Abstract
Purpose
We conducted a meta-analysis to systematically assess the prospective association between vitamin K and cardiovascular disease (CVD) events and all-cause mortality.
Methods
We searched PubMed and EMBASE through January 2019 for prospective studies that reported the association of vitamin K (assessed by dietary intake or circulating concentration) with CVD events [including total CVD, CVD mortality, total coronary heart disease (CHD), fatal CHD, nonfatal myocardial infarction (MI), and stroke] and all-cause mortality. Multivariable-adjusted hazard ratios (HRs) comparing top versus bottom tertiles of vitamin K were combined using random-effects meta-analysis.
Results
Twenty-one articles were included with 222,592 participants. A significant association was found between dietary phylloquinone and total CHD (pooled HR 0.92; 95% CI 0.84, 0.99; I2 = 0%; four studies), as well as menaquinone and total CHD (0.70; 95% CI 0.53, 0.93; I2 = 32.1%; two studies). No significant association was observed between dietary vitamin K and all-cause mortality, CVD mortality, or stroke. Elevated plasma desphospho-uncarboxylated MGP (dp-ucMGP), a marker of vitamin K deficiency, was associated with an increased risk of all-cause mortality (1.84; 95% CI 1.48, 2.28; I2 = 16.8%; five studies) and CVD mortality (1.96; 95% CI 1.47, 2.61; I2 = 0%; two studies). No significant association was observed between circulating total osteocalcin and all-cause mortality or total CVD.
Conclusions
Our findings showed that higher dietary vitamin K consumption was associated with a moderately lower risk of CHD, and higher plasma dp-ucMGP concentration, but not total circulating osteocalcin, was associated with increased risks of all-cause and CVD mortality. However, causal relations cannot be established because of limited number of available studies, and larger prospective studies and randomized clinical trials are needed to validate the findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD), including coronary heart disease (CHD) and stroke, is still the leading cause of death and disease burden in many countries around the world [1]. Mounting evidence has indicated that diet plays an important role in the development of CVD and eventually death [2,3,4,5].
Vitamin K, a family of fat-soluble compounds, comprises both phylloquinone and menaquinone. The former, also called as vitamin K1, is mainly derived from dark-green leafy vegetables; while the latter, also known as vitamin K2, is primarily derived from dairy products, meat, and eggs [6, 7]. Both phylloquinone and menaquinone can catalyze the γ-glutamate carboxylation of all vitamin K-dependent proteins [8], such as matrix Gla protein (MGP) and osteocalcin. MGP is synthesized by chondrocytes and vascular smooth muscle cells and categorized into various types according to their carboxylation or phosphorylation status, i.e., uncarboxylated MGP (ucMGP), carboxylated MGP (cMGP), dephosphorylated MGP (dpMGP), and phosphorylated MGP (pMGP) [9]. Osteocalcin is synthesized in bone during bone formation and includes both carboxylated and uncarboxylated forms [10]. However, no single biomarker is currently considered as a gold-standard clinical indicator of vitamin K status and reference ranges of those biomarkers remain to be established. Since vitamin K insufficiency leads to increase circulating concentration of desphospho-ucMGP (dp-ucMGP) and total osteocalcin, which have been indicated to be markers for the assessment of circulating vitamin K status [10, 11], with higher dp-ucMGP and osteocalcin concentrations reflecting lower vitamin K status [9,10,11]. The current evidence on vitamin K and risk of CVD events and death is equivocal because of limited observational studies and clinical trials [12,13,14]. A recent meta-analysis summarized data from cohort studies up to December 2017 [15], and reported that higher plasma concentrations of dp-ucMGP, but not dietary intakes of vitamin K, were associated with an increased risk of total and CVD mortality. However, the associations between dietary intake and incident CVD and other circulating vitamin K biomarkers such as osteocalcin were not reported.
Therefore, we performed a systematic review and quantitative evaluation of most updated evidence to add substantive new data and insights into the association of vitamin K exposure, involving studies of dietary vitamin K intake and circulating concentration of vitamin K (assessed by dp-ucMGP and osteocalcin), with risks of CVD events and all-cause mortality.
Methods
Search strategy
We conducted the meta-analysis following the PRISMA guidelines for performing and reporting meta-analyses of observational studies [16]. We searched the literature in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (http://www.embase.com) from inception until February 2018 in the first round, and we updated the articles from February 2018 to January 2019 and added “osteocalcin” as an exposure (from inception until January 2019) in the second round. Besides, we also reviewed the reference lists of all retrieved relevant articles and reviews [12,13,14,15]. Our study focused on the exposure (dietary vitamin K or vitamin K biomarkers) with multiple outcomes (CVD, type 2 diabetes, and mortality). Full search strategies are reported in the Supplemental material. This article focused on the association of dietary vitamin K, circulating dp-ucMGP and total osteocalcin with CVD events, and all-cause mortality, while the other blood biomarkers (e.g., cMGP, ucMGP, and carboxylated and undercarboxylated osteocalcin) and other outcomes (type 2 diabetes and cause-specific mortality other than CVD mortality) were not analyzed due to very limited numbers of articles.
Study selection
Eligibility of literature was independently assessed by two researchers (H-GC and L-TS), and any inconsistencies were settled by consensus or consultation with a third author (AP). Publications were included in the current study if they: (1) were peer-reviewed and reported original results (e.g., not editorial, letter, commentary, meeting abstract, or review article); (2) were cohort studies (i.e., the exposure status was measured before the onset of the outcome, including prospective or retrospective designs); (3) were published in English; (4) included non-institutionalized adults older than 18 years old without CVD at baseline; and (5) reported an association between dietary vitamin K, circulating dp-ucMGP or total osteocalcin, and at least one specific CVD events [i.e., total CVD, CVD mortality, total CHD, fatal CHD, nonfatal myocardial infarction (MI), and stroke] or all-cause mortality using multivariable-adjusted risk estimates. Potentially eligible publications were included through an initial screening of relevant titles or abstracts, accompanied by a full-text review.
Data extraction and quality assessment
The following information from each study was independently extracted by two researchers (H-GC and L-TS) using a standardized spreadsheet: citation, first author, publication year, study name, study location, participants characteristics (number, mean age or age range, and sex composition), follow-up time, main exposure (phylloquinone, menaquinone, dp-ucMGP, osteocalcin, and assessment method), main endpoints (CVD events, all-cause mortality, diagnostic methods, and number of cases), analysis strategy (statistical methods and confounding factors considered in the models), and multivariable-adjusted hazard ratio (HR) estimates with corresponding 95% confidence interval (CI). We contacted study authors when the data were unclear or unavailable from the identified papers. The Newcastle–Ottawa Scale [17], which included items related to the comparability of study design and analysis, selection bias, exposure and outcome measurements, exclusion of outcome at baseline, years of follow-up, response rate, confounding adjustment, and generalizability to other populations, was utilized to evaluate the study quality.
Statistical analysis
Hazard ratio estimates adjusted for the maximum number of covariates were pooled across studies separately for each outcome using a random-effects meta-analysis. Both categorical and continuous variables of vitamin K exposure status were used in the literature, and to achieve a consistent comparison of the results, we transformed the HR from each study to a risk estimate that compared the top with bottom tertiles of the exposure using methods described previously [18, 19]. In brief, these transformed estimates were calculated by multiplying the log risk ratio and the upper and lower confidence limits with a conversion factor (2.18 for a 1 SD increase, 2.18/2.54 for quartiles, and 2.18/2.80 for quintiles). The risk estimates were transformed assuming that a transformation of the exposure was normally distributed and a log-linear association with the outcome [18, 19].
Forest plots were drawn to intuitively visualize the HRs and corresponding 95% CIs across studies for each outcome using a random-effects model [20]. Heterogeneity of HRs was evaluated by calculating the Cochrane Q statistic (P < 0.10 was deemed to be statistically significant) and the I2 statistic (values of 0–25%, 26–50%, 51–75%, and 76–100% were considered as having low, modest, moderate, and high likelihood of heterogeneity, respectively) [21]. A sensitivity analysis was conducted using untransformed data from the comparisons of the extreme categories reported in the original papers, thus studies with continuous variables of vitamin K intake/status were not included in this analysis. We did not perform the sensitivity analysis of omitting one study a time because of limited number of studies. All analytical procedures were conducted with Stata version 13.1 (StataCorp, College Station, TX, USA); a two-sided α of 0.05 was chosen for the cut-off of significance.
Results
Literature search
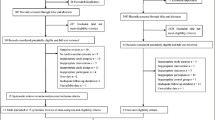
The literature search retrieved 19,027 publications, of which 282 were assessed in full-text following a selection of titles and abstracts. Besides, an extra two articles were found from the bibliographies of relevant reviews. After detailed examinations, 263 articles were excluded and 21 original articles met our inclusion criteria and involved a total of 222,592 participants [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] (Fig. 1). Among the 263 articles that were excluded based on a full-text review, four articles met the other inclusion criteria, but were excluded because of data format issues [43,44,45,46]. The characteristics of these four studies are depicted in Table S1 and the results of these studies were described in the respective text below. Among the 21 articles that were included in the meta-analysis, seven studies specifically reported dietary vitamin K as the exposure [22,23,24,25,26,27,28], eight studies specifically reported plasma dp-ucMGP as the exposure [29,30,31,32,33,34,35,36], and six studies specifically reported circulating total osteocalcin as the exposure [37,38,39,40,41,42].
Included study characteristics
All 21 eligible articles were derived from prospective cohort studies. Seven studies investigated associations of dietary vitamin K and CVD events, five studies investigated associations of plasma dp-ucMGP with CVD events, four studies investigated associations of circulating total osteocalcin with total CVD, and three dietary vitamin K studies, five plasma dp-ucMGP studies, and two circulating total osteocalcin studies explored the associations with all-cause mortality, respectively. In all studies, dietary vitamin K consumption was evaluated through validated food frequency questionnaires (FFQs), dp-ucMGP concentration was determined in plasma by ELISA method, and total osteocalcin was quantified using different assays such as electrochemiluminescent immunoassay, radioimmunoassay and enzyme immune assay and automated Elecsys assay, etc. Study samples ranged from 95 to 72,874, and the mean or median follow-up duration ranged from 1.9 to 16.8 years. Fifteen studies were conducted in European countries (The Netherlands, Spain, France, and Czech Republic), three studies were conducted in Asian countries (Japan and South Korea), and the other two and one studies were done in the United States (US) and Australia, respectively. Crude HR was transformed in study by Yeap et al. [37], two study adjusted for socio-demographic characteristics, and ten studies corrected for socio-demographic characteristics (e.g., age and sex) and established CVD risk factors (e.g., lifestyle factors and complications), and the other eight studies additionally corrected for dietary intakes or biomarker concentration of other vitamins (e.g., vitamin D) (Table 1). Overall, the Newcastle-Ottawa Scale awarded scores ranged from 6 to 9, indicating moderate-to-high methodological quality of all studies (Table S2).
Dietary vitamin K with CVD events and all-cause mortality
A total of seven articles reported on the associations of dietary vitamin K (phylloquinone and menaquinone) with CVD events (i.e., CVD mortality, total CHD, fatal CHD, nonfatal MI, and stroke) and all-cause mortality. For dietary phylloquinone, the association was only statistically significant with total CHD (pooled HR comparing top with bottom tertiles 0.92; 95% CI 0.84, 0.99; P = 0.035; I2 = 0%; 4249 cases from four studies). Modest associations were found between phylloquinone and fatal CHD (pooled HR comparing top with bottom tertiles 0.89; 95% CI 0.77, 1.02; P = 0.082; I2 = 0%; 1503 cases from four studies) and nonfatal MI (pooled HR comparing top with bottom tertiles 0.91; 95% CI 0.82, 1.02; P = 0.100; I2 = 0%; 2604 cases from three studies). No statistically significant associations were found with other CVD subtypes (i.e., stroke and CVD mortality) (Fig. 2). For dietary menaquinone, similar results were obtained for total CHD (pooled HR comparing top with bottom tertiles 0.70; 95% CI 0.53, 0.93; P = 0.014; I2 = 32.1%; 713 cases from two studies). There were no statistically significant associations of dietary menaquinone with fatal CHD and CVD mortality (Fig. 3). In the sensitivity analysis using untransformed data based on extreme categories of exposure, the findings were generally remained (Figs. S1 and S2). Three studies reported the association of both dietary phylloquinone and menaquinone as the exposure with all-cause mortality [22, 27, 28], and no significant associations were found (Fig. 4), nor in the sensitivity analysis using untransformed data (Fig. S3).
The study by Cheung et al. [43] was excluded because of data format issue. In this study, 3401 participants with chronic kidney disease (CKD) from the Third National Health and Nutrition Examination Survey (NHANES III) were classified into two groups based on their total vitamin K intake levels higher or lower than the recommended adequate intake level (90 μg/day for women and 120 μg/day for men, respectively), and it was reported that higher dietary vitamin K intake at baseline was associated with a 15% reduced risk of all-cause mortality (HR 0.85; 95% CI 0.72, 1.00; 1815 cases) and a 22% reduced risk of CVD mortality (HR 0.78; 95% CI 0.64, 0.95; 876 cases) during a median follow-up of 13.3 years (Table S1). Thus, our conclusion would not be substantially changed if this study was included.
The association of circulating dp-ucMGP and total osteocalcin with all-cause mortality
A significant positive association was observed between plasma concentrations of dp-ucMGP and all-cause mortality (pooled HR comparing top with bottom tertiles 1.84; 95% CI 1.48, 2.28; P < 0.001; 483 cases from five studies) with low heterogeneity (I2 = 16.8%) (Fig. 5). The results were not materially changed if the untransformed data were used in the sensitivity analysis (Fig. S4). However, it should be noted that all five studies were conducted in patients with certain diseases (e.g., symptomatic aortic stenosis [29], chronic kidney disease [30], type 2 diabetes [31], stable kidney transplantation [34], or heart failure patients with vascular disease [35]). Most of the patients were taking vitamin K antagonists, which may explain why their median dp-ucMGP concentrations ranged from 156 to 1038 pmol/L (average median = 784.8 pmol/L), much higher than the general population (for example, 114 pmol/L in the EPIC-NL study among general participants [33]). No statistically significant association was found between circulating total osteocalcin and all-cause mortality (pooled HR comparing top with bottom tertiles 1.07; 95% CI 0.59, 1.96; P = 0.816; 628 cases from two studies) (Fig. 6).
Two studies have examined the association between plasma dp-ucMGP concentrations and all-cause mortality in the general population, but they were not included in our meta-analysis because of data format issue (Table S1). Liu et al. [44] followed 2318 participants in Belgium for a median of 14.1 years and reported a J-shaped association with a nadir at 135 pmol/L, and dp-ucMGP was positively and linearly associated with all-cause mortality above the level of 135 pmol/L. Riphagen et al. [45] followed 4275 participants in The Netherlands for a median of 8.5 years and also reported a J-shaped association with a threshold at 414 pmol/L, and positive and linear association was found when dp-ucMGP levels were above 414 pmol/L, while the association was flat below this level. Given that these two studies both reported a J-shape association but with different nadir, more studies are still needed to confirm and quantify the association in the general population.
The association of circulating dp-ucMGP and total osteocalcin with CVD events
Three studies [31, 32, 36] investigated the association between plasma dp-ucMGP concentration and risk of total CVD; the overall pooled HR (95% CI) comparing top with bottom tertiles was 1.57 (1.19, 2.06; P < 0.001; I2 = 1.1%; 220 cases from three studies) (Fig. 5). The study by Shea et al. [46] was not included, because we cannot transform the HR to a risk estimate that compared the top with bottom tertiles based on existing data (Table S1). In this study, 635 community-dwelling adults aged 70–79 years from the US were classified into two groups based on their dp-ucMGP concentrations higher or lower than the predefined cut-off values (574 pmol/L), and it was reported that higher plasma dp-ucMGP concentration was not significantly associated with a higher risk of total CVD (HR 1.02; 95% CI 0.72, 1.45; 169 cases). We included this study in the sensitivity analysis using untransformed data based on extreme categories of exposure, and the pooled HR became insignificant (1.13; 95% CI 0.80, 1.62; I2 = 29.5%; 4 studies) (Fig. S4). Moreover, no statistically significant associations were found between circulating total osteocalcin and total CVD (pooled HR comparing top with bottom tertiles 1.02; 95% CI 0.76, 1.36; P = 0.917; 946 cases from four studies) (Fig. 6), nor in the sensitivity analysis using untransformed data (Fig. S5).
Two studies [31, 35] reported the association between plasma dp-ucMGP concentration and risk of CVD mortality; the overall pooled HR (95% CI) comparing top with bottom tertiles was 1.96 (1.47, 2.61; P < 0.001; I2 = 0%; 143 cases from two studies) (Fig. 5). A similar result was obtained in the sensitivity analysis (Fig. S4). The above-mentioned study by Liu et al. [44] was not included, because they reported that higher levels of dp-ucMGP were log-linearly associated with increased CVD mortality (HR for per doubling of the nadir 1.14; 95% CI 1.01, 1.28; 70 cases), but insignificantly associated with risk of total CVD (HR for per doubling of the nadir 0.99; 95% CI 0.94, 1.05; 180 cases). The above-mentioned study by Riphagen et al. [45] was also not included, because they reported a J-shaped association with CVD mortality, and positive and linear association was found when dp-ucMGP levels were above 557 pmol/L.
Regarding to other types of CVD events, a study from The Netherlands [33] reported insignificant associations of plasma dp-ucMGP concentration with risk of total CHD (HR comparing extreme quartiles 0.94; 95% CI 0.79, 1.13; 1252 cases) and stroke (HR comparing extreme quartiles 1.09; 95% CI 0.78, 1.51; 405 cases) in the general population. The above-mentioned study by Liu et al. [44] also reported that higher levels of dp-ucMGP were log-linearly associated with decreased risk of coronary events (HR for per doubling of the nadir 0.93; 95% CI 0.88, 0.99; 85 cases), but insignificantly associated with stroke risk (HR for per doubling of the nadir 0.98; 95% CI 0.81, 1.19; 29 cases). A study from South Korea [40] reported insignificant associations of circulating total osteocalcin with risk of incident CHD (HR comparing extreme tertiles 1.05; 95% CI 0.44, 2.50; 29 cases) and stroke (HR comparing extreme tertiles 0.69; 95% CI 0.34, 1.39; 47 cases) in middle-aged men.
Discussion
We systematically investigated the associations of vitamin K with CVD events and all-cause mortality using data from both dietary and biomarker studies. Our results suggested that higher dietary phylloquinone and menaquinone intakes were consistently associated with a lower risk of total CHD, and higher dp-ucMGP concentration was associated with a higher risk of all-cause and CVD mortality. In general, these data support potential benefits of vitamin K on the prevention of CVD events and all-cause mortality. However, given that no significant association was found for osteocalcin with the outcomes, and the number of included studies was small, consensus cannot be made, and more studies are still needed.
In our current meta-analysis, we found that higher dietary intakes of phylloquinone and menaquinone intake were associated with a lower risk of total CHD and a trend of lower risk but not statistically significant association with fatal CHD. The association appeared to be stronger for menaquinone. It is possible that phylloquinone and menaquinone are mainly transported by chylomicrons, from where phylloquinone is more effectively cleared by the liver. Long residence periods of higher menaquinone indicate that it is favorable for extrahepatic tissue such as arterial vessel uptake for much longer durations than phylloquinone [6]. However, the lower risk of CHD related to dietary phylloquinone could be a reflection of a healthy diet, because the main dietary sources are green vegetables and vegetable oils [6, 7]. By contrast, the associations between menaquinone and CHD risks were unlikely due to confounding by a healthier diet, since menaquinone is primarily derived from dairy products, meat, and eggs, and the associations between those food groups and CHD risks were not entirely consistent: no significant association was found for dairy [47] and egg [48], while increased risk was found for meat [49], particularly processed red meat. Therefore, the inverse association between menaquinone and CHD risks cannot be explained by the food sources. Given the limited number of identified articles, firm conclusions cannot be made, and further investigations are warranted to verify the associations of dietary vitamin K consumption and CHD risks.
Higher plasma dp-ucMGP concentration has been consistently reported to be associated with increased risks of all-cause mortality in several original studies [29,30,31, 34, 35]. Empirically, target biomarker measured in plasma may provide more objective nutritional assessment than dietary records [50]. In the dietary studies, the intakes of vitamin K were all self-reported using FFQs. The accuracy of FFQs data depends on memory and precise estimation of the frequency and portion size of the foods over a specified period (typically the past year), as well as the quality of the food composition table used to estimate the dietary vitamin K consumption [51]. It is possible that both random and systematic measurement errors were inevitable because of imperfect recalls, omissions of vitamin K containing food items in the FFQs (e.g., extended food composition data for vitamin K to support the notable contributions made by convenience foods and composite dishes), and inaccurate information in the dietary databases [52, 53]. Therefore, we cannot draw a firm conclusion regarding the role of dietary intakes of vitamin K on CVD events and all-cause mortality. On the other hand, plasma dp-ucMGP concentration is an objective biomarker that may reflect vitamin K status and can avoid the aforesaid measurement errors. However, the plasma dp-ucMGP concentration may only reflect short-term vitamin K exposure status owing to the short half-life of vitamin K [20, 54], and age, sex/ethnicity, lifestyle, and metabolic health status may influence of dp-ucMGP concentration. Although circulating total osteocalcin is thought to be a sensitive marker to reflect vitamin K status, we did not find any significant associations between total osteocalcin and all-cause mortality or total CVD. It is possible that dp-ucMGP is the main vitamin K status indicator of vascular calcification, while total osteocalcin is related to bone formation [55]. Therefore, consistent with the scientific opinion provided by European Food Safety Authority (EFSA) panel [56], we also considered that available data on dietary intake of phylloquinone or menaquinones and health outcomes cannot be utilized to derive dietary reference values (DRVs) for vitamin K, and no biomarkers of vitamin K intake or status are currently applicable to derive DRVs for vitamin K.
Very few randomized clinical trials have been performed to evaluate the effects of vitamin K supplementation on cardiovascular health. A 3-year double-blind, randomized controlled trial conducted among 388 community-dwelling men and women (60–80 years) in the US showed that daily supplementation of a multivitamin with 500 μg phylloquinone reduced the progression of existing coronary artery calcification (CAC) compared with a daily multivitamin without phylloquinone [57]. In a study with 244 healthy postmenopausal women in The Netherlands, Knapen et al. [58] revealed that long-term (36 months) supplementation with 180 μg/day menaquinone-7 improved arterial stiffness compared with those receiving placebo. Results from the two clinical trials support the view that vitamin K is beneficial in reducing CVD events. Mechanistically, phylloquinone and menaquinone act as cofactors to catalyze the carboxylation of glutamic acid residues (Glu) into γ-carboxyglutamate (Gla) [8], which binds with free calcium ions to inhibit vascular calcification [59]. Therefore, higher levels of vitamin K may reduce vascular calcification [60,61,62], and consequently lower the risks of CVDs and death. However, the current evidence is still limited to set optimal intake levels of vitamin K, and this needs to be confirmed in future studies.
Several limitations of this meta-analysis should be noted. First, although we conducted a comprehensive literature search, a relatively small number of articles were included, which limited our capacity to conduct more subgroup analyses (e.g., stratification by age, sex, participant characteristics, CVD subtypes, disease state, and medication use) and to identify the source of heterogeneity. More high-quality prospective studies are certainly needed. Second, we included plasma dp-ucMGP and total osteocalcin as biomarkers to reflect vitamin K status, but all included studies did not correct for total MGP concentrations and calculate the proportion of undercarboxylated osteocalcin in their analyses. Therefore, the observed association of dp-ucMGP and total osteocalcin with CVD events and all-cause mortality might be influenced, since dp-ucMGP and total osteocalcin concentrations may not necessarily reflect vitamin K intake. Third, we only included publications in English and excluded articles which did not meet our data format requirements, thus the possibility of bias cannot be ruled out. Fourth, the confounding factors adjusted for in the original articles varied across studies, and residual confounding cannot be completely excluded. The baseline levels for vitamin K intakes or circulating biomarker concentrations also varied across studies and we could not directly report the levels across tertiles in the pooled analyses. Notwithstanding, we examined the prospective associations of both dietary and biomarkers of vitamin K status with CVD outcomes and all-cause mortality by summarizing the most updated evidence. Compared to the prior meta-analysis [15], we additionally included studies of dietary vitamin K intakes with CVD risk, and also circulating osteocalcin concentrations with CVD outcomes and all-cause mortality. In addition, the prior meta-analysis [15] reported the pooled HR comparing the extreme categories, while we were able to transform risk estimates from most original studies to a consistent comparison of top versus bottom tertiles, which may provide more comparable estimates.
In summary, our meta-analysis provides further insight that higher vitamin K levels at baseline, as evaluated by both dietary intake and plasma dp-ucMGP concentration, may be associated with a lower risk of total CHD and all-cause mortality, respectively. However, we did not find significant associations between total osteocalcin and total CVD and all-cause mortality. Therefore, our results need to be interpreted with caution due to the limited number, inconsistent results and potential confounding issues of included studies. Since our study cannot infer causality, high-quality prospective cohort studies in different populations and randomized clinical trials are still required to draw a firm conclusion regarding the role of vitamin K in cardiovascular health and death.
References
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1211–1259. https://doi.org/10.1016/S0140-6736(17)32154-2
Pan A, Lin X, Hemler E, Hu FB (2018) Diet and cardiovascular disease: advances and challenges in population-based studies. Cell Metab 27(3):489–496. https://doi.org/10.1016/j.cmet.2018.02.017
Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, Sherliker P, Gao H, Chen Y, Yang L, Chen J, Wang S, Du R, Su H, Collins R, Peto R, Chen Z, China Kadoorie Biobank S (2016) Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med 374(14):1332–1343. https://doi.org/10.1056/NEJMoa1501451
Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB (2014) Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349:g4490. https://doi.org/10.1136/bmj.g4490
Scarborough P, Morgan R, Webster P, Rayner M (2011) Differences in coronary heart disease, stroke and cancer mortality rates between England, Wales, Scotland and Northern Ireland: the role of diet and nutrition. BMJ Open 1(1):e000263. https://doi.org/10.1136/bmjopen-2011-000263
Schurgers LJ, Vermeer C (2000) Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 30(6):298–307. https://doi.org/10.1159/000054147
Shearer MJ (1995) Vitamin K. Lancet 345(8944):229–234
Furie B, Bouchard BA, Furie BC (1999) Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood 93(6):1798–1808
Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JWJ (2013) Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem 24(4):624–628. https://doi.org/10.1016/j.jnutbio.2012.02.012
Shea MK, Booth SL (2016) Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients 8:8. https://doi.org/10.3390/nu8010008
Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C (2010) Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 104(4):811–822. https://doi.org/10.1160/TH09-11-0786
Rees K, Guraewal S, Wong YL, Majanbu DL, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH (2010) Is vitamin K consumption associated with cardio-metabolic disorders? A systematic review. Maturitas 67(2):121–128. https://doi.org/10.1016/j.maturitas.2010.05.006
Hartley L, Clar C, Ghannam O, Flowers N, Stranges S, Rees K (2015) Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 9:CD011148. https://doi.org/10.1002/14651858.CD011148.pub2
Harshman SG, Shea MK (2016) The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr Nutr Rep 5(2):90–98. https://doi.org/10.1007/s13668-016-0162-x
Zhang S, Guo L, Bu C (2019) Vitamin K status and cardiovascular events or mortality: a meta-analysis. Eur J Prev Cardiol 26:549–553. https://doi.org/10.1177/2047487318808066
Moher D, Liberati A, Tetzlaff J, Altman D (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (cited 1 June 2018)
Danesh J, Collins R, Appleby P, Peto R (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279(18):1477–1482. https://doi.org/10.1001/jama.279.18.1477
Chêne G, Thompson SG (1996) Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol 144(6):610–621. https://doi.org/10.1093/oxfordjournals.aje.a008971
Combettes E, Mazoit JX, Benhamou D, Beloeil H (2015) Modelling of vitamin K half-life in patients treated with vitamin K antagonists before hip fracture surgery. Anaesth Crit Care Pain Med 34(5):295–299. https://doi.org/10.1016/j.accpm.2015.06.003
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC (2004) Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr 134(11):3100–3105. https://doi.org/10.1093/jn/134.11.3100
Erkkila AT, Booth SL, Hu FB, Jacques PF, Manson JE, Rexrode KM, Stampfer MJ, Lichtenstein AH (2005) Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr 59(2):196–204. https://doi.org/10.1038/sj.ejcn.1602058
Erkkila AT, Booth SL, Hu FB, Jacques PF, Lichtenstein AH (2007) Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis 17(1):58–62. https://doi.org/10.1016/j.numecd.2006.03.008
Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT (2009) A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis 19(7):504–510. https://doi.org/10.1016/j.numecd.2008.10.004
Vissers LE, Dalmeijer GW, Boer JM, Monique Verschuren WM, van der Schouw YT, Beulens JW (2013) Intake of dietary phylloquinone and menaquinones and risk of stroke. J Am Heart Assoc 2(6):e000455. https://doi.org/10.1161/jaha.113.000455
Juanola-Falgarona M, Salas-Salvado J, Martinez-Gonzalez MA, Corella D, Estruch R, Ros E, Fito M, Aros F, Gomez-Gracia E, Fiol M, Lapetra J, Basora J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Munoz MA, Ruiz-Gutierrez V, Fernandez-Ballart J, Bullo M (2014) Dietary intake of vitamin K is inversely associated with mortality risk. J Nutr 144(5):743–750. https://doi.org/10.3945/jn.113.187740
Zwakenberg SR, den Braver NR, Engelen AI, Feskens EJ, Vermeer C, Boer JM, Verschuren WM, van der Schouw YT, Beulens JW (2016) Vitamin K intake and all-cause and cause specific mortality. Clin Nutr 36(5):1294–1300. https://doi.org/10.1016/j.clnu.2016.08.017
Ueland T, Gullestad L, Dahl CP, Aukrust P, Aakhus S, Solberg OG, Vermeer C, Schurgers LJ (2010) Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med 268(5):483–492. https://doi.org/10.1111/j.1365-2796.2010.02264.x
Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA (2010) The circulating inactive form of matrix Gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol 5:568–575. https://doi.org/10.2215/cjn.07081009
Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW (2013) Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care 36(11):3766–3771. https://doi.org/10.2337/dc13-0065
van den Heuvel EG, van Schoor NM, Lips P, Magdeleyns EJ, Deeg DJ, Vermeer C, den Heijer M (2014) Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas 77(2):137–141. https://doi.org/10.1016/j.maturitas.2013.10.008
Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW (2014) Circulating desphospho-uncarboxylated matrix gamma-carboxyglutamate protein and the risk of coronary heart disease and stroke. J Thromb Haemost 12(7):1028–1034. https://doi.org/10.1111/jth.12609
Keyzer CA, Vermeer C, Joosten MM, Knapen MH, Drummen NE, Navis G, Bakker SJ, de Borst MH (2015) Vitamin K status and mortality after kidney transplantation: a cohort study. Am J Kidney Dis 65(3):474–483. https://doi.org/10.1053/j.ajkd.2014.09.014
Mayer O Jr, Seidlerova J, Vanek J, Karnosova P, Bruthans J, Filipovsky J, Wohlfahrt P, Cifkova R, Windrichova J, Knapen MH, Drummen NE, Vermeer C (2016) The abnormal status of uncarboxylated matrix Gla protein species represents an additional mortality risk in heart failure patients with vascular disease. Int J Cardiol 203:916–922. https://doi.org/10.1016/j.ijcard.2015.10.226
Zwakenberg SR, van der Schouw YT, Vermeer C, Pasterkamp G, den Ruijter HM, Beulens JWJ (2018) Matrix Gla protein, plaque stability, and cardiovascular events in patients with severe atherosclerotic disease. Cardiology 141:32–36. https://doi.org/10.1159/000493006
Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Hankey GJ, Beilby JP, Norman PE (2012) Associations of total osteocalcin with all-cause and cardiovascular mortality in older men. The Health In Men Study. Osteoporos Int 23:599–606. https://doi.org/10.1007/s00198-011-1586-1
Yamashita T, Okano K, Tsuruta Y, Akiba T, Nitta K (2013) Serum osteocalcin levels are useful as a predictor of cardiovascular events in maintenance hemodialysis patients. Int Urol Nephrol 45:207–214. https://doi.org/10.1007/s11255-012-0156-6
Holvik K, van Schoor NM, Eekhoff EM, den Heijer M, Deeg DJ, Lips P, de Jongh R (2014) Plasma osteocalcin levels as a predictor of cardiovascular disease in older men and women: a population-based cohort study. Eur J Endocrinol 171:161–170. https://doi.org/10.1530/eje-13-1044
Hwang YC, Kang M, Cho IJ, Jeong IK, Ahn KJ, Chung HY, Lee MK (2015) Association between the circulating total osteocalcin level and the development of cardiovascular disease in middle-aged men: a mean 8.7-year longitudinal follow-up study. J Atheroscler Thromb 22:136–143. https://doi.org/10.5551/jat.25718
Miyake H, Kanazawa I, Sugimoto T (2018) Association of bone mineral density, bone turnover markers, and vertebral fractures with all-cause mortality in type 2 diabetes mellitus. Calcif Tissue Int 102:1–13. https://doi.org/10.1007/s00223-017-0324-x
Zwakenberg SR, van der Schouw YT, Schalkwijk CG, Spijkerman AMW, Beulens JWJ (2018) Bone markers and cardiovascular risk in type 2 diabetes patients. Cardiovasc Diabetol 17:45. https://doi.org/10.1186/s12933-018-0691-2
Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC (2015) Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr 34(2):235–240. https://doi.org/10.1016/j.clnu.2014.03.011
Liu YP, Gu YM, Thijs L, Knapen MH, Salvi E, Citterio L, Petit T, Carpini SD, Zhang Z, Jacobs L, Jin Y, Barlassina C, Manunta P, Kuznetsova T, Verhamme P, Struijker-Boudier HA, Cusi D, Vermeer C, Staessen JA (2015) Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension 65(2):463–470. https://doi.org/10.1161/hypertensionaha.114.04494
Riphagen IJ, Keyzer CA, Drummen NEA, de Borst MH, Beulens JWJ, Gansevoort RT, Geleijnse JM, Muskiet FAJ, Navis G, Visser ST, Vermeer C, Kema IP, Bakker SJL (2017) Prevalence and effects of functional vitamin K insufficiency: the PREVEND Study. Nutrients 9(12):1334. https://doi.org/10.3390/nu9121334
Shea MK, Booth SL, Weiner DE, Brinkley TE, Kanaya AM, Murphy RA, Simonsick EM, Wassel CL, Vermeer C, Kritchevsky SB (2017) Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the Health, Aging, and Body Composition Study (Health ABC). J Nutr 147(5):888–895. https://doi.org/10.1161/strokeaha.117.01720610.3945/jn.117.249375
Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS (2017) Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 32:269–287. https://doi.org/10.1007/s10654-017-0243-1
Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, Sands A, Hu FB, Liu L (2013) Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ 346:e8539. https://doi.org/10.1136/bmj.e8539
Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, De Henauw S, Michels N, Devleesschauwer B, Schlesinger S, Schwingshackl L (2019) Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 59:1071–1090. https://doi.org/10.1080/10408398.2017.1392288
Holick CN, Michaud DS, Stolzenberg-Solomon R, Mayne ST, Pietinen P, Taylor PR, Virtamo J, Albanes D (2002) Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol 156(6):536–547. https://doi.org/10.1093/aje/kwf072
Dietary Assessment Primer (2016) Food frequency questionnaire at a Glance. National Cancer Institute, National Institutes of Health. https://dietassessmentprimer.cancer.gov/profiles/questionnaire/ (cited 1 June 2018)
Lawlor DA, Smith GD, Bruckdorfer KR, Kundu D, Ebrahim S (2004) Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? Lancet 363(9422):1724–1727. https://doi.org/10.1016/s0140-6736(04)16260-0
Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB (2012) alpha-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 96(6):1262–1273. https://doi.org/10.3945/ajcn.112.044040
Jones KS, Bluck LJ, Wang LY, Coward WA (2008) A stable isotope method for the simultaneous measurement of vitamin K1 (phylloquinone) kinetics and absorption. Eur J Clin Nutr 62(11):1273–1281. https://doi.org/10.1038/sj.ejcn.1602859
van Ballegooijen AJ, Beulens JW (2017) The role of vitamin K status in cardiovascular health: evidence from observational and clinical studies. Curr Nutr Rep 6:197–205. https://doi.org/10.1007/s13668-017-0208-8
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), Turck D, Bresson JL, Burlingame B, Dean T, Fairweather-Tait S, Heinonen M, Hirsch-Ernst KI, Mangelsdorf I, McArdle HJ, Naska A, Nowicka G, Pentieva K, Sanz Y, Siani A, Sjodin A, Stern M, Tome D, Van Loveren H, Vinceti M, Willatts P, Lamberg-Allardt C, Przyrembel H, Tetens I, Dumas C, Fabiani L, Ioannidou S, Neuhauser-Berthold M, Nutr EPDP (2017) Scientific Opinion on the dietary reference values for vitamin K. EFSA J 15(5):4780. https://doi.org/10.2903/j.efsa.2017.4780
Shea MK, O’Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL (2009) Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 89(6):1799–1807. https://doi.org/10.3945/ajcn.2008.27338
Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C (2015) Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost 113(5):1135–1144. https://doi.org/10.1160/TH14-08-0675
Schurgers LJ, Uitto J, Reutelingsperger CP (2013) Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol Med 19(4):217–226. https://doi.org/10.1016/j.molmed.2012.12.008
Cranenburg EC, Schurgers LJ, Vermeer C (2007) Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost 98(1):120–125. https://doi.org/10.1160/TH07-04-0266
Spronk HM, Soute BA, Schurgers LJ, Thijssen HH, De Mey JG, Vermeer C (2003) Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J Vasc Res 40(6):531–537. https://doi.org/10.1159/000075344
Tsugawa N (2015) Cardiovascular diseases and fat soluble vitamins: vitamin D and vitamin K. J Nutr Sci Vitaminol (Tokyo) 61(Suppl):S170–S172. https://doi.org/10.3177/jnsv.61.S170
Acknowledgements
We thank Geertje W. Dalmeijer and Sabine R. Zwakenberg (Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands) for providing re-analyzed data on dp-ucMGP and total CVD.
Funding
The work was supported by the National Key Research and Development Program of China (2017YFC0907500 and 2017YFC0907504), and Hubei Province Science Fund for Distinguished Young Scholars (2018CFA033).
Author information
Authors and Affiliations
Contributions
H-GC performed the statistical analysis; contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. H-GC and L-TS conducted the research, screened the references and extracted the data. Y-BZ, A-LC, and Y-WL researched and proofed the data. SKK transformed the data to comparison of top third with bottom third. LJ and AP planned and designed the study. H-GC and AP had full access to the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically reviewed, discussed, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AP reported receiving a research grant from the BY-HEALTH CO., LTD, outside the submitted work. Other authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, HG., Sheng, LT., Zhang, YB. et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr 58, 2191–2205 (2019). https://doi.org/10.1007/s00394-019-01998-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01998-3