Abstract
Purpose
Polycystic ovary syndrome (PCOS) is a common endocrine disorder among reproductive-age women. Insulin resistance and dyslipidemia are linked to PCOS. l-Carnitine supplementation as a management strategy for women with PCOS has been proposed. The effect of l-carnitine supplementation on insulin resistance, sex hormone-binding globulin (SHBG) and lipid profile in overweight/obese women with PCOS was investigated.
Methods
This randomized, double-blind, controlled clinical trial, was conducted on 62overweight/obese women with PCOS. Participants were randomly assigned into two groups to receive 1000 mg/day l-carnitine or placebo (1000 mg starch) for 12 weeks.
Results
l-Carnitine supplementation compared to the placebo showed a significant improvement in insulin [− 0.7 (− 7.3 to 4.0) vs. 0.7 (− 3.0 to 5.2); P = 0.001], homeostatic model assessment for insulin resistance [− 0.4 (− 1.7 to 1.1) vs. 0.0 (− 0.7 to 1.3); P = 0.002], quantitative insulin sensitivity check index (+ 0.01 ± 0.02 vs. − 0.01 ± 0.01; P = 0.02) and a non-significant change toward improvement in SHBG (+ 11.5 ± 40.2 vs. − 3.2 ± 40.2; P = 0.2). However, there was no significant differences between the two groups in serum levels of fasting plasma glucose, total cholesterol, triglyceride, low density lipoprotein-cholesterol and high density lipoprotein cholesterol (P > 0.05).
Conclusion
12-week l-carnitine supplementation in overweight or obese women with PCOS ameliorate insulin resistance, but has no effect on SHBG and lipid profile. Studies with higher dosages and duration of l-carnitine intake are required. The trial was registered on 30 December 2019 at Iranian Registry of Clinical Trials IRCT20191016045131N1.
Trial registration
Registered on 30th December 2019 at Iranian Registry of Clinical Trials (IRCT20191016045131N1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among reproductive-age women, and is associated with increased risk of infertility, type 2 diabetes mellitus (T2DM), nonalcoholic fatty liver disease (NAFLD), and cardiovascular disease (CVD) [1,2,3]. The prevalence of PCOS in the general population has been estimated to be 3–10% [4, 5]. There are three different sets of diagnostic criteria for PCOS as follows: 1; the National Institutes of Health (NIH), 2; the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine (ESHRE/ASRM), which is also known as the Rotterdam criteria, and 3; the Androgen Excess Society and PCOS Society (AES-PCOS) [6,7,8,9]. Anovulation, hyperandrogenism, amenorrhea or oligomenorrhea, hirsutism, acne, and obesity are the most common clinical features of PCOS [10]. Moreover, dyslipidemia is a common disorder among women with PCOS [11]. Insulin resistance is closely linked to the PCOS pathogenesis [12, 13]. Insulin resistance by mechanisms such as suppressing the hepatic sex hormone-binding globulin (SHBG) production, and subsequently increasing free testosterone, increasing the luteinizing hormone (LH) pulse amplitude, stimulating the adrenal P450c17α activity and inducing hyperandrogenism is involved in the pathogenesis of PCOS [13]. Currently, diet modification and weight loss are the important strategies in the management of PCOS [14].
l-Carnitine as a conditionally essential amino acid synthesized from lysine (diaminohexanoic acid-26) and methionine (amino-4-(methylthio) butanoic acid-2), is found in almost every cell in the body (specially muscles) and plays a critical role in the energy production, lipid and glucose metabolism [15,16,17,18]. Carnitine is obtained from endogenous synthesis or diet [16, 19]. Meat products, fishes, and dairy foods are rich sources, and most fruits and vegetables are poor sources of carnitine [15, 19]. Several studies have investigated the effects of l-carnitine on osteoarthritis, CVD, hypothyroidism, obesity and T2DM [20,21,22,23,24,25]. In addition, the evidence demonstrated the therapeutic effects of l-carnitine on insulin resistance and dyslipidemia [26, 27]. l-Carnitine by increasing the glucose transporter GLUT4 expression, and the serum levels of adiponectin improved insulin resistance in metabolic syndrome‐induced rats [28]. Moreover, oral administration of l-carnitine led to a decrease in the levels of cholesterol, triglyceride (TG), low-density lipoprotein-cholesterol (LDL-c) and very low density lipoprotein-cholesterol (VLDL-c) as well as an increase in the levels of high-density lipoprotein-cholesterol (HDL-c) in metabolic syndrome‐induced rats [28]. The benefits of l-carnitine in the management of infertility have been demonstrated [29, 30]. l-Carnitine can attenuate the effect of high LDL and oxidized LDL on spermatogenesis and improve the serum testosterone and LH levels in rats [31]. The studies reported a direct relationship between serum carnitine and SHBG, which is a transporter of sex hormones [32,33,34]. Moreover, a negative correlation between serum carnitine and free androgen index (FAI) was found [34]. To date, there is no clinical trial evaluating the effect of l-carnitine on SHBG. In addition, there are limited number of clinical trials (with contradictory findings) investigating the effects of l-carnitine on insulin resistance and dyslipidemia in women with PCOS [35, 36]. According to the current evidence, the present clinical trial was designed to investigate the effect of 1000 mg/day oral l-carnitine intake on insulin resistance, SHBG and lipid profile in overweight/obese women with PCOS.
Methods
Recruitment and eligibility screening
From May 2019 to August 2019, 75women with PCOS referred to diabetes research center in Yazd, Iran were screened. The diagnose of PCOS was performed based on the Rotterdam criteria by an endocrinologist. In the PCOS defined by Rotterdam criteria, the women must represent symptoms in two out of three categories, which include oligo/anovulation, hyperandrogenism, and the presence of polycystic ovaries [9, 10]. A total of 62women diagnosed with PCOS met the inclusion criteria, which is defined as follows: aged 18–45 years, and body mass index (BMI) ≥ 25. The exclusion criteria included T2DM, thyroid diseases, psychiatric diseases, smoking, pregnancy, taking letrazole, glucose-lowering drugs (metformin, rosiglitazone, pioglitazone, glimepiride, nateglinide), and lipid lowering medications, insulin infusion, adherence to a specific diet, and unwillingness to continue the study. In addition, the participants were excluded if their l-carnitine and placebo capsules consumption (the compliance rate) was less than 80%.
Study design
We conducted a double-blind, randomized, controlled clinical trial. The registration of the protocol was performed at Iranian clinical trials website (http://www.irct.ir) with code number: IRCT20191016045131N1. After a detailed description of the potential benefits and side effects of study participation, the participants signed a written informed consent. At the baseline, the participants were randomly assigned into the treatment and control groups by a trained person, using a simple randomization sampling method. Randomization list was prepared using a computer-generated random number table. The follow-up was done for 12 weeks from September 2019 to December 2019. The participants, and investigators remained blinded for the randomization and intervention assignment until the end of the study.
Intervention
The treatment group received one l-carnitine capsule (1000 mg l-carnitine) daily and the control group received daily one placebo capsule containing 1000 mg starch. All subjects received clomiphene citrate, and were advised to the common healthy dietary recommendations during follow-up. l-carnitine and placebo capsules were prepared by Karen Pharmaceuticals Co., Yazd, Iran. The odor, shape and color of the l-carnitine and the placebo capsules were similar. l-Carnitine and placebo capsules were given to participants every 2 weeks, and the compliance rate was monitored.
Physical activity and dietary intake measurement
Using the international physical activity questionnaire (IPAQ), the level of physical activity was measured at the baseline and the end of the study. In addition, using a 3-day (1 weekend day and 2 nonconsecutive weekdays) 24-h recall questionnaire, evaluating energy intake and diet composition was performed at the baseline and the end of the study.
Blood sampling and biochemical measurements
At the baseline and the end of the trial, 5 cc blood was taken from each participant after 12 h fasting, and the blood samples were centrifuged for 10 min at a speed of 3600 rpm. The microtubes containing serum samples were stored at − 80 °C. Insulin and SHBG were measured by ELISA method using the Q-1-DiaPlus, USA kits. Fasting plasma glucose (FPG), total cholesterol (TC), TG, LDL-c, and HDL-c were measured using Pars Azmoon, Iran kits by an autoanalyzer. The laboratory measurements were done in the diabetes research center laboratory, based on the standard protocols.
Glucose homeostasis
To determine insulin resistance, the homeostatic model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = [fasting insulin (mU/L) × fasting plasma glucose (mg/dL)]/405. In addition, the quantitative insulin sensitivity check index (QUICKI) was calculated based on the following formula:1/[log (fasting insulin (mU/L)) + log (fasting plasma glucose (mg/dL))].
Anthropometric measurements
Height, weight, waist circumference (WC), and hip circumference (HC) were measured under the standard protocols at the baseline and the end of the trial. Height, WC and HC were assessed using a measuring tape. Measuring weight was performed by a portable digital scale (Omeron BF511, Japan) with an accuracy of 100 g. The participants’ height, WC, HC and weight were measured in standing position without shoes. To measure weight, participants had light clothes. Using the following formula, body mass index (BMI) was calculated: weight (kg)/height squared (m2).
Sample size and statistical analysis
The sample size was calculated based on insulin, as the main variable of the study of Samimi et al. [36], with α = 0.05, power = 80%. The minimum sample size was 28 participants in each group. The sample size increased to 31 participants per group, after considering ∼10% dropout. The normal distribution of variables was assessed using Kolmogorov–Smirnov test. An independent t-test was used to compare the means of normal variables at the baseline and the end of the study, as well as comparing mean changes of normal data between two groups. To compare the abnormal data between the two groups at the baseline and after the intervention as well as the mean changes of abnormal data between two groups Mann–Whitney U test was used. We used paired t test to compare the mean of normal variables in each group, and if the distribution of data was not normal, Wilcoxon test was utilized. ANCOVA was also carried out to control the covariates. Data were analyzed using SPSS version 24 (SPSS, Inc.). P < 0.05 was considered as significance level.
Results
Characteristics of the participants
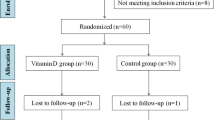
Sixty-two women who met inclusion criteria were enrolled in the trial. During follow-up, three women due to pregnancy, and three women due to refuse to continue were excluded. 28 participants in the control group and 28 participants in the treatment group completed the trial (Fig. 1).
Baseline and anthropometric variables
At the baseline, no significant differences were found between the two groups in terms of age, physical activity, energy intake, anthropometric indices, glycemic variables, SHBG and lipid profile (except for HDL-c with P-value = 0.007) (Table 1). There was no significant difference between the two groups in the levels of energy intake, diet composition and physical activity during follow-up (P > 0.05) (Table 2). In addition, we found no significant difference between the two groups in terms of weight, BMI, and HC during follow-up; but, a significant change in WC (P = 0.001) was observed (Table 3). There was no serious side effect related to consuming supplements during follow-up.
Outcomes
In the treatment group compared to the control group, a significant improvement was seen in the terms of insulin (changes: − 0.7 (− 7.3 to 4.0) vs. 0.7 (− 3.0 to 5.2); P = 0.001), HOMA-IR (changes: − 0.4 (− 1.7 to 1.1) vs. 0.0 (− 0.7 to 1.3); P = 0.002) and QUICKI (mean changes: + 0.01 ± 0.02 vs. − 0.01 ± 0.01; P = 0.02), after adjusting for changes of WC and baseline values. However, FPG remained without significant change (changes: − 6.0 (− 9.4 to 5.0) vs. − 1.0 (− 4.0 to 1.0); P = 0.44) (Table 4).
No significant improvement was found in the treatment group compared to the control group in serum SHBG (mean changes: + 11.5 ± 40.2 vs. − 3.2 ± 40.2; P = 0.2) (Table 4). In addition, serum levels of TC (mean changes: − 3.0 ± 29.5 vs. − 6.7 ± 15.5; P = 0.54), TG (changes: − 3.0 (− 20.1 to 22.0) vs. 0.0 (− 20.8 to 3.5); P = 0.72), LDL-c (mean changes: − 6.4 ± 30.0 vs. − 10.7 ± 13.6; P = 0.48) and HDL-c (changes: 3.0 (− 6.1 to 9.2) vs. 5.0 (0.8–9.8); P = 0.44) remained without significant change (Table 4).
Discussion
In the present study that conducted among overweight/obese women with PCOS, 12-week l-carnitine intake can reduce insulin resistance; however, SHBG and lipid profile without significant change. The study of Samimi et al. [34] found a significant reduction in insulin, FPG and HOMA-IR among women with PCOS consuming 250 mg/day l-carnitine for 12 weeks. In addition, the study of Ismail et al. [33] reported that l-carnitine intake (3 g/day) can reduce levels of insulin and FPG in women with PCOS. We demonstrated a non-significant change toward improvement in the term of FPG. It seems, higher doses of l-carnitine and longer duration of supplementation with L-carnitine could improve the levels of FPG. l-Carnitine by stimulating glucose metabolism via increasing mitochondrial oxidation of acyl-coenzyme A (Acyl-CoA), modulating pyruvate dehydrogenase complex activity, stimulating insulin and insulin like growth factor-1 cascade, as well as regulating glycolytic and gluconeogenic enzymes gene expression, can improve insulin resistance [26, 37].
For the first time, we investigated the effect of l-carnitine on SHBG in women with PCOS. Evidence suggested a direct correlation of serum total l-carnitine with SHBG, but a negative correlation of serum l-carnitine with FAI [34]. Furthermore, SHBG as a strong predictor of serum total l-carnitine level, is inversely correlated with insulin resistance [34]. We found a non-significant change toward improvement in SHBG, and if the intervention duration was longer, we could probably see a significant improvement. A study revealed that after 12-week l-carnitine intake, levels of free testosterone and dehydroepiandrosterone (DHEA) remained without significant change in women with PCOS [38]. In addition, a clinical trial demonstrated that l-carnitine intake (250 mg/day) for 12 weeks has no effect on levels of free testosterone, but can significantly reduce DHEA [36].
The present study did not find any change in lipid profile after l-carnitine intake. The study of Samimi et al. [36] reported that 12-week l-carnitine supplementation (250 mg/day) has no effect on lipid profile of women with PCOS. A study revealed that l-carnitine supplementation cannot improve TC, TG and HDL-c in hemodialysis patients [39]. In addition, Lee et al. [40] showed that l-carnitine supplementation (1 g/day) has no significant effect on TC, TG and LDL-c in coronary artery disease. In addition, the study of Malaguarnera et al. [41] reported that supplementation with l-carnitine (2 g/day) has a beneficial effect on lipid profile in patients with T2DM. A logical reason for this discrepancy can be the differences in dosage of l-carnitine. It seems, higher dosages of l-carnitine can improve lipid profile. A recent meta-analysis showed that l-carnitine less than 2 g/day has no effect on TC, TG and LDL-c [42]. Previously, some studies suggested that l-carnitine by stimulating the production of apolipoprotein-A1, modulating the TG synthesis and esterification toward the formation of acetylcarnitines, can decrease the TG and VLDL-c plasma concentration [43,44,45,46].
Our study has some strengths. The present study was the first clinical trial evaluating SHBG in women with PCOS. In addition, we considered the significant changes of anthropometric variables from baseline as covariates. However, we used the low dose of l-carnitine as well as small sample size, which are the important limitations of the present study. In addition, use of instruments to measure body composition that are not the gold standard tools was another limitation of the present study.
Conclusions
Overall, 1000 mg/day oral l-carnitine supplementation ameliorated insulin resistance, however, has no effect on SHBG and lipid profile. Further clinical trials with higher dosages of l-carnitine and longer intervention durations are required.
Abbreviations
- Acyl-CoA:
-
Acyl-coenzyme A
- AES:
-
Androgen Excess Society
- ASRM:
-
American Society for Reproductive Medicine
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DHEA:
-
Dehydroepiandrosterone
- ESHRE:
-
European Society of Human Reproduction and Embryology
- FAI:
-
Free androgen index
- FPG:
-
Fasting plasma glucose
- HC:
-
Hip circumference
- HDL-c:
-
High density lipoprotein-cholesterol
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- IPAQ:
-
International physical activity questionnaire
- LDL-c:
-
Low-density lipoprotein-cholesterol
- LH:
-
Luteinizing hormone
- MET-h:
-
Metabolic equivalent task hours
- NAFLD:
-
Nonalcoholic fatty liver disease
- NIH:
-
National Institutes of Health
- PCOS:
-
Polycystic ovary syndrome
- QUICKI:
-
Quantitative insulin sensitivity check index
- SHBG:
-
Sex hormone-binding globulin
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- VLDL:
-
Very low-density lipoprotein
- WC:
-
Waist circumference
References
Condorelli RA, Calogero AE, Di Mauro M, Mongioi’ LM, Cannarella R, Rosta G et al (2018) Androgen excess and metabolic disorders in women with PCOS: beyond the body mass index. J Endocrinol Invest 41(4):383–388
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS et al (2016) Polycystic ovary syndrome. Nat Rev Dis Primers 2:16057
Harris HR, Terry KL (2016) Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract 2:14
Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R (1998) Prevalence of the polycystic ovary syndrome in unselected black and white women of the Southeastern United States: a prospective study. J Clin Endocrinol Metab 83(9):3078–3082
Kauffman RP, Baker VM, DiMarino P, Gimpel T, Castracane VD (2002) Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: a comparison of two distinct populations. Am J Obstet Gynecol 187(5):1362–1369
Amato MC, Galluzzo A, Finocchiaro S, Criscimanna A, Giordano C (2008) The evaluation of metabolic parameters and insulin sensitivity for a more robust diagnosis of the polycystic ovary syndrome. Clin Endocrinol (Oxf) 69(1):52–60
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W et al (2009) The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91(2):456–488
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L et al (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 110(3):364–379
Carmina E (2004) Diagnosis of Polycystic ovary syndrome: from NIH criteria to ESHRE-ASRM guidelines. Minerva Ginecol 56(1):1–6
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Wild RA, Rizzo M, Clifton S, Carmina E (2011) Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 95(3):1073–1079
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7(4):219–231
Barber TM, Dimitriadis GK, Andreou A, Franks S (2016) Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin Med (Lond) 16(3):262–266
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E (2015) American association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—Part 1. Endocr Pract 21(11):1291–1300
Steiber A, Kerner J, Hoppel CL (2004) Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med 25(5–6):455–473
El-Hattab AW, Scaglia F (2015) Disorders of carnitine biosynthesis and transport. Mol Genet Metab 116(3):107–112
Stephens FB, Constantin-teodosiu D, Greenhaff PL (2007) New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 581(Pt 2):431–444
Evans AM, Fornasini G (2003) Pharmacokinetics of L-carnitine. Clin Pharmacokinet 42(11):941–967
Demarquoy C, Demarquoy J (2019) Autism and carnitine: a possible link. World J Biol Chem 10(1):7–16
Ruggenenti P, Cattaneo D, Loriga G, Ledda F, Motterlini N, Gherardi G et al (2009) Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: effects of acetyl-l-carnitine therapy. Hypertension 54(3):567–574
Power RA, Hulver MW, Zhang JY, Dubois J, Marchand RM, Ilkayeva O et al (2007) Carnitine revisited: potential use as adjunctive treatment in diabetes. Diabetologia 50(4):824–832
Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C (2004) Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: a review. Ann N Y Acad Sci 1033:79–91
Barzegar A, Alipour B, Panahi F, Karamzad N (2013) Effect of L-Carnitine supplementation on serum adipokines (Leptin and Visfatin) levels in obese type II diabetes mellitus women with Hypocaloric diet. Life Sci J 10:359–365
Malek Mahdavi A, Mahdavi R, Kolahi S, Zemestani M, Vatankhah AM (2015) L-Carnitine supplementation improved clinical status without changing oxidative stress and lipid profile in women with knee osteoarthritis. Nutr Res 35(8):707–715
Askarpour M, Hadi A, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E (2020) Beneficial effects of L-carnitine supplementation for weight management in overweight and obese adults: an updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol Res 151:104554
Ringseis R, Keller J, Eder K (2012) Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur J Nutr 51(1):1–18
Emami Naini A, Moradi M, Mortazavi M, Amini Harandi A, Hadizadeh M, Shirani F et al (2012) Effects of oral L-carnitine supplementation on lipid profile, anemia, and quality of life in chronic renal disease patients under hemodialysis: a randomized, double-blinded, placebo-controlled trial. J Nutr Metab. https://doi.org/10.1155/2012/510483
Zayed EA, AinShoka AA, El Shazly KA, Abd El Latif HA (2018) Improvement of insulin resistance via increase of GLUT4 and PPARγ in metabolic syndrome-induced rats treated with omega-3 fatty acid or l-carnitine. J Biochem Mol Toxicol 32(11):e22218
Agarwal A, Sengupta P, Durairajanayagam D (2018) Role of L-carnitine in female infertility. Reprod Biol Endocrinol 16(1):5
Dunning KR, Robker RL (2012) Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim Reprod Sci 134(1–2):69–75
Ghanbarzadeh S, Garjani A, Ziaee M, Khorrami A (2014) CoQ10 and L-Carnitine attenuate the effect of high LDL and oxidized LDL on spermatogenesis in male rats. Drug Res (Stuttg) 64(10):510–515
Zhu J, Chen Z, Feng W, Long S, Mo ZC (2019) Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta 499:142–148
Hammond GL, Wu TS, Simard M (2012) Evolving utility of sex hormone-binding globulin measurements in clinical medicine. Curr Opin Endocrinol Diabetes Obes 19(3):183–189
Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N (2008) Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod 23(7):1602–1606
Ismail AM, Hamed AH, Saso S, Thabet HH (2014) Adding L-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. Eur J Obstet Gynecol Reprod Biol 180:148–152
Samimi M, Jamilian M, Ebrahimi FA, Rahimi M, Tajbakhsh B, Asemi Z (2016) Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 84(6):851–857
Maleki V, Jafari-Vayghan H, Kashani A, Moradi F, Vajdi M, Kheirouri S et al (2019) Potential roles of carnitine in patients with polycystic ovary syndrome: a systematic review. Gynecol Endocrinol 35(6):463–469
Salehpour S, Nazari L, Hoseini S, Moghaddam PB, Gachkar L (2019) Effects of L-carnitine on polycystic ovary syndrome. JBRA Assist Reprod 23(4):392–395
Huang H, Song L, Zhang H, Zhang H, Zhang J, Zhao W (2014) Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: a systematic review and meta-analysis. Kidney Blood Press Res 38(1):31–41
Lee BJ, Lin JS, Lin YC, Lin PT (2016) Effects of L-carnitine supplementation on lipid profiles in patients with coronary artery disease. Lipids Health Dis 15:107
Malaguarnera M, Vacante M, Avitabile T, Malaguarnera M, Cammalleri L, Motta M (2009) L-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. Am J Clin Nutr 89(1):71–76
Askarpour M, Hadi A, Symonds ME, Miraghajani M, Sadeghi O, Sheikhi A et al (2019) Efficacy of L-carnitine supplementation for management of blood lipids: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 29(11):1151–1167
Montjean D, Entezami F, Lichtblau I, Belloc S, Gurgan T, Menezo Y (2012) Carnitine content in the follicular fluid and expression of the enzymes involved in beta oxidation in oocytes and cumulus cells. J Assist Reprod Genet 29(11):1221–1225
Longo N, Frigeni M (1863) Pasquali M (2016) Carnitine transport and fatty acid oxidation. Biochim Biophys Acta 10:2422–2435
Sirtori CR, Calabresi L, Ferrara S, Pazzucconi F, Bondioli A, Baldassarre D et al (2000) L-carnitine reduces plasma lipoprotein(a) levels in patients with hyper Lp(a). Nutr Metab Cardiovasc Dis 10(5):247–251
Hoppel C (2003) The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis 41(Suppl4):S4–S12
Acknowledgements
We acknowledge the contribution of the co-researchers. We also thank the participants for their cooperation throughout recruitment and data collection processes.
Funding
Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran supported this study. This was a financial support for student thesis process including laboratory works, and provide kits as well as supplements.
Author information
Authors and Affiliations
Contributions
FP and MH: conducted the study; AG: provided material and technical support, AS: interpreted the finding and drafted the manuscript; MH and AN: critically revised the manuscript; HF: carried out the statistical analysis; and MH: supervised the study. The final version of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interests
The authors have declared no competing interests.
Ethical approval
The research council of Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences and Health Services approved the study protocol. The ethical committee of Shahid Sadoughi University of Medical Sciences and Health Services in Yazd approved the written informed consent (code number: IR.SSU.SPH.REC.1397.014).
Informed consent
Written informed consent was obtained from all participants before the data collection.
Consent for publication
Not applicable.
Data availability
The data and materials of the current study is available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Sangouni, A.A., Pakravanfar, F., Ghadiri-Anari, A. et al. The effect of l-carnitine supplementation on insulin resistance, sex hormone-binding globulin and lipid profile in overweight/obese women with polycystic ovary syndrome: a randomized clinical trial. Eur J Nutr 61, 1199–1207 (2022). https://doi.org/10.1007/s00394-021-02659-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02659-0