to COVID-19 patients given the high rate of infection among lung alveolar epithelial cells and the involvement of lung tissue inflammation and alveolar damage in COVID-19 pathology [38]. Third, UPF consumption may increase exposure to chemicals used in packaging and production and many non-natural ingredients and additives such as flavours, colours, emulsifiers, and other cosmetic additives, which could lead to adverse health outcomes [39, 40]. Fourth, a higher proportion of UPF consumption denoted a lower proportion of fresh vegetables, fruits, and essential micronutrients, which plays key roles in supporting the human immune system and reducing the risk of infections [41, 42].
In this study, we further discovered that UPFs were still associated with a higher risk of COVID-19 after adjusting for commonly used healthy diet scores. This shed insight on the adverse effect of food processing not merely the effect of nutrient quality on COVID-19 susceptibility. UPFs have drastically deconstructed food matrices that cause modified kinetics of release within the digestive tract and altered bio-accessibility and bioavailability [16]. A previous study showed that based on a data set of 98 ready-to-eat foods, the degree of food processing would correlate with the satiety index and glycaemic response [43]. This shed light on other mechanical pathways of the impact of UPFs on the immune system and infectious risk.
Additionally, our current study indicates that BMI accounted for 13.2% of the association between UPF consumption and COVID-19. Previous studies have shown that overweight or obesity increases the risk of infections from pathogens, such as influenza and coronavirus [44, 45], which might partly support our findings. The underlying mechanism of the effect of obesity on susceptibility might be that obesity could impair the activity of helper T lymphocytes, cytotoxic T lymphocytes, B lymphocytes, and natural killer cells, and reduce antibody and interferon-γ production [46, 47]. On the other hand, evidence has suggested that consumption of UPFs could cause obesity [48, 49]. A randomized cross-over control trial suggested that people consumed more calories when exposed to the ultra-processed diet as compared to the unprocessed diet, despite presented daily intakes of calories, sugar, fat, fibre, and macronutrients being matched [48]. Observational studies suggested that higher consumption of UPF was associated with a gain in BMI and higher risks of overweight and obesity [50]. Our study added the role of BMI in the association between UPF and COVID-19 and showed that BMI was an important mediator.
A recent study showed that during COVID-19 lockdown, the consumption of UPF had highly increased, which might hurt immunity, and people would be more susceptive to COVID-19 [51]. Under this circumstance, a proposal of a healthy diet and lower intake of UPF would be of great importance.
Our study contributes to ongoing research efforts to better understand the potential risk factors of COVID-19 infection. And this is the first study evaluating the association between UPF intake and the risk of COVID-19 adjusting for lifestyle, socio-demographic factors, and social physical measurements. We identified that UPF consumption was associated with an increased risk of COVID-19. Specifically, we found that BMI was a partial mediator in this relationship. These findings reinforce the value of a healthy diet and reveal a potential adverse effect of UPFs on infectious diseases.
We acknowledge several limitations. First, we could not confirm the causal relationship between UPF and the risk of COVID-19 since our research was an observational study. Second, the included population was not a random sample of all participants in UK Biobank since only a limited sample had COVID- 19 tests. Therefore, the generalizability of our findings needs to be confirmed in additional studies. Third, social desirability bias might induce underestimation of UPF consumption, which might dilute the studied associations. Nevertheless, online administration of the dietary questionnaire is expected to minimize any reporting bias due to social desirability. And study proved that this way of dietary assessment is acceptable to the public and a feasible strategy for large population-based studies [25]. Fourth, selection bias due to the exclusion of participants with missing values in covariates may have influenced the results. However, the results were robust in sensitivity analyses. Fifth, misclassifications in the NOVA categories cannot be ruled out. UK Biobank study collected limited information about food processing procedures. Thus, insufficient information might lead to misclassification.
Conclusion
Higher UPF consumption in the diet was associated with a significantly increased risk of COVID-19 in this large prospective cohort. This association could be partly mediated by the effect of UPF consumption on BMI. Our findings suggest that public health interventions to improve nutrition and poor metabolic health may be important for reducing the burden of the COVID-19 pandemic. Further evidence on the underlying mechanism is needed.
Data availability
This research has been conducted using the UK Biobank Resource under Application Number 45676. The UK Biobank data are available on application to the UK Biobank (www.ukbiobank.ac.uk/).
References
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Dong E, Du H, Gardner L (2020) An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20(5):533–534. https://doi.org/10.1016/S1473-3099(20)30120-1
World Health Organization (2022) A clinical case definition of post COVID-19 condition by a Delphi consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed 18 February 2022
Leong A, Cole JB, Brenner LN, Meigs JB, Florez JC, Mercader JM (2021) Cardiometabolic risk factors for COVID-19 susceptibility and severity: a Mendelian randomization analysis. Plos Med. https://doi.org/10.1371/journal.pmed.1003553
The Lancet Diabetes E (2021) Metabolic health: a priority for the post-pandemic era. Lancet Diabetes Endocrinol 9(4):189. https://doi.org/10.1016/S2213-8587(21)00058-9
Singh S, Khan A (2020) Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 159(2):768-771.e3. https://doi.org/10.1053/j.gastro.2020.04.064
Wood S, Harrison SE, Judd N, Bellis MA, Hughes K, Jones A (2021) The impact of behavioural risk factors on communicable diseases: a systematic review of reviews. BMC Public Health 21(1):2110. https://doi.org/10.1186/s12889-021-12148-y
Mozaffarian D (2016) Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 133(2):187–225. https://doi.org/10.1161/CIRCULATIONAHA.115.018585
Calder PC (2020) Nutrition, immunity and COVID-19. BMJ Nutr Prev Health 3(1):74–92. https://doi.org/10.1136/bmjnph-2020-000085
Belanger MJ, Hill MA, Angelidi AM, Dalamaga M, Sowers JR, Mantzoros CS (2020) Covid-19 and disparities in nutrition and obesity. N Engl J Med 383(11):e69. https://doi.org/10.1056/NEJMp2021264
Moallemian Isfahani M, Emam-Djomeh Z, Rao IM, Rezaei N (2021) Nutrition and immunity in COVID-19. Adv Exp Med Biol 1318:485–497. https://doi.org/10.1007/978-3-030-63761-3_28
Storm I, den Hertog F, van Oers H, Schuit AJ (2016) How to improve collaboration between the public health sector and other policy sectors to reduce health inequalities? - a study in sixteen municipalities in the Netherlands. Int J Equity Health 15:97. https://doi.org/10.1186/s12939-016-0384-y
Merino J, Joshi AD, Nguyen LH et al (2021) Diet quality and risk and severity of COVID-19: a prospective cohort study. Gut 70(11):2096–2104. https://doi.org/10.1136/gutjnl-2021-325353
Deschasaux-Tanguy M, Srour B, Bourhis L et al (2021) Nutritional risk factors for SARS-CoV-2 infection: a prospective study within the NutriNet-Santé cohort. BMC Med 19(1):290. https://doi.org/10.1186/s12916-021-02168-1
Monteiro CA, Cannon G, Levy RB et al (2019) Ultra-processed foods: what they are and how to identify them. Public Health Nutr 22(5):936–941. https://doi.org/10.1017/S1368980018003762
Fardet A, Rock E (2022) Chronic diseases are first associated with the degradation and artificialization of food matrices rather than with food composition: calorie quality matters more than calorie quantity. Eur J Nutr. https://doi.org/10.1007/s00394-021-02786-8
Briguglio M, Pregliasco FE, Lombardi G, Perazzo P, Banfi G (2020) The malnutritional status of the host as a virulence factor for new coronavirus SARS-CoV-2. Front Med 7:146. https://doi.org/10.3389/fmed.2020.00146
Gibney MJ, Forde CG, Mullally D, Gibney ER (2017) Ultra-processed foods in human health: a critical appraisal. Am J Clin Nutr 106(3):717–724. https://doi.org/10.3945/ajcn.117.160440
Myles IA (2014) Fast food fever: reviewing the impacts of the western diet on immunity. Nutr J 13:61. https://doi.org/10.1186/1475-2891-13-61
Sideleva O, Suratt BT, Black KE et al (2012) Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 186(7):598–605. https://doi.org/10.1164/rccm.201203-0573OC
Fiolet T, Srour B, Sellem L et al (2018) Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. BMJ (Clinical research ed) 360:k322. https://doi.org/10.1136/bmj.k322
Narula N, Wong ECL, Dehghan M et al (2021) Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ (Clinical research ed) 374:n1554. https://doi.org/10.1136/bmj.n1554
Rico-Campa A, Martinez-Gonzalez MA, Alvarez-Alvarez I et al (2019) Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 365:l1949. https://doi.org/10.1136/bmj.l1949
Srour B, Fezeu LK, Kesse-Guyot E et al (2019) Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante). BMJ 365:l1451. https://doi.org/10.1136/bmj.l1451
Bycroft C, Freeman C, Petkova D et al (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562(7726):203–209. https://doi.org/10.1038/s41586-018-0579-z
Bonaccio M, Costanzo S, Di Castelnuovo A et al (2022) Ultra-processed food intake and all-cause and cause-specific mortality in individuals with cardiovascular disease: the Moli-sani Study. Eur Heart J 43(3):213–224. https://doi.org/10.1093/eurheartj/ehab783
Galante J, Adamska L, Young A et al (2016) The acceptability of repeat Internet-based hybrid diet assessment of previous 24-h dietary intake: administration of the Oxford WebQ in UK Biobank. Br J Nutr 115(4):681–686. https://doi.org/10.1017/S0007114515004821
Perez-Cornago A, Pollard Z, Young H et al (2021) Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur J Nutr 60(7):4019–4030. https://doi.org/10.1007/s00394-021-02558-4
Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC (2018) The UN Decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr 21(1):5–17. https://doi.org/10.1017/S1368980017000234
Moubarac JC, Parra DC, Cannon G, Monteiro CA (2014) Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep 3(2):256–272. https://doi.org/10.1007/s13679-014-0092-0
Armstrong J, Rudkin JK, Allen N et al (2020) Dynamic linkage of COVID-19 test results between public health England’s second generation surveillance system and UK Biobank. Microbial genomics. https://doi.org/10.1099/mgen.0.000397
Pazoki R, Dehghan A, Evangelou E et al (2018) Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood pressure levels and cardiovascular events. Circulation 137(7):653–661. https://doi.org/10.1161/circulationaha.117.030898
Wang M, Zhou T, Li X et al (2020) Baseline vitamin D status, sleep patterns, and the risk of incident Type 2 diabetes in data from the UK Biobank study. Diabetes Care 43(11):2776–2784. https://doi.org/10.2337/dc20-1109
H. Pinchen, N. Powell, S. Church, P. Finglas P.H. England (Ed.) (2021) McCance and Widdowson’s The Composition of Foods Integrated Dataset 2021, User Guide. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/971021/McCance_and_Widdowsons_Composition_of_Foods_integrated_dataset_2021.pdf. Accessed 8 June 2022
Christ A, Lauterbach M, Latz E (2019) Western diet and the immune system: an inflammatory connection. Immunity 51(5):794–811. https://doi.org/10.1016/j.immuni.2019.09.020
De Bandt JP, Monin C (2021) Obesity, nutrients and the immune system in the era of COVID-19. Nutrients. https://doi.org/10.3390/nu13020610
Statovci D, Aguilera M, MacSharry J, Melgar S (2017) The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol 8:838. https://doi.org/10.3389/fimmu.2017.00838
Shi Y, Wang Y, Shao C et al (2020) COVID-19 infection: the perspectives on immune responses. Cell Death Differ 27(5):1451–1454. https://doi.org/10.1038/s41418-020-0530-3
Buckley JP, Kim H, Wong E, Rebholz CM (2019) Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National health and nutrition examination survey, 2013–2014. Environ Int 131:105057. https://doi.org/10.1016/j.envint.2019.105057
Lawrence MA, Baker PI (2019) Ultra-processed food and adverse health outcomes. BMJ 365:l2289. https://doi.org/10.1136/bmj.l2289
Ha TW, Jung HU, Kim DJ et al (2021) Association between environmental factors and asthma using mendelian randomization: increased effect of body mass index on adult-onset moderate-to-severe asthma subtypes. Front Genet 12:639905. https://doi.org/10.3389/fgene.2021.639905
Vu TT, Rydland KJ, Achenbach CJ, Van Horn L, Cornelis MC (2021) Dietary behaviors and incident COVID-19 in the UK Biobank. Nutrients 13(6):2114. https://doi.org/10.3390/nu13062114
Fardet A (2016) Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct 7(5):2338–2346. https://doi.org/10.1039/c6fo00107f
Moser JS, Galindo-Fraga A, Ortiz-Hernandez AA et al (2019) Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir Viruses 13(1):3–9. https://doi.org/10.1111/irv.12618
Petrakis D, Margina D, Tsarouhas K et al (2020) Obesity a risk factor for increased COVID19 prevalence, severity and lethality (Review). Mol Med Rep 22(1):9–19. https://doi.org/10.3892/mmr.2020.11127
Honce R, Schultz-Cherry S (2019) Impact of Obesity on Influenza A virus pathogenesis, immune response, and evolution. Front Immunol 10:1071. https://doi.org/10.3389/fimmu.2019.01071
Frasca D, Diaz A, Romero M, Blomberg BB (2017) Ageing and obesity similarly impair antibody responses. Clin Exp Immunol 187(1):64–70. https://doi.org/10.1111/cei.12824
Hall KD, Ayuketah A, Brychta R et al (2019) Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab 30(1):67-77.e3. https://doi.org/10.1016/j.cmet.2019.05.008
Poti JM, Braga B, Qin B (2017) Ultra-processed food intake and obesity: what really matters for health-processing or nutrient content? Curr Obes Rep 6(4):420–431. https://doi.org/10.1007/s13679-017-0285-4
Beslay M, Srour B, Mejean C et al (2020) Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Sante cohort. Plos Med 17(8):e1003256. https://doi.org/10.1371/journal.pmed.1003256
López-Gil JF, García-Hermoso A, Tárraga-López PJ, Brazo-Sayavera J (2021) Dietary patterns, adherence to the food-based dietary guidelines, and ultra-processed consumption during the COVID-19 lockdown in a sample of Spanish young population. Front Pediatr 9:702731. https://doi.org/10.3389/fped.2021.702731
Acknowledgements
The authors would like to express their gratitude to the participants and staff involved in data collection and management in the UK Biobank. This research has been conducted using the UK Biobank Resource under project number 45676.
Funding
This study was funded by the National Natural Science Foundation of China (No. 71910107004, 91746205). The funding sources had no role in the design, execution, analyses, and interpretation of the data or decision to submit the results of this study.
Author information
Authors and Affiliations
Contributions
LZ and HL designed the study and drafted the statistical analysis plan. LZ compiled the data, did the statistical analysis, interpreted the data, and drafted the manuscript. HL and SZ interpreted the data, and critically revised the manuscript. HY critically revised the manuscript. YM compiled the data. YW conceived the study, critically revised the manuscript, and acquired the data and funding. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflicts of interest.
Ethical approval
The ethical approvals of UK Biobank were obtained from the North West Multi-Centre Research Ethics Committee, as a Research Tissue Bank (RTB) approval. This study was approved under the UK Biobank application number 45676.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The manuscript is approved by all authors for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, L., Li, H., Zhang, S. et al. Impact of ultra-processed food intake on the risk of COVID-19: a prospective cohort study. Eur J Nutr 62, 275–287 (2023). https://doi.org/10.1007/s00394-022-02982-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02982-0