Abstract
Purpose
Coffee is rich in bioactive compounds with health beneficial properties, with green coffee presenting higher phenol content than roasted. We evaluated the effects of regularly consuming realistic amounts of a green/roasted coffee blend on cardiovascular health-related biomarkers.
Methods
A randomized, cross-over, controlled study was carried out in 25 normocholesterolemic [total cholesterol (TC) < 200 mg/dL] and 27 hypercholesterolemic (TC 200–240 mg/dL) subjects. During 8 weeks, volunteers consumed 6 g/day of soluble green/roasted (35:65) coffee or a control beverage (water or an isotonic drink). Blood pressure, heart rate and body weight were monitored at the end of each intervention, and serum lipids [TC, HDL-C, LDL-C, VLDL-C, triglycerides and phospholipids], cytokines and chemokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, MCP-1, MIP-1β, TNF-α, INF-γ), adhesion molecules (ICAM-1, VCAM-1), and C-reactive protein were measured. Plasma antioxidant capacity (FRAP, ORAC and ABTS methods), and lipid (malondialdehyde, MDA) and protein (carbonyl groups, CG) oxidation were also determined.
Results
Attending to the general lineal model of variance for repeated measures, after the green/roasted coffee intervention significant reductions in TC, LDL-C, VLDL-C and triglycerides levels (p = 0.006, 0.001, 0.003 and 0.017, respectively), and a significant group effect were observed (0.001, < 0.001, 0.019 and 0.027, respectively). Only within the hypercholesterolemic group, attending to the Bonferroni test, the aforementioned lipid parameters were significantly lower after regular green/roasted coffee intake compared to baseline values. Moreover, after the coffee stage, plasma antioxidant capacity improved, according to the increase in ORAC and FRAP values (p < 0.001 and p < 0.001, respectively) and decrease of MDA (p = 0.015) and CG (p < 0.001) levels, without differences between groups. Systolic (p = 0.001) and diastolic (p < 0.001) blood pressure, heart rate (p = 0.035), and body weight (p = 0.017) were reduced in both normo- and hypercholesterolemic groups.
Conclusion
Regular consumption of moderate amounts of a soluble green/roasted (35:65) coffee blend may contribute to improve cardiovascular health in moderately hypercholesterolemic people, as reducing serum lipids, blood pressure and body weight effects, as well as increasing plasma antioxidant capacity, have been observed. Moreover, positive influences on blood pressure, body weight, and plasma antioxidant capacity were obtained in the healthy group. Therefore, incorporation of green coffee beans into the coffee brew can be recommended as part of a dietary strategy to protect from cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coffee is a complex beverage containing a large range of phytochemicals with potential health effects, including methylxanthines (mostly caffeine), dietary fiber and minor components such as the diterpenes, cafestol and kahweol or trigonelline [1]. However, phenolic compounds are one of the most abundant phytochemicals in coffee, mainly hydroxycinnamoylquinic acids, also known as chlorogenic acids or caffeoylquinic acids (CQAs). Polyphenols may amount to up to 10% of the dry weight in the green beans, although they are drastically lost during roasting, with losses over 50% of the initial phenolic content depending on the degree of roasting [1, 2]. The major polyphenols are hydroxycinnamoylquinic acids (caffeoylquinic, feruloylquinic and p-coumaroylquinic acids), dicaffeoylquinic acids and caffeoylferuloylquinic acids, in addition to caffeoylquinic lactones formed during roasting [3, 4]. Other components are also produced during roasting of the green beans such as melanoidins (up to 29% in roasted beans) [5], hydroxyhydroquinone (HHQ) [6], or N-methylpyridinium (NMP), a degradation product of trigonelline [7].
Coffee consumption has been reported to have an inverse relationship with certain chronic disorders such as cancer, type 2 diabetes or metabolic syndrome [8,9,10]. However, it is still a habitual practice to limit or even to totally ban caffeinated coffee consumption to patients suffering from hypertension, arrhythmia or other cardiovascular conditions in spite of the absence of recommendation of coffee withdrawal in recent guidelines for the management of heart failure [11]. Data in the literature concerning the effect of coffee on biomarkers of cardiovascular risk (i.e. serum lipids, blood pressure, endothelial function, inflammation, etc.) are controversial [12]. There is evidence from randomized clinical trials (RCT) showing that coffee, especially boiled, unfiltered coffee, is associated with increased serum lipids [total cholesterol (TC), LDL-cholesterol (LDL-C) and triglycerides (TG)] [13, 14]. Coffee had been associated to increased blood pressure in several meta-analyses of RCT [15,16,17], although recent systematic reviews and meta-analysis of RCT and prospective observational studies did not support an association between habitual coffee consumption and increased risk of hypertension [18,19,20]. Similarly, negative acute effects in vascular function [increased arterial stiffness and arterial wave reflection, reduced flow mediated dilation (FMD)] have also been reported in healthy individuals [21,22,23], although long-term studies show positive effects on peripheral endothelial function [24].
Studies on the health effect of coffee have been performed with caffeinated vs. decaffeinated coffee or pure caffeine, coffees differing in brewing type (boiled, filtered, espresso, etc.), coffee blends differing in the degree of roasting, etc., the complexity and variability of the coffee beverage and the content of bioactive phytochemicals largely explaining the controversial results in the literature. In a recent review on the effects of coffee components on cardiovascular health, caffeine was identified as the compound responsible for the increase in blood pressure and other endothelial disorders, diterpenoids for the hyperlipidemic effects of unfiltered coffee, and hydroxyhydroquinones for the production of reactive oxygen species (ROS) [25]. In contrast, CQAs seem to mitigate the negative effects of coffee thanks to their antioxidant, chemopreventive, antihypertensive and hypoglycemic properties [25]. This has drawn attention to green coffee polyphenols, and several animal and human intervention studies using chlorogenic acids, polyphenol-rich green coffee bean extracts or green coffee have shown promising results in body weight control [26, 27], plasma lipids [26, 28, 29], blood pressure [30,31,32], vascular function [24, 28, 33, 34], and glucose metabolism [29, 35].
An easy, straightforward way of increasing CQA content of the coffee brew is to blend roasted coffee with unroasted green coffee beans. This is an alternative already commercially available in several countries as a soluble instant coffee, although to the best of our knowledge the health effects of such coffee blend on cardiovascular disease have not been established. Thus, the aim of the present study was to evaluate the effects of the sustained consumption of moderate, realistic amounts (three cups a day during 8 weeks) of a soluble green/roasted coffee product containing 35% green coffee beans on cardiometabolic parameters. The study was performed in healthy and cardiovascular risk subjects; considering the reported higher susceptibility of hyperlipidemic individuals to the cholesterol-rising effect of coffee [13, 14]. Volunteers with mild hypercholesterolemia (total cholesterol 200–240 mg/dL) were selected as subjects with cardiovascular risk.
Subjects and methods
Subjects
The study met the guidelines laid down in the Declaration of Helsinki of 1975 (revised in 1983) and was approved by the Clinical Research Ethics Committee of Hospital Puerta de Hierro (Madrid, Spain). Volunteers’ recruitment was carried out through our website (http://www.ictan.csic.es), as well as by giving short talks between lectures at Complutense University faculties, and placing advertisements at the University campus, in language and professional schools and medical centers.
The inclusion criteria were as follows: 18–45 years old men and women with body mass index (BMI) under 25 kg/m2, non-smokers, non-vegetarian, non-pregnant women, not-having vitamins or dietary supplements, not-having been on antibiotic treatment 6 months before the start of the study and not suffering chronic pathologies or disorders other than the moderate hypercholesterolemia in the cardiovascular risk subjects, who should not be under medical treatment on statins or any other medication. Participants were separated in two groups attending to their total cholesterol (TC) levels determined in a pre-screen consented blood analysis: normocholesterolemic (TC < 200 mg/dL) and hypercholesterolemic (TC > 200–240 mg/dL) subjects.
Taking total cholesterol as the main variable, a sample size of 23 participants per group (i.e. normocholesterolemic subjects vs. hypercholesterolemic subjects) was calculated based on the assumption that the within-patient standard deviation of the response variable was 10, the difference between treatments was 6 mg/dL and that the study was randomized, controlled and cross-over. The statistical power was established at 80% and the significance level at 0.05.
Fifty-four subjects fulfilling the inclusion criteria accepted to participate in the study and gave informed written consent. Only two individuals withdrew due to personal and professional reasons. Of the remaining volunteers, 25 were normocholesterolemic and 27 were hypercholesterolemic. The baseline characteristics of the 52 volunteers who completed the study are presented in Table 1.
Study design
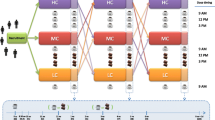
A randomized, controlled, cross-over study was carried out in free-living subjects. After a 2-week run-in stage, during which participants had to follow the food restrictions mentioned below, subjects were randomly distributed in two groups, so that half the participants consumed the coffee product first and the other half had the control drink for 8 weeks. Then, after a 3-week wash-out period with the same food restrictions, participants consumed the other beverage during the same 8-week intervention period. Along the coffee intervention, volunteers were indicated to consume three servings of 2 g of the coffee blend dissolved in 200 mL of hot water, three times a day, preferably at breakfast, midday and after lunch. Volunteers were instructed to consume the coffee without milk or sugar, but if indispensable, to add a small amount. The control drink consisted of water or an isotonic drink, caffeine and polyphenol free, which should be consumed three times a day, instead of coffee. Blood samples, blood pressure, heart rate and body weight measurements were taken at baseline (i.e. at the end of the run-in period), control, wash-out and coffee stages.
Coffee composition
The soluble coffee product was a commercial green/roasted blend containing 35% green beans, provided to the volunteers in blind individual sachets. Total soluble polyphenols in the green/roasted coffee brew were analyzed by HPLC-DAD using an Agilent 1200 HPLC (Agilent Technologies, CA, USA) as described before [4]. Methylxanthine content was analyzed by HPLC-DAD following a method previously described [36]. The content of the major hydroxycinnamoylquinic acids and methylxanthines in coffee is shown in Table 2. Coffee presented 74.2 mg/g (dry matter) of total hydroxycinnamic acids and 20.2 mg/g (dry matter) of total methylxanthines. Therefore, the daily consumption of a 2 g sachet of instant coffee three times a day, which can be considered a realistic, moderate consumption of coffee, provided a total amount of 445.2 mg CQAs and 121.2 mg of caffeine. This dose of phenolic compounds was higher than that used by Ochiai et al. [33] (140 mg/day of polyphenols as a green coffee bean extract, GCBE) or Kozuma et al. [30] (46–185 mg/day GCBE), and comparable to doses used by Bakuradze et al. [7] (435 mg/day of CQAs provided as an antioxidant-rich coffee brew), but lower than the amounts used in other interventions studies with GCBE (600 mg/day [24], up to 1050 mg/day [27]), pure chlorogenic acid (2 g in an acute study [37]), or coffee brews providing total CQAs doses of 665.8 mg/day [38], 1200 mg/day [39] or as high as 8200 mg/day [32].
Dietary control and compliance
Along the whole study, participants were asked to maintain their habitual lifestyle and dietary habits, and to refrain from consuming other coffee products, certain fruits and vegetables rich in polyphenols, particularly those rich in hydroxycinnamic acids (artichokes, chard, broccoli, eggplant, soya, oranges, grapes, plums or cherries among others) as well as their derived products and beverages, including wine and juices. Tea, cocoa and yerba mate, as well as whole wheat products and caffeine-rich drinks were also restricted. The consumption of potatoes, apples, pomegranates, rice or corn was reduced to once a week. These restrictions were aimed at reducing inter-individual differences in polyphenol and methylxanthine intake. A list of alternative foods was provided to the volunteers to facilitate their food choice during the study.
In the last week of each stage of the study, volunteers were asked to complete a 72-h detailed food intake record, specifying the ingredients, amounts, and serving weights when possible. Volunteers were instructed on how to fill in the dietary records before starting the study. Compliance of food restrictions and coffee intake was controlled by calling the volunteers every week, and counting the number of coffee servings before and after the intervention. To estimate the energy intake and dietary composition, the programme DIAL [Department of Nutrition and Bromathology I, School of Pharmacy, Complutense University of Madrid (UCM), Spain] was used.
Physical activity and body weight
Participants were asked not to change their usual level of physical activity during the study. At baseline and at the end of each stage of the study, volunteers filled out a questionnaire on three representative days to evaluate their physical activity including that involved in their occupation. They were asked to record the minutes dedicated to work, sports and free-time, and also their sleeping time, and then their physical activity factor was calculated using the program ADN (Department of Nutrition and Bromathology I, Pharmacy Faculty, Complutense University of Madrid (UCM), Spain). Also, at baseline and at the end of each stage, volunteer’s body weight was measured using a BC-418 MA (Tanita Corporation) equipment.
Cardiovascular parameters
At the end of the stages of the study, after an overnight fast and before blood sampling, volunteers rested for at least 5 min before measuring systolic (SBP) and diastolic (DBP) blood pressure and heart rate. Measures were taken using an automatic arm sphygmomanometer (Pic Indolor Diagnostic, BS 150, Artsana, Italy) in triplicates, waiting for 3 min between measurements. Readings were compared, and if not in agreement within 10–15 mmHg in blood pressure and 5 beats per minute (bpm) in heart rate was not accepted.
Blood sampling
After 8–10 h overnight fast, blood samples were collected using BD Vacutainer® tubes (Becton, Dickinson and Company, New Jersey, USA) with EDTA or without anticoagulant to separate plasma and serum, respectively. Plasma samples were immediately centrifuged after blood collection, whereas serum samples were maintained 30 min at room temperature after collection. After centrifugation for 10 min at 1500g, 4 °C (Biofuge Primo R Haereus centrifuge, IMLAB, Lille, France), aliquots were separated and stored at − 80 °C until analysis.
Biochemical analysis
Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), phospholipids and triglycerides were determined in serum samples following reference methods or methods recommended by Sociedad Española de Bioquímica Clínica y Patologóa Molecular (SEQC) using a Roche Cobas Integra 400 plus analyser (Roche Diagnostics, Mannheim, Germany). The enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were analyzed according to standardized spectrophotometric techniques.
Inflammatory biomarker analysis
High-sensitive C-reactive protein (CRP) was determined in serum using an automatized ultrasensible turbidimetric method (AU2700 Biochemistry Analyzer, Olympus). In plasma samples, using Bio-Plex kit Pro Human Cytokine, Chemokine and Growth Factor Assay (group I), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, INF-γ MCP-1, MIP-1β, and TNF-α were analyzed, where intra-assay %CV were 6, 7, 9, 8, 7, 6, 5, 6, 8, 8, 10, 12, 15, 9, 8 and 8 and inter-assay %CV were 8, 9, 8, 10, 11, 8, 6, 6, 7, 6, 5, 6, 9, 7, 8 and 6, respectively. Bio-Plex kit Pro Human Cytokine, Chemokine and Growth Factor Assay (group II) was used to analyze cell adhesion molecules ICAM-1 and VCAM-1, where intra-assay %CV were 4.3 and 6.7 and inter-assay %CV were 3.8 and 5.5, respectively. All analytes were measured in duplicates on a MAGPIX™ Multiplex reader fitted to a Bio-Plex Pro Wash Station and software Bio-Plex Manager™ MP (Luminex Corporation, Austin, USA) was used for data processing. Results were expressed as pg/mL plasma, except adhesion molecules which were expressed as ng/mL and CRP as mg/dL.
Analysis of antioxidant capacity and oxidation biomarkers
Free radical scavenging activity of plasma was measured using the ABTS radical cation [40] and the oxygen radical absorbance capacity (ORAC) [41] methods, and the reducing capacity was determined by the ferric reducing/antioxidant power (FRAP) assay [42]. Trolox was used as standard and results were expressed as µM of Trolox equivalent (TE). All samples were analysed in triplicates on a Beckman DU 640 spectrophotometer (Beckman Coulter, California, USA),
Lipid peroxidation was measured in serum by determining malondialdehyde (MDA) levels as described by Mateos et al. [43]. Concentrations were expressed as nmol MDA/mL serum. Protein peroxidation was measured in serum by determining the concentration of carbonyl groups according to Ritcher et al. [44]. Protein concentration was analysed spectrophotometrically using the Bradford reagent. Results were expressed as nmol/mg protein.
Statistical analysis
Results are shown as means ± standard errors. Statistical analyses were carried out using the program SPSS version 23.0 (IBM Company, NY, USA). Normality of distribution and homogeneity of variance were evaluated using the Kolmogorov–Smirnov and Levene tests, respectively, for all variables studied. As aforementioned, measurements of all the biomarkers studied were taken at baseline (i.e. at the end of the run-in period) and at the end of the control, wash-out and coffee stages. To exclude the presence of a carryover effect, the 3-week wash-out period data were used to compare if there were any differences between the subgroup that started with the control intervention and the subgroup that started with the coffee intervention. As we did not observe differences between the subgroups, the wash-out data were not included in the present work. To assess changes in outcome variables in response to the intervention treatments (intra-subject factor), the baseline, coffee and control data were statistically studied using the general linear model of variance for repeated measures and, to evaluate the effects of belonging to the normocholesterolaemic or hypercholesterolaemic group, the group was considered as an inter-subject factor. Furthermore, differences within each group were studied using paired t-tests with Bonferroni corrections (Bonferroni Post hoc test). Significance level was established at p < 0.05.
Results
Dietary control and compliance: body weight
The 72-h intake reports were pre-filtered to avoid including non-reliable data and were contrasted with the volunteers whenever necessary. In this sense, reports that showed daily estimations 70% below light-activity energy intake recommendations or 130% above normal-activity energy intake recommendations attending to age and gender were discarded [45]. The reports of four volunteers were discarded for both the coffee and control interventions. Volunteers were called weekly to follow-up their fulfillment of dietary indications and food restrictions. At the end of the study, when they were asked to return any unused sachet, only one volunteer returned some coffee sachets. Attending to their feedback, it was assumed that compliance was appropriate.
The intake of most nutrients were below the dietary recommendations [45], such as the intake of carbohydrates that was under 50–60% (37.9–41.2 and 35.3–38.8% in the normocholesterolemic and hypercholesterolemic groups, respectively) and PUFA, which was below 17% (4.8–5.1% in the normocholesterolemic participants and 5.0–5.3% in the hypercholesterolemic group). In contrast, proteins (17.5–18.6 and 16.8–18.4% in the normocholesterolemic and hypercholesterolemic groups, respectively), lipids (37.9–39.4 and 35.0–39.3% in the normocholesterolemic and hypercholesterolemic groups, respectively) and saturated fatty acids (SFA; 12.4–13.5 and 11.5–13.5% in the normocholesterolemic and hypercholesterolemic groups, respectively) were higher than the recommended intakes (proteins: 10–15%; lipids: 30–35%; SFA < 7% of daily energy intake) (Table 3). MUFA intake (16.4–18.4% in the normocholesterolemic group and 14.2–16.2% in the hypercholesterolemic participants) was quite higher than the recommended intake (3–6%) due to the majority of volunteers using exclusively olive oil as cooking fat. Intake of protein (p = 0.025) was lower at the end of the coffee intervention, whereas none of the other dietary intake parameters showed statistical differences along the study. There were no differences between the normocholesterolemic and hypercholesterolemic groups.
Volunteers showed a physical activity factor between 1.6 and 1.8; therefore, attending to the FAO/WHO/UNU Expert Consultation Report on Energy and Protein Requirements [46], their physical activity was moderate. As instructed, participants did no modify their habitual physical activity and, in agreement, these factors did not change along the study. Interestingly, however, the consumption of the coffee blend significantly reduced body weight (Table 3).
Cardiovascular parameters
All subjects were normotensive and their heart rate was within the normal range (60–100 bpm) (Table 1), although values of systolic and diastolic blood pressure and heart rate were slightly higher in the hypercholesterolemic subjects compared to the normocholesterolemic, a tendency that was maintained along the study. Interestingly, SBP and DBP significantly decreased in both normocholesterolemic (p = 0.001) and hypercholesterolemic (p < 0.001) groups (Table 4). In the hypercholesterolemic subjects, both parameters were statistically lower after the coffee intervention compared to baseline, although not different to control values. Accordingly, heart rate decreased after the coffee intervention (p = 0.035).
Biochemical analysis
At baseline, serum levels of lipids were within the range established by Sociedad Española de Bioquímica Clínica y Patología Molecular (SEQC) in the normocholesterolemic group, whereas in the hypercholesterolemic these biomarkers were higher in agreement with their pathology (Table 5). A significant reduction of TC, LDL-C, VLDL-C and TG (p = 0.006, 0.001, 0.003 and 0.017, respectively) related to coffee consumption was observed. There was a significant difference in these parameters due to belonging to the normocholesterolemic or hypercholesterolemic group (p = 0.001, < 0.001, 0.019 and 0.027, respectively), so that the reductions of these biomarkers only took place in the hypercholesterolemic subjects. According to the paired test, the aforementioned serum lipids levels as well as phospholipids in this group were lower after the control and coffee interventions compared to baseline. In contrast, in the normocholesterolemic group, HDL-C levels slightly increased after coffee intervention, although the change was not statistically significant. The levels of alanine aminotransferase and aspartate transaminase were not affected during the intervention (Table 5).
Inflammatory biomarker analysis
Inflammatory biomarkers did not show significant changes along the study according to the repeated measures test. However, in some parameters there was a small response in opposite directions in the two study groups. In the healthy subjects, plasma levels of IL-2 significantly decreased according to the Bonferroni test and the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α tended to be reduced, whereas in the hypercholesterolemic participants there was no modification of these cytokines. In contrast, anti-inflammatory markers IL-4, IL-10 and IL-13 tended to be increased in the high-risk subjects, with a trend for reduction in healthy participants (Table 6). In normocholesterolemic subjects, other pro-inflammatory cytokine concentrations that showed a slight trend to decrease after coffee supplementation were IL-5, IL-7, IL-17 and IFN-γ, whereas in the hypercholesterolemic group such tendency was not observed. Interestingly, plasma levels of CRP decreased after green/roasted coffee intake in both study groups, being statistically lower than baseline and control values according to the Bonferroni test. Regarding other pro-inflammatory molecules and chemokines, there was no clear tendency. In healthy volunteers, G-CSF values were significantly lower at the control and coffee stages compared to baseline according to the paired test, and MCP-1 levels decreased, although not statistically, after coffee intake, whereas MIP-1β values were slightly increased and GM-CSF were not modified (Table 6). In the hypercholesterolemic group, there were no relevant changes in any of these inflammatory mediators. Adhesion molecules ICAM-1 and VCAM-1 did not change along the study; only a slight reduction was observed in the healthy subjects after consuming coffee (Table 6).
Oxidation and antioxidant biomarker analysis
After the coffee intervention, the antioxidant capacity measured by ORAC (p < 0.001) and FRAP (p < 0.001) assays significantly increased in both groups (Table 7). According to the paired test, after coffee consumption ORAC values were significantly higher than at baseline in the healthy group and higher than the control values in the hypercholesterolemic, whereas FRAP values after the coffee intervention were significantly higher than the control in both groups. However, no change was observed when the antioxidant capacity was measured by the ABTS assay.
Oxidative damage to serum lipids and proteins (Table 7), measured by MDA and carbonyl groups, respectively, significantly decreased after the coffee intervention (p = 0.015 and p < 0.001, respectively). According to the Bonferroni test, MDA levels significantly decreased after the coffee intervention compared to baseline values in the healthy group and carbonyl values were statistically lower than at baseline in both groups, showing an effect due to the group (p = 0.011).
Discussion
For many years, coffee consumption has been associated with increased cardiovascular risk. In contrast, this study shows that regular consumption of a soluble coffee product containing 35% of green coffee may reduce cardiovascular risk in hypercholesterolemic subjects by lowering blood pressure and heart rate, improving lipid profile and increasing plasma antioxidant capacity. The study was carried out in free-living subjects who only restricted the consumption of certain polyphenol- and methylxanthine-rich foods and daily consumed three cups of a green/roasted coffee blend (2 g/serving), which represents a moderate, realistic coffee consumption rate.
It is well known that enrolling in a nutrition study raises awareness of participants in their food consumption and influences dietary behaviour, which is a background effect in nutrition intervention trials that might affect the results. To circumvent this, a randomized, crossover and controlled study design was chosen, with measurements taken at the beginning of the intervention after a 2-week run-in period (i.e. baseline values), at the end of each intervention stage and at the end of a 3-week wash-out period between both intervention stages. Prior to further analysis of the results, we used the 3-week wash-out data to ensure there were no carry-over effect from the previous treatment period. Since no differences were found, baseline values, and the outcome data observed after the control and the green/roasted coffee interventions for all volunteers were comparatively studied, irrespectively of which intervention they were first assigned.
On the other hand, volunteers were asked to restrain from consuming certain polyphenol- and methylxanthine-rich foods along the study to minimize the potential contribution of these bioactive food components. We observed no major modifications in energy intake and physical activity along the study. In contrast, there was a slight yet significant reduction in body weight (0.5–1 kg) after coffee intake (Table 3), which was less marked during the control stage, pointing to a direct effect of coffee. As mentioned above, coffee is a complex beverage rich in different phytochemicals with potential effects on weight control. Methylxanthines and dietary fiber are known to induce satiety, and favor lipolysis and fatty acid beta-oxidation, whereas caffeine induces higher thermogenesis and CQAs reduce glucose absorption and the activity of SREBPs, therefore, avoiding lipogenesis [47]. These mechanisms may explain the weight loss observed after the intake of the green/roasted coffee product, contributing to reduce cardiovascular risk. Some authors observed a similar effect in overweight subjects after the intake of a green coffee extract [7, 27]. However, to the best of our knowledge, this is the first study reporting weight reduction by consuming a green/roasted coffee blend beverage in normoweight subjects, which is relevant outcome that deserves further investigation.
The effect of coffee and caffeine on blood pressure has been extensively studied in the literature, with important discrepancies due to dissimilar results in acute vs. long-term studies, different responses in habitual vs. non-habitual coffee drinkers, and also to the different response to administration of caffeine vs. coffee. The pressor effect of caffeine both in normotensive and hypertensive subjects is well established [17, 48]. However, long-term consumption of coffee is not associated with higher blood pressure or incident hypertension [18]. On the other hand, acute responses to coffee are milder than after similar doses of caffeine [17, 48], pointing to other components in coffee counteracting the pressor effect of caffeine. Chlorogenic acid (400 mg) has shown to have antihypertensive effects in an acute study in healthy subjects, reducing SBP and DBP (− 2.41 and − 1.53 mmHg, respectively) [49], which suggests CQAs may be responsible for this effect on blood pressure and justify the recent interest on CQAs-rich green coffee.
Few studies in the literature report on the effect of green coffee on blood pressure. In two long-term studies, consuming during 3–4 months a green coffee bean extract (GCBE) providing 140 mg CQAs/day decreased systolic (− 4.6 mmHg) and diastolic (− 3.2 mmHg) blood pressure in healthy subjects [33], with higher reductions in mildly hypertensive individuals (up to − 10 mmHg SBP and − 7 mmHg DBP) [31]. A dose-dependent decrease in SBP (− 3.2 to − 5.6 mmHg) and DBP (− 2.9 to − 3.9 mmHg) in mildly hypertensive individuals was reported after consuming different doses of a GCBE (providing 46–185 mg CQAs/day, respectively) for 28 days [30]. Similarly, drinking caffeinated green coffee for 2 weeks also reduced SBP (− 2.65 mmHg) and DBP (− 3.1 mmHg) in healthy subjects [32].
In the present study, the effect of coffee consumption was assessed after intake of the green/roasted coffee blend during 8 weeks in habitual coffee consumers. The high content of hydroxycinnamic acids in the soluble coffee blend, providing 445.2 mg/day CQAs, might account for the observed reduction in blood pressure both in normocholesterolemic and hypercholesterolemic subjects (Table 4). In the later, reductions in SBP and DBP (− 5.2 and − 5.6 mmHg, respectively, compared to baseline) were more marked than in the normocholesterolemic volunteers (− 3.4 and − 2.3 mmHg, respectively). These reductions were in line with the observations by Kozuma et al. [30] and Ochiai et al. [33] in long-term assays with lower doses of CQAs in caffeine-free GCBE (up to 185 mg CQAs/day), and were higher than in the pilot study by Revuelta-Iniesta and Al-Dujaili [32] in healthy individuals who consumed a much higher amount of CQAs (up to 8200 mg/day for 2 weeks) from a green coffee brew. Although participants in both groups in the present study were normotensive (Table 1), blood pressure was marginally higher in the hypercholesterolemics (Table 4), who would benefit from the slightly more marked reduction in this CVD risk factor after consuming the green/roasted coffee blend.
Many studies have shown that drinking coffee has a negative impact on serum lipids, corroborated in several meta-analyses of RCT showing a dose-dependent effect, with higher increments in studies with boiled, unfiltered coffee and evidencing a higher susceptibility of hyperlipemic subjects to the lipid-enhancing effect of coffee [13, 14]. In contrast, in the present study we observed no significant modification in lipid levels in the healthy, normocholesterolemic subjects save for a small, not statistically significant increase in HDL-C levels (4% compared to baseline values). On the contrary, we found important improvements in the lipid profile of the hypercholesterolemic group, who significantly reduced their serum levels of TC, LDL-C and TG approximately in 20 mg/dL and VLDL-C in 5 mg/dL after consuming three cups per day of the soluble green/roasted coffee during 8 weeks (Table 5). This resulted in an improved LDL/HDL ratio in this risk subjects, changing from 2.45 at baseline to 2.17 after the coffee intervention. Although still far from values in the normocholesterolmic group (LDL/HDL ratio 1.59 at baseline; 1.54 after coffee intake), this reduction was remarkable (0.28 units after 8 weeks).
The lipid-soluble diterpenes kahweol and cafestol in coffee have been described as the main contributors to the beverage’s hyperlipidemic effects [25]. The concentration of these diterpenes is lower in filtered and instant coffee, explaining the higher increments in serum lipids after unfiltered coffee consumption. Although kahweol and cafestol contents were not determined in the instant green/roasted coffee product here studied, the possible negative effects of these compounds could have been neutralized by the high phenolic content in the coffee blend. Indeed, intake of a green coffee bean extract providing 50 or 100 mg/day chlorogenic acid during 28 days showed reductions in TC and LDL-C levels (between 8 and 10 and 3.5–5.5 mg/dL, respectively) in hypercholesterolemic subjects [30], in agreement with the results observed in the present study. Also, in a recent study daily consumption of two types of coffees providing 420 and 780 mg CQAs for 8 weeks did not modify blood lipids in healthy subjects [28], while HDL-C levels were increased in healthy participants drinking eight cups/day filtered coffee [50], also in agreement with the results reported here. Animal studies have shown a strong inhibitory effect of chlorogenic acid on the formation of cholesterol micelles also inhibiting pancreatic lipase, hydroxymethyl glutaryl Co-A reductase and acyl-cholesterol acyltransferase while increasing fatty acid beta-oxidation activity and peroxisome proliferator-activated receptor alpha expression in the liver [26, 29]. This would result in the inhibition of intestinal absorption of lipids and modified hepatic metabolism of cholesterol and fatty acids, as potential mechanisms of action of CQAs in reducing blood lipids.
Levels of inflammation-related cytokines, chemokines and cell adhesion molecules showed little changes (mostly tendencies) in the present study, observing an influence due to the group in some parameters. In the healthy participants, levels of the pro-inflammatory cytokine IL-2 decreased significantly after coffee intake compared with baseline values (Table 6), as did concentrations of other interleukins (IL-5, IL-6, IL-7, IL-17) and the inflammatory mediators TNF-α and INF-γ, although these variations were not statistically significant. In contrast, in the cardiovascular risk subjects, anti-inflammatory interleukins IL-4, IL-10 and IL-13 tended to increase. CRP, a marker of systemic inflammation that has been shown to have a direct role in CVD, significantly decreased in healthy and at risk subjects. This reduction was concomitant with a trend to decrease MCP-1 levels in healthy participants but the contrary was observed in hypercholesterolemic subjects, although these changes were not statistically significant. Other chemokines such as MIP-1β and GM-CSF were not affected during the study, although G-CSF levels were lower in healthy subjects after consuming the green/roasted coffee blend (Table 6). Cell adhesion molecules (VCAM-1 and ICAM-1), which are more specific markers of endothelial function, were not modified during the intervention. Although the plasma levels of these chemoattractant proteins and colony-stimulating factors quantified by a bead-based multiplex technology differed from values reported in the literature determined by ELISA assays [51, 52], they were in line with published values for these chemokines quantified by the Luminex technology [53, 54].
Few studies have approached the effects of coffee consumption on the inflammatory response and, to the best of our knowledge, this is the first study reporting the effect of green coffee blended with roasted beans on inflammation. In vitro studies in cell culture models suggest the positive although moderate effects of coffee could be mediated by CQAs, since hydroxycinnamic acids such as chlorogenic, caffeic or ferulic acids have shown to suppress the expression of interleukins and cell adhesion molecules (VCAM-1, ICAM-1 and E-selectin) induced by IL-1β, [55] lipopolysaccharide [56], the adipokine resistin [57] or gamma-irradiation [58] in human endothelial cells, and suppressing adhesion of monocytes to activated endothelial cells.
Coffee is rich in phenolic compounds and possesses higher antioxidant capacity than other beverages such as tea and red wine [59]. Thanks to its high consumption, coffee is one of the major dietary sources of antioxidants with a contribution higher than that of fruits and vegetables [60]. The soluble coffee product used in the present study, containing 35% green beans, is especially rich in phenolic compounds that conferred significant protection against oxidative stress, modulating cell cytotoxicity, ROS generation, antioxidant defenses and macromolecular damage in a cell model of oxidative stress in human hepatoma HepG2 cells [61]. The regular consumption of three cups of the green/roasted coffee blend, providing 445.2 mg CQAs distributed along the day, had a positive effect enhancing plasma antioxidant capacity determined by the ORAC and FRAP methods (i.e. increasing free radical scavenging and reducing capacities of plasma). In addition, MDA levels were also significantly lower after coffee intake, with serum MDA concentrations in hypercholesterolemic subjects 10% lower compared to baseline values, a reduction even higher in healthy volunteers (− 16%, Table 7). This decrease in MDA levels was higher than that reported in a previous study after consuming a coffee product rich in hydroxycinnamic acids (1200 mg/day) for 5 days, with a non-significant 8% reduction in MDA [39]. These authors also found decreased excretion of 8-isoprostane in urine, another marker of lipid oxidation, together with a moderate increase in total antioxidant capacity, reduced levels of oxidized LDL, and a significant 16% reduction (p = 0.027) in 3-nitrotyrosine in plasma as a marker of protein oxidation. In the present study, protein damage significantly (p < 0.001) decreased after consuming the green/roasted coffee blend (about − 35% compared to baseline values in healthy and hypercholesterolemic subjects).
This work presents certain limitations. The lack of blinding of the coffee product may have induced certain bias. As mentioned above, as in any long-term study volunteers may have introduced certain changes in their diet during the interventions, being a potential confounding factor that has not been specifically assessed. Also, the possibility that other food components may have contributed to the effects observed cannot be ruled out. The content of other bioactive phytochemicals in coffee such as diterpenes, hydroxyhydroquinone or N-methylpyridinium were not measured in the soluble green/roasted coffee product and in consequence it is not possible to confirm their potential contribution to the observed effects. Importantly, we did not analyze phenolic metabolites in blood or urine after consumption of coffee. However, in a previous acute study in healthy volunteers with the same coffee product used in the present intervention, the pharmacokinetics of caffeine and phenolic compounds’ metabolism were assessed. Caffeine showed to be quickly and completely absorbed in the small intestine and extensively metabolized in the liver, with plasma concentrations of caffeine up to 10 µM 1.2 h after the intake of a single coffee serving [36]. Furthermore, CQAs were partially absorbed and extensively metabolized reaching plasma concentrations of 1 µM in a biphasic kinetic, with low concentrations (nM) of de-esterified hydroxycinnamic acid metabolites approximately 1 h after intake and higher (µM) concentrations of the main metabolites (dihydrocaffeic and dihydroferulic acids and their phase II conjugates among others) about 6 h after the intake of coffee [62]. The regular consumption of three cups of the coffee blend distributed along the day could have resulted in raised plasmatic concentrations of coffee-derived compounds, maintaining them relatively high, thus contributing to the cardioprotective effects observed in the present study.
Conclusions
Regular consumption of moderate amounts of a soluble green/roasted (35:65) coffee blend may contribute to improve cardiovascular health in moderately hypercholesterolemic people, as reducing serum lipids, blood pressure and body weight effects, as well as increasing plasma antioxidant capacity, have been observed. Moreover, positive influence on blood pressure, body weight, and plasma antioxidant capacity was obtained in the healthy group. Therefore, incorporation of green coffee beans into the coffee brew can be considered as part of a dietary strategy to protect from cardiovascular disease.
Abbreviations
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CG:
-
Carbonyl groups
- CQAs:
-
Caffeoylquinic acids
- CRP:
-
C-reactive protein
- DBP:
-
Diastolic blood pressure
- FRAP:
-
Ferric reducing/antioxidant power
- G-CSF:
-
Granulocyte colony-stimulating factor
- ICAM-1:
-
Intercellular cell adhesion molecule-1
- IFNγ:
-
Interferon gamma
- IL:
-
Interleukin
- LC-MS:
-
Liquid chromatography–mass spectrometry
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDA:
-
Malondialdehyde
- MIP-1β:
-
Macrophage inflammatory protein-1 beta
- ORAC:
-
Oxygen radical absorbance capacity
- RCT:
-
Randomized clinical trial
- SBP:
-
Systolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TNF-α:
-
Tumor necrosis factor alpha
- VCAM-1:
-
Vascular cell adhesion molecule-1
References
Farah A (2012) Coffee constituents. In: Chu YF (ed) Coffee: emerging health effects and disease prevention. Blackwell Publishing Ltd, Oxford, pp 21–58
Jaiswal R, Matei MF, Subedi P, Kuhnert N (2014) Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food Res Int 61:214–227
Alonso-Salces RM, Serra F, Reniero F, Heberger K (2009) Botanical and geographical characterization of green coffee (Coffea arabica and Coffea canephora): chemometric evaluation of phenolic and methylxanthine contents. J Agric Food Chem 57:4224–4235
Baeza G, Sarriá B, Bravo L, Mateos R (2016) Exhaustive qualitative LC-DAD-MSn analysis of Arabica green coffee beans: cinnamoyl-glycosides and cinnamolyshikimic acids as new polyphenols in green coffee. J Agric Food Chem 64:9663–9674
Morales FJ, Somoza V, Fogliano V (2012) Physiological relevance of dietary melanoidins. Amino Acids 42:1097–1109
Ochiai R, Chikama A, Kataoka K, Tokimitsu I, Maekawa Y, Ohishi M, Rakugi M, Mikami H (2009) Effects of hydroxyhydroquinone-reduced coffee on vasoreactivity and blood pressure. Hypertens Res 32:969–974
Bakuradze T, Boehm N, Janzowski C, Lang R, Hofmann T, Stockis JP, Albert FW, Stiebitz H, Bytof G, Lantz I et al (2011) Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: results from an intervention study. Mol Nutr Food Res 55:793–797
Lopez-Garcia E, Guallar-Castillon P, Leon-Muñoz L, Graciani A, Rodriguez-Artalejo F (2014) Coffee consumption and health-related quality of life. Clin Nutr 33:143–149
Ohnaka K, Ikeda M, Maki T, Okada T, Shimazoe T, Adachi M, Nomura M, Takayanagi R, Kono S (2012) Effects of 16-week consumption of caffeinated and decaffeinated instant coffee on glucose metabolism in a randomized controlled trial. J Nutr Metab 207426
Sarriá B, Martínez-López S, Sierra-Cinos JL, García-Diz L, Mateos R, Bravo L (2016) Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome. Eur J Nutr. https://doi.org/10.1007/s00394-016-1316-8
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Gerazi SA, Horwich T, Januzzi JL et al (2013) ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128:e240–e327
Bravo L, Mateos R, Sarriá B (2017) Preventive effect of coffee against cardiovascular diseases. In: Farah A (ed) Coffee: chemistry, quality and health implications. Royal Society of Chemistry, Oxford
Jee SH, He J, Appel LJ, Whelton PK, Suh I, Klag MJ (2001) Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol 153:353–362
Cai L, Ma D, Zhang Y, Liu Z, Wang P (2012) The effect of coffee consumption on serum lipids: a meta-analysis of randomized controlled trials. Eur J Clin Nutr 66:872–877
Jee SH, He J, Whelton PK, Suh I, Klag MJ (1999) The effect of chronic coffee drinking on blood pressure. A meta-analysis of controlled clinical trials. Hypertension 33:647–652
Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM (2005) Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens 23:921–928
Nurminen M-L, Niittyenen L, Korpela R, Vapaatalo V (1999) Coffee, caffeine and blood pressure: a critical review. Eur J Clin Nutr 53:831–839
Mesas AE, Leon-Muñoz LM, Rodriguez-Artalejo F, Lopez-Garcia E (2011) The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr 94:1113–1126
Steffen M, Kuhle C, Hensrud D, Erwin PJ, Murad MH (2012) The effect of coffee consumption on blood pressure and the development of hypertension: a systematic review and meta-analysis. J Hypertens 30:2245–2254
Zhang Z, Hu G, Caballero B, Apple L, Chen L (2011) Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am J Clin Nutr 93:1212–1219
Karatzis E, Papaioannou TG, Aznaouridis K, Karatzi K, Stamatelopoulos K, Zampelas A, Papamichael C, Lekakis J, Mavrikakis M (2005) Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: should we consider monitoring central blood pressure? Int J Cardiol 98:425–430
Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME (2005) Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci 109:55–60
Buscemi S, Verga S, Batsis JA, Donatelli M, Tranchina MR, Belmonte S, Mattina A, Re A, Cerasola G (2010) Acute effects of coffee on endothelial function in healthy subjects. Eur J Clin Nutr 64:483–489
Ochiai R, Sugiura Y, Otsuka K, Katsuragi Y, Hashiguchi T (2014) Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutr Res 34:155–159
Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A (2014) Coffee: biochemistry and potential impact on health. Food Funct 5:1695–1717
Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK (2010) Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induce-obese mice. Food Chem Toxicol 48:937–943
Vinson JA, Burnham BR, Nagendran MV (2012) Randomized, double-blind, placebo-controlled, linear dose, crossover study to evaluate the efficacy and safety of a green coffee bean extract in overweight subjects. Diabetes Metab Syndr Obes Target Ther 5:21–27
Agudelo-Ochoa GM, Pulgarín-Zapata IC, Velásquez-Rodríguez CM, Duqye-Ramírez M, Naranjo-Cano M, Quintero-Ortiz MM, Lara-Guzmán OJ, Muñoz-Durango K (2016) Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J Nutr 146:524–531
Meng S, Cao J, Feng Q, Peng J, Hu Y (2013) Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complem Alt Med 2013:801457
Kozuma K, Tsuchiya S, Kohori J, Hase T, Tokimitsu I (2005) Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens Res 28:711–718
Watanabe T, Arai Y, Mitsui Y, Kusaura T, Okawa W, Kajihara Y, Saito I (2006) The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin Exper Hypert 28:439–449
Revuelta-Iniesta R, Al-Dujaili EAS (2014) Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: a pilot crossover study using green and black coffee. Bio Med Res Int 482704
Ochiai R, Jokura H, Suzuki A, Tokimitsu I, Ohishi M, Komai N, Rakugi H, Ogihara T (2004) Green coffee bean extract improves human vasoreactivity. Hypertens Res 27:731–737
Ward NC, Hodgson JM, Woodman RJ, Zimmermann D, Poquet L, Leveques A, Actis-Goretta L, Puddey IB, Croft KD (2016) Acute effects of chlorogenic acids on endothelial function and blood pressure in healthy men and women. Food Funct 7:2197–2203
Sarriá B, Martínez-López S, Mateos R, Bravo L (2016) Long-term consumption of a green/roasted coffee blend positively affects glucose and insulin resistance in humans. Food Res Int 89:1023–1028
Martinez-Lopez S, Sarria B, Baeza G, Mateos R, Bravo-Clemente L (2014) Theobromine, caffeine, and theophylline metabolites in human plasma and urine after consumption of soluble cocoa products with different methylxanthine contents. Food Res Int 63:446–455
Olthof MR, Hollman PC, Zock PL, Katan MK (2001) Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am J Clin Nutr 73:532–538
Kempf K, Kolb H, Gärtner B, Bytof G, Stiebitz H, Lantz I, Lang R, Hofmann T, Martin S (2015) Cardiometabolic effects of two coffee blends differing in content form major constituents in overweight adults: a randomized controlled trial. Eur J Nutr 54:845–854
Hoelzl C, Knasmüller S, Wagner KH, Elbling L, Huber W, Kager N, Ferk F, Ehrlich V, Nersesyan A, Neubauer O et al (2010) Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol Nutr Food Res 54:1722–1733
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 26:1231–1237
Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL (2002) High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem 50:4437–4444
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified FRAP assay. J Agric Food Chem 48:3396–3402
Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L (2005) Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J Chrom B 827:76–82
Ritcher S, Wehr NB, Stadtman ER, Levine RL (2002) Assessment of skin carbonyl content as a noninvasive measure of biological age. Arch Biochem Biophys 397:430–432
Moreiras O, Carbajal A, Cabrera L, Cuadrado C (2016) Ingestas diarias recomendadas de energía y nutrientes para la población española. Tablas de Composición de Alimentos. Ed. Pirámide (Grupo Anaya, SA), 18th edn. Madrid
FAO/WHO/UNU (1985) Expert Consultation Report. Energy and Protein Requirements. Technical Report Series 724. WHO, Geneva
Greenberg JA, Boozer CN, Geliebter A (2006) Coffee, diabetes, and weight control. Am J Clin Nutr 84:682–693
Riksen NP, Rongen GA, Smits P (2009) Acute and long-term cardiovascular effects of coffee: implications for coronary heart disease. Pharmacol Ther 121:185–191
Mubarak A, Bondonno CP, Liu AH, Considine MJ, Rich L, Mas E, Croft KD, Hodgson JM (2012) Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: a randomized trial. J Agric Food Chem 60:9130–9136
Kempf K, Herder C, Erlund I, Kolb H, Martin S, Cartensen M, Koenig W, Sundvall J, Bidel S, Kuha S, Tuomilehto J (2010) Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr 91:950–957
Koh KK, Quon MJ, Han SH, Chung W-J, Ahn JY, Seo Y-H, Kang MH, Ahn TH, Choi IS, Shin EK (2004) Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation 110:3687–3692
Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental.Mendia LE, Majeed M, Sahebkar A (2016) Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: a post-hoc analysis of a randomized controlled trial. Biomed Pharmacother 82:578–582
Correa TAF, Rogero MM, Mioto BM, Tarasoutchi D, Tuda VL, Cesar LAM, Torres EAFS. (2013) Paper-filtered coffee increases cholesterol and inflammation biomarkers independent of roasting degree: a clinical trial. Nutrition 29:977–981
Therkelsen SP, Hetland G, Lyberg T, Lygren I, Johnson E (2016) Cytokine levels after consumption of a medicinal Agaricus blazei Murill-based mushroom extract, AndoSanTM, in patients with Chron’s disease and ulcerative colitis in a randomized single-blinded placebo-controlled study. Human Immunol 84:323–331
Chang WC, Chen CH, Lee MF, Chang T, Yu YM (2010) Chlorogenic acid attenuates adhesion molecules up-regulation in IL-1β-treated endothelial cells. Eur J Nutr 49:267–275
Hwang SJ, Kim Y-W, Park Y, Lee H-J, Kim K-W (2014) Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RWA 264.7 cells. Inflamm Res 63:81–90
Lee E-S, Park S-H, Kim MS, Han S-Y, Kim H-S, Kang Y-H (2012) Caffeic acid disturbs monocyte adhesion onto cultured endothelial cells stimulated by adipokine resistin. J Agric Food Chem 60:2730–2739
Ma Z-C, Hong Q, Wang Y-G, Tan H-L, Xiao C-R, Liang Q-D, Cai S-H, Gao Y (2010) Ferulic acid attenuates adhesion molecule expression in gamma-radiated human umbilical vascular endothelial cells. Biol Pharm Bull 33:752–758
Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F (2003) Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr 133:2812–2819
Svilaas A, Sakhi AK, Andersen LF, Svilaas T, Ström EC, Jacobs DR, Ose L, Bloomhoff R (2004) Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr 134:562–567
Baeza G, Amigo-Benavent M, Sarria B, Goya L, Mateos R, Bravo L (2014) Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res Int 62:1038–1046
Gómez-Juaristi M, Martínez-López S, Sarria B, Bravo L, Mateos R (2018) Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct 9:313–334
Acknowledgements
The financial support of projects AGL2010-18269 and AGL2015-69986-R from the Spanish Ministry of Economy, Industry and Competitivity is acknowledged. We want to thank the volunteers who participated in the study, J. L. Sierra Cinos and L. García-Diz for their assistance in anthropometric measurements, M. Jimenez and L. T. Cayuelas for their assistance in dietary records analysis and M. Barba and C. Oeo for their assistance in lab analysis. Nestle, S. A. provided and packed the soluble coffee product for free. S. M.-L. thanks the Spanish National Research Council for her pre-doctoral fellowship under the JAE-Pre programme co-funded by CSIC and the European Social Fund. L.B. designed the trial and has the primary responsibility for final content. B.S. and S.M.-L carried out the statistical analysis of data. B.S., R.M. and S.M.-L. conducted the research and interpreted the results. S.M.-L wrote the initial draft and all authors revised and approved the final version of the manuscript.
Funding
This study was funded by Projects AGL2010-18269 and AGL2015-69986-R from the Spanish Ministry of Economy and Competitivity and approved by Ethical Committee Hospital Puerta de Hierro (Majadahonda, Madrid).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Martínez-López, S., Sarriá, B., Mateos, R. et al. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur J Nutr 58, 865–878 (2019). https://doi.org/10.1007/s00394-018-1726-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1726-x