Abstract
Purpose
To explore whether muscle strength, the insulin-like growth factor axis (IGF-axis), height, and body composition were associated with serum 25-hydroxyvitamin D [25(OH)D] and affected by winter vitamin D supplementation in healthy children, and furthermore to explore potential sex differences.
Methods
We performed a double-blind, placebo-controlled, dose–response winter trial at 55ºN. A total of 117 children aged 4–8 years were randomly assigned to either placebo, 10, or 20 µg/day of vitamin D3 for 20 weeks. At baseline and endpoint, we measured muscle strength with handgrip dynamometer, fat mass index (FMI), fat free mass index (FFMI), height, plasma IGF-1, IGF-binding protein 3 (IGFBP-3), and serum 25(OH)D.
Results
At baseline, serum 25(OH)D was positively associated with muscle strength, FFMI, and IGFBP-3 in girls only (all p < 0.01). At endpoint, baseline-adjusted muscle strength, FMI and FFMI did not differ between intervention groups. However, baseline-adjusted IGF-1 and IGFBP-3 were higher after 20 µg/day compared to placebo (p = 0.043 and p = 0.006, respectively) and IGFBP-3 was also higher after 20 µg/day compared to 10 µg/day (p = 0.011). Children tended to be taller after 20 µg/day compared to placebo (p = 0.064). No sex interactions were seen at endpoint.
Conclusions
Avoiding the winter-related decline in serum 25(OH)D may influence IGF-1 and IGFBP-3 in children. Larger trials are required to confirm these effects, and the long-term implication for linear growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During winter months, no cutaneous synthesis of vitamin D3 occurs in northern latitudes due to the large zenith angle of the sun [1]. We have previously reported that young Danish children without winter vitamin D supplementation had a marked decrease in serum 25-hydroxyvitamin D [25(OH)D] and that all of these children had concentrations < 50 nmol/L by the end of winter [2]. However, the potential implications of this reduced winter vitamin D status among young children remain to be elucidated.
Besides its well-established role in skeletal health [3, 4], vitamin D also has a role in muscle function. Muscle weakness may co-exist with severe vitamin D deficiency [5] and impaired muscle function may be present before bone disease develops [6]. In accordance with this, human skeletal muscle has vitamin D receptors (VDR’s) in the plasma membrane and nucleus through which the active metabolite of vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D], elicits its effects [7]. In a number of cross-sectional studies in children serum 25(OH)D was positively associated with muscle strength [8,9,10,11]. However, few randomized controlled trials have investigated the effect of vitamin D supplementation on muscle strength in healthy children [12,13,14]. The results are conflicting, and the studies were all conducted in girls older than 10 years. Thus, as also suggested by others, studies in younger children are needed [14, 15].
Vitamin D is also suggested to stimulate liver production of insulin-like growth factor 1 (IGF-1) and its binding protein IGF-binding protein 3 (IGFBP-3) [16], and vitamin D supplementation has been shown to increase circulating IGF-1 and IGFBP-3 in vitamin D-deficient infants [17, 18]. Since IGF-1 mediates the effect of growth hormone on linear growth, and stimulates hypertrophy of skeletal muscle [19], this may affect growth and development of children. However, the effect of the winter decline in serum 25(OH)D on IGF-1, IGFBP-3, and growth in healthy children is not well-investigated. Moreover, it is unknown whether potential effects of vitamin D supplementation on the IGF-axis differ in boys and girls, as has been shown for other nutrients [20, 21]. With a possible role of vitamin D in both muscle strength and the IGF-axis, it is likely that also lean mass may be affected by vitamin D supplementation. In accordance with this, a 1-year vitamin D supplementation trial found an increased lean mass in adolescent females [12]. In addition, obesity is often seen to associate with low vitamin D status [22], but the underlying explanation and potential direction of causality is not well-studied.
Thus, using secondary and exploratory outcomes from the ODIN Junior randomized, controlled trial, [2] we investigated whether muscle strength, fat free mass index (FFMI), fat mass index (FMI), height, plasma IGF-1, IGFBP-3, and the IGF-1:IGFBP-3 molar ratio were associated with serum 25(OH)D at baseline and affected by 20 weeks of winter vitamin D3 supplementation in 4–8 year-old white Danish children. Moreover, we assessed if associations and effects were different in boys and girls.
Participants and methods
Study design
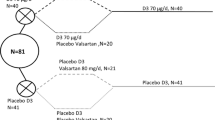
The ODIN (Food-Based Solutions for Optimal Vitamin D Nutrition and Health through the Life Cycle) Junior trial was a randomized, double-blind, placebo-controlled, dose–response trial with changes in serum 25(OH)D as primary outcome [2]. A total of 130 Danish children (including 7 pairs of siblings) were randomly assigned to receive supplements with 0 (placebo), 10, or 20 µg/day of vitamin D3 for 20 weeks during winter. Baseline and endpoint measurements took place Sep–Oct 2014 and Feb–Mar 2015, respectively, at Department of Nutrition, Exercise, and Sports, University of Copenhagen, Denmark. The study protocol was approved by the Committees on Biomedical Research Ethics for the Capital Region of Denmark (H-3-2014-022), and the study was conducted in accordance with the Declaration of Helsinki [23].
Participants
Children 4–8 years of age of white Danish/European origin living in greater Copenhagen (55°N), and not planning a winter vacation below 51° N were recruited as previously described [2]. Children with diseases or intake of medicine known to affect vitamin D or calcium metabolism or taking vitamin D supplements prior to the intervention were not eligible. We obtained informed written consent from all parents.
Measurements
Muscle strength
Muscle strength was measured as hand grip strength which is considered representative of whole body strength [24] and has a high reproducibility in children aged 4–11 years with a reported intraclass correlation coefficient (ICC; measure of agreement between test and retest) of 0.91–0.93 [25]. We used a digital hand dynamometer (SAEHAN Corporation, South Korea). Children sat with a 90° knee and elbow flexion, and the forearm in neutral position as previously recommended [26]. A minimum of three measurements were conducted with each hand with 90 s break between measurements. The children had additional trials if the last trial was highest (max 5 per hand). The instructor gently supported below the instrument to minimize the effect of gravity [26]. Muscle strength was calculated as (1) the mean of the highest value obtained with the right and left hand, respectively, (averaged peak strength) and (2) as the highest value obtained irrespective of hand (max peak strength) [27].
Anthropometry and body composition
Weight and height were measured as previously described [2] and body mass index (BMI) was calculated as body weight in kg /(height in m)2. Sex- and age-adjusted z-scores for BMI were calculated with WHO AnthroPlus software [28]. Body composition was measured in duplicate with bioelectrical impedance analysis (BIA) when lying down and with electrodes placed on right hand and foot (Quantum III; RJL Systems, Michigan, USA). The reliability of BIA measurements in children aged 5–14 years has previously been reviewed and reported as high with an ICC ≥ 0.96 [29]. Fat mass index (FMI) and fat free mass index (FFMI) was calculated as FM and FFM in kg/(height in m)2, respectively.
Blood sampling and analyses
After 2–4 h of fasting, a venous 25 ml blood sample was taken at the baseline and endpoint examination and centrifuged and stored as previously described [2]. Serum 25(OH)D was analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS) at University College Cork, Ireland. Intra- and inter-assay coefficient of variation (CV) was < 5% and < 6%, respectively. Plasma IGF-1 and IGFBP-3 were measured in one batch on Immulite 1000 (Siemens Medical Solutions Diagnostics; Los Angeles, USA) and analyzed at University of Copenhagen. Intra- and inter-assay CV was < 5% and < 3% for IGF-1 and < 4% and < 2% for IGFBP-3, respectively. As a reflection of the free biologically active IGF-1, we calculated the molar ratio of IGF-1: IGFBP-3 with the conversion equivalents: 1 µg/L IGF-1 = 0.133 nM IGF-1, and 1 mg/L IGFBP-3 = 33 nM IGFBP-3.
Questionnaires on parental education, physical activity, diet, and puberty
We assessed parental education by questionnaire at baseline, and categorized into highest level of education in the household. We also determined screen time and physical activity level by questionnaires at baseline, and the latter was categorized as either sedentary, light, moderate or vigorous, as previously done [30]. We merged the categories moderate and vigorous due to few children in the latter category. Dietary vitamin D and calcium intakes were estimated using a validated and interviewer-administered food frequency questionnaire (FFQ) [31]. Pubertal stage (Tanner stages I-V) was assessed in girls aged 7–8 years with a parent-administered questionnaire. All boys and all girls < 7 years were assumed not to have reached puberty [32, 33].
Statistical analyses
Descriptive data are presented as means and standard deviations, medians and interquartile ranges or n (%) when appropriate. Baseline differences between boys and girls were tested with two-sample t-test for continuous variables (Kruskal–Wallis test for non-normally-distributed variables) and chi2 for categorical variables.
Baseline associations between serum 25(OH)D and muscle strength, height, body composition, and markers of the IGF-axis were investigated in a linear mixed model. Initial analyses showed a significant interaction term of serum 25(OH)D × sex for the variables FFMI and IGFBP-3. Thus, all baseline associations were performed and presented stratified by sex. Analyses were both performed unadjusted and adjusted for covariates and potential confounders, which were included as fixed effects. Height and age were included as covariates to account for biological variation. Parental education, physical activity level, and screen time were included as confounders, since initial analyses of bivariate associations confirmed that these were associated with both serum 25(OH)D and some of the outcome variables.
Differences at endpoint between intervention groups were analyzed by pairwise comparisons in linear mixed models with intervention group as fixed factor, siblings as random effect and adjustment for baseline value of the outcome (baseline-adjusted model). Moreover, analyses were performed with additional adjustment for baseline height, age, and sex to account for biological variation as well as baseline vitamin D status, since this may influence the response to supplementation as previously reported [2]. Finally, we added the interaction term treatment × sex to the baseline-adjusted model to check for sex differences.
Due to the exploratory nature of the study, we did not adjust for multiple comparisons. We checked model assumptions with residual and normal-probability plots. IGF-1 was logarithm transformed in the analysis to meet model assumptions and results back-transformed. Statistical analyses were carried out using STATA software (version 14.0, StataCorp LP, Texas, USA) and p < 0.05 was considered statistically significant.
Power calculation
The initial power calculation was based on the expected slope of the relation between total vitamin D intake and serum 25(OH)D as the primary outcome as previously described [2]. For the secondary exploratory outcomes, a difference of 0.65 SD was detectable between any two groups, with n = 38 completing participants per group assuming a power and significance level of 0.8 and 0.05, respectively. Post hoc power calculations showed that we would be able to detect a difference of 2.1 kg between two groups in average peak strength.
Results
Compliance and protocol adherence
In total, 4 children (3%) withdrew from the study due to refusal of blood sampling. In addition, 9 children were excluded from analyses due to travels south of 51ºN during the intervention (n = 4), compliance < 80% (n = 2), refusal of muscle strength measurements (n = 2), and unsuccessful blood sampling (n = 1). Consequently, the per-protocol analyses were based on n = 117 children, i.e., 40, 38, and 39 children from the placebo, 10 and 20 µg/day groups, respectively. The included and non-included children did not differ with respect to baseline age, sex, BMI-for-age z-score, physical activity level, screen time, or parental education. Tablet compliance, calculated from the number of tablets in the bottle at endpoint, among the 117 children was 96% (IQR 93, 99) with no differences between groups (p = 0.60).
Baseline characteristics
Baseline characteristics of the 130 children in the three intervention groups was reported previously and showed that randomization was overall successful [2]. Table 1 shows baseline characteristics for girls and boys separately. Children were 6.6 ± 1.5 y at baseline, with an equal distribution of boys and girls. Most of the children (82%) were normal weight. Boys had higher FFMI and lower plasma IGF-1 and IGF-1: IGFBP-3 than girls. Average peak strength was 10.3 ± 3.3 kg (4.4–18.2 kg) with no sex differences (Table 1). Baseline serum 25(OH)D was 56.8 ± 12.5 nmol/L (28.7–101.4 nmol/L), and dietary vitamin D intake was around 2 µg/day, with no sex differences (Table 1). Only 4 girls had entered puberty, 2 in the placebo group and 1 in each of the vitamin D supplement groups, and pubertal status was, therefore, not included in any analyses.
Baseline associations between serum 25(OH)D and muscle strength, height, body composition, and markers of the IGF-axis
In the cross-sectional analyses stratified by sex, serum 25(OH)D was positively associated with muscle strength in girls (p = 0.005 and p = 0.006 for the two strength outcomes, respectively) also when adjusted for height, age, physical activity level, screen time, and parental education (Table 2). Moreover, serum 25(OH)D and FFMI was positively associated in girls (p = 0.001 and p = 0.006 for unadjusted and adjusted analyses, respectively; Table 2). IGFBP-3 was also positively associated with serum 25(OH)D in girls (p = 0.010), but not in the adjusted model (p = 0.078; Table 2). Neither FMI, height, IGF-1, or IGF-1: IGFBP-3 were associated with serum 25(OH)D.
Effect of vitamin D supplementation on serum 25(OH)D
Baseline and endpoint serum 25(OH)D are presented in Table 3. As previously shown [2], serum 25(OH)D at baseline did not differ between groups. Mean serum 25(OH)D decreased after placebo (p < 0.0001), and in this group, 45% became vitamin D-deficient (< 30 nmol/L) and 55% vitamin D insufficient (30–50 nmol/L). Supplementation with 10 µg/day increased serum 25(OH)D (p < 0.001), and consequently, none were vitamin D-deficient and 8% insufficient. After 20 µg/day, serum 25(OH)D increased (p < 0.0001), and all children had a vitamin D status above the sufficiency cut-off at endpoint (≥ 50 nmol/L). Endpoint serum 25(OH)D differed between all three groups when adjusted for baseline value (all p < 0.001; Table 3) with 29.7 (95% CI: 26.4; 32.9) nmol/L and 43.0 (95% CI: 39.8; 46.2) nmol/L higher serum 25(OH)D after 10 and 20 µg/day compared to placebo. In addition, after 20 µg/day, serum 25(OH)D was 13.3 (95% CI: 10.1; 16.6) nmol/L higher than after 10 µg/day.
Effect of vitamin D supplementation on muscle strength and body composition
At endpoint, muscle strength did not differ between any of the intervention groups when adjusted for baseline muscle strength either when expressed as average peak strength or maximum peak strength (all p > 0.23; Table 3). Moreover, the three groups did not differ in FFMI, or FMI at endpoint. Additional analyses with adjustment for baseline vitamin D status, height, age, and sex did not change the level of significance for any of the mentioned outcomes. Also, no sex interactions were seen with regard to muscle strength or body composition (all p > 0.30).
Effect of vitamin D supplementation on markers of the IGF-axis and height
At endpoint, baseline-adjusted IGF-1 was higher (1.1 (95% CI: 1.0; 1.2) µg/L) after 20 µg/day compared to placebo (p = 0.043) and also tended to be higher after 20 µg/day compared to 10 µg/day (p = 0.055), (Table 3; Fig. 1a). After additional adjustment for baseline vitamin D status, height, age, and sex, the 20 µg/day group had higher IGF-1 compared to both placebo and 10 µg/day (p = 0.026 and p = 0.016, respectively). For IGFBP-3, the group receiving 20 µg/day had a higher baseline-adjusted IGFBP-3 at endpoint than both the placebo group (0.2 (95% CI: 0.1; 0.4) mg/L) and the 10 µg/day group (0.2 (95% CI: 0.0: 0.4) mg/L), (p = 0.006 and p = 0.011, respectively, Table 3; Fig. 1b), also after adjustment (p = 0.003 and p = 0.004, respectively). Endpoint height tended to be slightly higher (0.2 (95% CI: − 0.0; 0.4) cm) after 20 µg/day compared to placebo when adjusted for baseline height (p = 0.064, Table 3; Fig. 1c) as well as in the fully adjusted model (p = 0.062). No sex interactions existed in the response to supplementation (all p > 0.34).
Change (median and interquartile range) in a insulin-like growth factor 1 (IGF-1) (n = 114), b IGF-binding protein-3 (IGFBP-3) (n = 114), and c height (n = 117) from baseline to endpoint in the three intervention groups. P values for differences between groups after adjustment for baseline value of the outcome. # indicate within-group difference at endpoint compared to baseline (p < 0.05)
Discussion
Avoiding the winter decline in serum 25(OH)D did not affect muscle strength or body composition in healthy 4–8 year-olds. However, children who received 20 µg/day, which avoided vitamin D insufficiency, had higher circulating IGF-1 and IGFBP-3 at endpoint and tended to increase more in height than the placebo group. At baseline, serum 25(OH)D associated positively with both muscle strength, FFMI, and IGFBP-3 in girls only.
Few randomized controlled trials have investigated the effect of vitamin D supplementation on muscle strength in children. In a 1-year randomized trial by Ward et al., 12–14 year-old girls had improved jumping efficacy, but not improved grip strength after 4 mega-doses of vitamin D2 compared to placebo [13]. In another 1-year randomized trial in 10–17 year-old girls who received either placebo, 35 or 350 µg/week of vitamin D3, grip strength did not differ between groups [12]. One explanation of the inconsistency between effects on muscle strength may be the method. Although the hand dynamometer has a high reproducibility in children [25] and is a widely used tool to measure whole body muscle strength, [25, 34] it has been suggested that vitamin D deficiency primarily affects the muscles of the lower limbs [6], which may explain why effects are seen in studies using jumping mechanography. In support of this, a meta-analysis previously found a positive effect of vitamin D supplementation on lower limb muscle strength but not on grip strength [35]. However, jumping mechanography requires considerable coordination and motor skills, and may be difficult to replicate. Thus, this method is not suitable for children as young as 4 years. It is also possible that an effect on muscle strength is more likely in children with low muscle strength, and/or muscle weakness and low serum 25(OH)D. In the randomized trial by Ward et al., which showed a positive effect on jumping efficacy, only girls with a serum 25(OH)D < 37.5 nmol/L were included [13]. In our trial, mean baseline serum 25(OH)D was 56.8 ± 12.5 nmol/L and only 5% of the children had a baseline serum 25(OH)D < 30 nmol/L [2] which is defined as deficiency by the Institute of Medicine [3]. The short duration of our study may also explain the lack of effect. The group receiving 20 µg/day reached a serum 25(OH)D of 76.0 ± 1.8 nmol/L after 20 weeks, but it is likely that this concentration has not been present for a sufficient period of time to affect muscle strength in the children. However, we did not intent to investigate long-term supplementation in children or effects in vitamin D-deficient children or children with muscle weakness, but rather explore the effect of avoiding a decrease in vitamin D status during winter with supplementation in healthy children. We did, however, find an association between baseline vitamin D status and muscle strength but only in girls. This is in line with the findings of others who showed cross-sectional positive associations in adolescent females with muscle strength measured by jumping mechanography [8] and hand grip strength [9] as well as studies where adolescents with the highest serum 25(OH)D had the highest hand grip strength [10, 11].
The effect of vitamin D supplementation on markers of the IGF-axis are in agreement with the hypothesis that 1,25(OH)2D stimulates liver production of IGF-1 and IGFBP-3 [16] and that it may also increase the synthesis of cytoskeletal proteins, including IGFBP-3 in muscle cells [15]. Binding of IGF-1 to IGFBP-3 can stabilize IGF-1 in the circulation and prolong its half-life substantially and thus ensure a steady supply to tissues [16, 36]. Treatment with 175 µg/week of vitamin D3 for 12 weeks has been shown to increase circulating IGF-1 in older adults [37], and both IGF-1 [17, 18] and IGFBP-3 in vitamin D-deficient infants treated with a single mega-dose of vitamin D3 [18]. Cross-sectionally, IGF-1 has been reported to associate positively with serum 25(OH)D in healthy adults [38]. We did not observe this association but instead a positive association between serum 25(OH)D and IGFBP-3 in girls. There may be a larger variation in growth in the group of girls due to their earlier puberty and growth spurt in general compared to boys, and it is possible that this may contribute to the observed sex interaction. To our knowledge, this sex-specific association has not been investigated previously in children.
A clinical implication of an effect on markers of the IGF-axis could be a stimulatory effect on linear growth which was also observed in one study in which growth velocity in vitamin D-treated infants increased and was correlated with the increased IGF-1 and serum 25(OH)D [17]. In addition, others have reported a positive correlation between serum 25(OH)D and height in post-pubertal females with no signs of rickets [39]. The tendency of an effect on height after 20 µg/day compared to placebo in our study may imply that winter vitamin D supplementation could affect height during childhood. However, larger and more long-term trials are needed to confirm this and to explore the possible mediating factor of the IGF-axis. As we have previously reported, plasma PTH was higher at endpoint in the placebo group compared to both supplemented groups, and with no differences in serum calcium between groups [2]. However, from this study, we cannot determine if the placebo group experienced increased bone turnover.
Even though not transferred into an effect on muscle strength, it is possible that vitamin D affects muscle size, maybe through an upregulation of IGF-1 and IGFBP-3. In a 1-year randomized trial, vitamin D supplementation increased lean mass compared to placebo. In our study, we did not see an effect of vitamin D supplementation on FFMI, although IGF-1 and IGFBP-3 were increased. However, baseline serum 25(OH)D was positively associated with FFMI in girls, as also seen by others [9, 40]. As for IGFBP-3, it could be speculated that this association seen in girls but not boys is due to an earlier growth and development among girls and thereby larger diversity within the group.
Not only lean mass but also fat mass has been linked to vitamin D status, and low serum 25(OH)D has been associated with obesity in a number of studies [22]. However, we did not see either a baseline association between serum 25(OH)D and FMI or an effect of supplementation on FMI. One explanation may be that the majority of the children in the present study were normal weight. Moreover, the direction of a potential causation between obesity and low serum 25(OH)D most likely is that high BMI or body fat leads to a low serum 25(OH)D and not the opposite [41, 42] and it has been suggested that this is due to volumetric dilution, i.e., an uptake and distribution of vitamin D3 by body fat [41].
A strength of the present study is the placebo-controlled winter-design, which ensured that no dermal vitamin D synthesis occurred during the intervention. Furthermore, compliance was high and drop-out rate was low. In addition, serum 25(OH)D was analyzed with the gold standard LC-MS/MS method [43]. The study is limited by the relatively small sample size. However, it was originally designed and statistically powered to investigate changes in serum 25(OH)D in response to vitamin D supplementation, whereas outcomes as muscle strength, height, body composition, and markers of the IGF-axis are secondary and exploratory. In addition, the results should be interpreted with caution, since we did not adjust for multiple comparisons due to the exploratory nature of the study.
Conclusions
In conclusion, the winter-related decrease in serum 25(OH)D in the placebo group and the increase in serum 25(OH)D in both vitamin D-supplemented groups did not affect muscle strength or body composition. However, after supplementation with 20 µg/day, circulating IGF-1 and IGFBP-3 was higher compared to placebo and for IGFBP-3, the difference was also evident between the two supplementation groups. Moreover, in girls baseline serum 25(OH)D was positively associated with muscle strength, FFMI and IGFBP-3. Larger intervention trials are required to confirm the effect of vitamin D supplementation on these outcomes, as well as their long-term implication for linear growth, and studies should be performed in both boys and girls to further explore differences between sexes.
Abbreviations
- BIA :
-
Bioelectrical impedance analysis
- BMI :
-
Body mass index
- CV :
-
Coefficient of variation
- FFM :
-
Fat free mass
- FFMI :
-
Fat free mass index
- FM :
-
Fat mass
- FMI :
-
Fat mass index
- ICC :
-
Intraclass correlation coefficient
- IGF-1 :
-
Insulin-like growth factor 1
- IGFBP-3 :
-
IGF-binding protein-3
- LC-MS/MS :
-
Liquid chromatography tandem mass spectrometry
- ODIN :
-
Food-based solutions for optimal vitamin D nutrition and health through the life cycle
- VDR :
-
Vitamin D receptor
- 1,25(OH) 2 D :
-
1,25-dihydroxyvitamin D
- 25(OH)D :
-
25-hydroxyvitamin D
References
Webb AR, Kline L, Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in boston and edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67:373–378. https://doi.org/10.1210/jcem-67-2-373
Mortensen C, Damsgaard CT, Hauger H et al (2016) Estimation of the dietary requirement for vitamin D in white children aged 4–8 y: a randomized, controlled, dose-response trial. Am J Clin Nutr 104:1310–1317. https://doi.org/10.3945/ajcn.116.136697
Institute of Medicine Food and Nutrition Board (2011) Dietary reference intakes for calcium and vitamin D. National Academies Press, Washington, DC
Nordisk Ministerråd (2014) Nordic Nutrition Recommendations 2012, integrating nutrition and physical activity, 5th edn, 1. oplag. Nordic Council of Ministers
Shaw NJ, Mughal MZ (2013) Vitamin D and child health: part 2 (extraskeletal and other aspects). Arch Dis Child 98:368–372. https://doi.org/10.1136/archdischild-2012-302585
Glerup H, Mikkelsen K, Poulsen L et al (2014) Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int 66:419–424. https://doi.org/10.1007/s002230010085
Ceglia L (2008) Vitamin D and skeletal muscle tissue and function. Mol Aspects Med 29:407–414. https://doi.org/10.1016/j.mam.2008.07.002
Ward KA, Das G, Berry JL et al (2009) Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab 94:559–563. https://doi.org/10.1210/jc.2008-1284
Valtueña J, Gracia-Marco L, Huybrechts I et al (2013) Cardiorespiratory fitness in males, and upper limbs muscular strength in females, are positively related with 25-hydroxyvitamin D plasma concentrations in European adolescents: the HELENA study. QJM 106:809–821. https://doi.org/10.1093/qjmed/hct089
Foo LH, Zhang Q, Zhu K et al (2009) Low vitamin D status has an adverse influence on bone Mass, bone turnover, and muscle strength in Chinese adolescent girls. J Nutr 139:1002–1007. https://doi.org/10.3945/jn.108.102053
Carson EL, Pourshahidi LK, Hill TR et al (2015) Vitamin D, muscle function, and cardiorespiratory fitness in adolescents from the young hearts study. J Clin Endocrinol Metab 100:4621–4628. https://doi.org/10.1210/jc.2015-2956
El-Hajj Fuleihan G, Nabulsi M, Tamim H et al (2006) Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab 91:405–412. https://doi.org/10.1210/jc.2005-1436
Ward KA, Das G, Roberts SA et al (2010) A randomized, controlled trial of vitamin D Supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab 95:4643–4651. https://doi.org/10.1210/jc.2009-2725
McCarthy EK, Kiely M (2015) Vitamin D and muscle strength throughout the life course: a review of epidemiological and intervention studies. J Hum Nutr Diet 28:636–645. https://doi.org/10.1111/jhn.12268
Hazell TJ, DeGuire JR, Weiler HA (2012) Vitamin D: an overview of its role in skeletal muscle physiology in children and adolescents. Nutr Rev 70:520–533. https://doi.org/10.1111/j.1753-4887.2012.00510.x
Ameri P, Giusti A, Boschetti M et al (2013) Interactions between vitamin D and IGF-I: from physiology to clinical practice. Clin Endocrinol (Oxf) 79:457–463. https://doi.org/10.1111/cen.12268
Soliman AT, Al Khalaf F, AlHemaidi N et al (2008) Linear growth in relation to the circulating concentrations of insulin-like growth factor I, parathyroid hormone, and 25-hydroxy vitamin D in children with nutritional rickets before and after treatment: endocrine adaptation to vitamin D deficiency. Metabolism 57:95–102. https://doi.org/10.1016/j.metabol.2007.08.011
Bereket A, Cesur Y, Özkan B et al (2010) Circulating insulin-like growth factor binding protein-4 (IGFBP-4) is not regulated by parathyroid hormone and vitamin D in vivo: evidence from children with rickets. J Clin Res Pediatr Endocrinol 2:17–20
Hamilton B (2010) Vitamin D and human skeletal muscle. Scand J Med Sci Sports 20:182–190. https://doi.org/10.1111/j.1600-0838.2009.01016.x
Closa-Monasterolo R, Ferré N, Luque V et al (2011) Sex differences in the endocrine system in response to protein intake early in life. Am J Clin Nutr 94:1920S–1927S. https://doi.org/10.3945/ajcn.110.001123
Damsgaard CT, Harsløf LBS, Andersen AD et al (2016) Fish oil supplementation from 9 to 18 months of age affects the insulin-like growth factor axis in a sex-specific manner in Danish infants. Br J Nutr 115:782–790. https://doi.org/10.1017/S0007114515004973
Earthman CP, Beckman LM, Masodkar K, Sibley SD (2012) The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes 36:387–396. https://doi.org/10.1038/ijo.2011.119
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194. https://doi.org/10.1001/jama.2013.281053
Newman DG, Pearn J, Barnes A et al (1984) Norms for hand grip strength. Arch Dis Child 59:453–459. https://doi.org/10.1136/adc.59.5.453
Scientist WA, van den BHM, Epidemiologist, Scientist WA van den BHM, et al (2006) Validity and reproducibility of the Jamar dynamometer in children aged 4–11 years. Disabil Rehabil 28:1303–1309. https://doi.org/10.1080/09638280600631047
Molenaar HM (Ties), Selles RW, Zuidam JM et al (2009) Growth diagrams for grip strength in children. Clin Orthop Relat Res 468:217. https://doi.org/10.1007/s11999-009-0881-z
Hager-Ross C, Rosblad B (2002) Norms for grip strength in children aged 4–16 years. Acta Paediatr 91:617–625
World Health Organization Application tools WHO AnthroPlus software. In: WHO. http://www.who.int/growthref/tools/en/. Accessed 4 Jan 2016
Talma H, Chinapaw MJM, Bakker B et al (2013) Bioelectrical impedance analysis to estimate body composition in children and adolescents: a systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obes Rev 14:895–905. https://doi.org/10.1111/obr.12061
Andersen LB, Schnohr P, Schroll M, Hein HO (2000) All-cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Arch Intern Med 160:1621–1628. https://doi.org/10.1001/archinte.160.11.1621
Kiely M, Collins A, Lucey AJ et al (2016) Development, validation and implementation of a quantitative food frequency questionnaire to assess habitual vitamin D intake. J Hum Nutr Diet 1–10. https://doi.org/10.1111/jhn.12348
Aksglaede L, Sørensen K, Petersen JH et al (2009) Recent Decline in Age at Breast Development: The Copenhagen Puberty Study. Pediatrics 123:e932–e939. https://doi.org/10.1542/peds.2008-2491
Sørensen K, Aksglaede L, Petersen JH, Juul A (2010) Recent changes in pubertal timing in healthy danish boys: associations with body mass index. J Clin Endocrinol Metab 95:263–270. https://doi.org/10.1210/jc.2009-1478
Van den Beld WA, Van der Sanden GAC, Janssen AJWM. et al (2011) Comparison of 3 instruments to measure muscle strength in children: A prospective study. Eur J Paediatr Neurol 15:512–518. https://doi.org/10.1016/j.ejpn.2011.05.006
Beaudart C, Buckinx F, Rabenda V et al (2014) The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 99:4336–4345. https://doi.org/10.1210/jc.2014-1742
Juul A, Dalgaard P, Blum WF et al (1995) Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab 80:2534–2542. https://doi.org/10.1210/jcem.80.8.7543116
Ameri P, Giusti A, Boschetti M et al (2013) Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency. Eur J Endocrinol 169:767–772. https://doi.org/10.1530/EJE-13-0510
Bogazzi F, Rossi G, Lombardi M et al (2010) Vitamin D status may contribute to serum insulin-like growth factor I concentrations in healthy subjects. J Endocrinol Invest 34:e200–e203. https://doi.org/10.3275/7228
Kremer R, Campbell PP, Reinhardt T, Gilsanz V (2009) Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab 94:67–73. https://doi.org/10.1210/jc.2008-1575
Foo LH, Zhang Q, Zhu K et al (2009) Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos Int 20:417–425. https://doi.org/10.1007/s00198-008-0667-2
Drincic AT, Armas LAG, Van Diest EE, Heaney RP (2012) Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obes Silver Spring Md 20:1444–1448. https://doi.org/10.1038/oby.2011.404
Vimaleswaran KS, Berry DJ, Lu C et al (2013) Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 10:e1001383
Arneson WL, Arneson DL (2013) Current methods for routine clinical laboratory testing of vitamin D levels. Lab Med 44:e38–e42. https://doi.org/10.1309/LMONQZQ27TIN7XFS
Acknowledgements
We thank all the participating children and their parents.
Funding
This project was funded by the European Commission (FP7/2007–2013) under Grant Agreement 613977 for the ODIN Integrated Project [Food-based solutions for optimal vitamin D nutrition and health through the life cycle http://www.odin-vitd.eu/].
Author information
Authors and Affiliations
Contributions
CTD and CMø: Designed the study; CMo, CTD, HH, and CMø: Conducted the research; CMo: Performed the statistical analyses and drafted the manuscript; CTD, CMø, HH, and MK: Assisted in the manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
This study involving humans was approved by the Committees on Biomedical Research Ethics for the Capital Region of Denmark (H-3-2014-022), and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We obtained informed written consent from all parents. Any details that may have disclosed the identity of the participants were omitted. The study was registered at http://www.clinicaltrials.gov as NCT02145195.
Rights and permissions
About this article
Cite this article
Mortensen, C., Mølgaard, C., Hauger, H. et al. Winter vitamin D3 supplementation does not increase muscle strength, but modulates the IGF-axis in young children. Eur J Nutr 58, 1183–1192 (2019). https://doi.org/10.1007/s00394-018-1637-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1637-x