Abstract
Objectives
The aim of this study was to investigate the effects of intrauterine growth retardation (IUGR) and Bacillus subtilis PB6 supplementation in formula milk (FORM) on growth performance, intestinal development and immune function of neonates using a porcine model.
Methods
Fourteen pairs of normal birth weight and IUGR piglets (7 days old) were randomly assigned to receive FORM or FORM supplemented with B. subtilis PB6 (FORM-BsPB6) for a period of 21 days. Blood samples, intestinal tissues and digesta were collected at necropsy and analysed for morphology, digestive enzyme activities, immune cell abundance, expression of genes associated with innate immunity and barrier function and microbial populations.
Results
Regardless of diet, IUGR significantly decreased average daily dry matter intake and average daily weight gain (P < 0.05). Moreover, IUGR significantly decreased plasma concentrations of immunoglobulin A, interleukin 1β, count and percentage of blood lymphocytes (P < 0.05). Meanwhile, IUGR markedly decreased villous height and maltase activity, as well as mRNA abundance of Toll-like receptor 9 and Toll-interacting protein in the ileum (P < 0.05). Regardless of body weight, FORM-BsPB6 markedly decreased the feed conversion ratio (P < 0.05), due to better intestinal development, as indicated by increased villous height (P < 0.05), activities of maltase and sucrase in the intestine (P < 0.10). Moreover, both mRNA and protein abundances of zonula occludens-1 and claudin-1 in the ileum as well as the copy number of Bacillus in colonic digesta were increased (P < 0.05) in piglets fed FORM-BsPB6 relative to FORM.
Conclusion
The results of this study indicate that IUGR delayed growth, intestinal development and immune function of piglets, while FORM-BsPB6 improved digestive capability and intestinal barrier function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrauterine growth retardation (IUGR) impairs growth and development of the mammalian embryo/foetus or its organs during gestation [1]. IUGR in neonates is characterized by delayed postnatal growth, permanent mal-development and increased susceptibility to infection, resulting in high morbidity and mortality during the early life period [2]. Several studies have demonstrated that IUGR is associated with impaired intestinal development and poses a high risk of intestinal diseases in neonates [3–5]. Also, our previous studies found that both excessive and restricted nutrient intake impaired intestinal development and immune function in neonates with IUGR [6, 7]. The intestine is important for digestion and absorption of nutrients, and the gut-associated lymphoid tissue is the largest immune organ in the body [8]. Thus, new strategies to promote growth and intestinal function in neonates with IUGR are urgently needed.
Establishment of intestinal microbiota after birth plays an important role in the development of the gastrointestinal and immune systems [9]. Previous studies have reported that gut colonization by bacteria and the fermentation activity of the resulting intestinal microbiota are altered in neonates with IUGR, as compared with normal neonates, because of the effect of IUGR on the small intestine [10, 11]. Many recent studies have highlighted the beneficial role of probiotics in intestinal motor function. In humans, probiotics supplementation was found to reduce both the incidence and severity of necrotizing enterocolitis in newborns with IUGR [12]. In animals, moreover, probiotics seem to be a good alternative to the use of antibiotics to promote growth [13]. As we know, probiotics as microbial supplementation convey beneficial effects when administered in adequate quantities [14]. However, little is known about the effects of probiotics supplementation on growth, intestinal development and immune function in neonates with IUGR.
Bacillus subtilis is a facultative anaerobe that plays the vital roles in intestinal microecological balance by consumption of intestinal oxygen, which creates an anaerobic environment [15, 16]. Moreover, B. subtilis is preferred due to the high resistance of its spores to harsh environments and capacity for long-term storage at ambient temperatures [17]. Previous studies have reported that some Bacillus species improved porcine intestinal health by regulation of immune function to protect against pathogenic challenge [18, 19]. Moreover, some species can be used as potent producers of extracellular degrading enzymes to promote nutrient digestion and utilization [16, 18]. Pigs are multifoetal animals and exhibit serious IUGR occurrence, which has been recognized as an ideal model for the study of clinical nutrition [20]. In the present study, therefore, we investigated the effects of IUGR and B. subtilis PB6 supplementation on the growth performance, intestinal development and immune function of piglets during the suckling period.
Materials and methods
The animal experiment followed the actual law of animal protection and was approved by the Animal Care and Use Committee of the Sichuan Agricultural University and was performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Animal and treatment
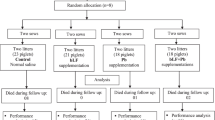
Piglets with a birth weight near the mean litter birth weight (SD 0.5) were identified as normal birth weight (NBW), whereas those with at least 1.5 SD lower birth weight were defined as IUGR according to our previous study [6, 21]. A total of 14 pairs newborn boars (Pig Improvement Company 327 × 1050) of NBW with body weight (BW) at 1.49 (SD 0.06) kg and IUGR with BW at 0.92 (SD 0.03) kg were selected from 14 healthy sows (10 piglets/litter). All piglets were weaned at 7 days of age and moved to be individually fed with formula milk by bottle feeding every 3 h between 06:00 and 24:00 hours in nursing cages (0.8 m × 0.7 m × 0.4 m). In each litter, one of the IUGR and NBW piglets received the formula milk (FORM) or formula milk supplemented with B. subtilis PB6 (FORM-BsPB6), respectively. In total, four groups (BW-FORM) of piglets were created and studied: NBW with FORM; IUGR with FORM; NBW with FORM-BsPB6; IUGR with FORM-BsPB6 (n = 7 per group). The FORM was formulated according to previous study [6]. FORM-BsPB6 was prepared by supplementing the spores of B. subtilis PB6 (Kemin Industries, Inc., Des Moines, IA) at 60 g per 100 kg FORM powder, containing 2 × 109 cfu/kg. The liquid formula milk was prepared by mixing 1 kg of formula powder (DM 87.5 %) with 4 L of water, in which nutrients composition and levels were similar as sow milk [7]. All piglets had free access to drinking water. Room temperature was maintained at approximately 30 °C, and the humidity was controlled between 50 and 60 %. The BW and milk intake of piglets were recorded daily. The average daily DM intake (ADMI) was calculated via multiplying the average daily intake of milk by its DM content (%), while milk intake was calculated as the difference between the offered amounts and the refusals.
Blood sampling and analyses
Blood samples were collected by venepuncture in the morning (08:00) of day 21 after an overnight fast and were injected into two vacuum tubes containing sodium heparin. The vacuum tubes were immediately placed on ice until the examination of leucocytes and flow cytometry analysis, respectively (within 2 h). The differential leucocyte count was obtained using an ADVIA 2120 Hematology System (Bayer HealthCare, Tarrytown, NY). Total peripheral blood lymphocytes were separated from heparinized peripheral blood by separation medium and then were stained with mouse anti-porcine CD3e-SPRD (PE-Cy5) (catalogue no. 4510-13), CD4a-FITC (catalogue no. 4515-02) and CD8a-PE (catalogue no. 4520-09), which were purchased from Southern Biotechnology Associates (Birmingham, AL). PBS (1×, Gibco, Carlsbad, CA) and 1.0 % BSA (ICN Biomedicals, Aurora, OH) were used as diluent and washing buffer. Flow cytometry analysis was performed on a FACSCalibur flow cytometer (Becton–Dickinson, San Jose, CA) and repeated for the same sample.
Tissue sample collection
After blood sampling, all piglets were anaesthetized with an intravenous injection of pentobarbital sodium (50 mg/kg BW) and slaughtered. Piglets were weighed, and crown-rump length (CRL) was taken (the supine length of the piglet from the crown of its head to the base of its tail). Body mass index (BMI; BW/CRL2) was calculated for each piglet. The liver, spleen, kidney, heart and pancreas of each piglet were weighed immediately after slaughter. The length and weight of small intestine were measured after the removal of luminal contents. Duodenal, jejunal and ileal samples of approximately 2 cm in length were stored in 4 % paraformaldehyde solution for histological analyses. The other pieces of the jejunum and ileum (approximately 2 cm) were snap-frozen and then stored in fridge with −80 °C until further analysis. Finally, colonic digesta were collected immediately and frozen at −80 °C.
Small-intestinal morphology and goblet cell countings
The duodenal, jejunal and ileal samples were preserved in 4 % paraformaldehyde solution and then embedded in paraffin. Each of the samples (duodenum, jejunum and ileum) was used to prepare five slides, and each slide had three sections (5 µm thickness), which were stained with eosin and haematoxylin for intestinal morphology measurement by 20 well-oriented villi and crypts each section (Optimus software version 6.5; Media Cybergenetics), and villi–crypt ratio (VCR) was calculated. The goblets cells number per villi was measured (NIS-Elements BR 2.3; Nikon France SAS), and the values obtained from 10 villi by each small-intestinal segment were averaged.
Measurement of plasma immunoglobulin subset and cytokines
Commercially available enzyme immunoassays were performed according to the instructions from the manufacturer for the following markers: IgA (Bethyl Lab. Inc., Montgomery, USA), IL-1β (R&D Systems, Oxford, UK), TNF-α (R&D Systems, Oxford, UK), IL-10 (Bio Source/Med Probe, Camarillo, CA). Absorbance (450 nm) was determined using a Bio-Tek synergy HT microplate reader (Bio-Tek Instruments, VT). The detection limits were 12.5 ng/mL for IgA, 7.0 pg/mL for TNF-α, 30.0 pg/mL for IL-1β and 8.0 pg/mL for IL-10, respectively; the inter- and intra-assay coefficients of variation were less than 10 %.
Digestive enzyme activities
After thawing, the frozen jejunal tissue was weighed and homogenized (5 min) in the 9 times volume of 50 mM Tris–HCl buffer, pH 7.0, centrifuged (3000×g, 10 min). The supernatant was collected and stored at −20 °C for enzyme assay. Total proteins were extracted, and their concentration was determined according to the procedure of bicinchoninic acid (Solarbio, Inc.), with bovine serum albumin as standard. Disaccharidases (including maltase, sucrase and lactase) were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions. The absorbance was determined with spectrophotometer (Beckman Coulter DU-800; Beckman Coulter, Inc.). The activities of disaccharidases were expressed as U/mg protein. One unit (U) was defined as 1 nmol maltase, sucrase and lactase as substrate for the enzymatic reaction.
Microbial population determination
Bacterial DNA was extracted from colonic digesta using the Stool DNA Kit (Omega Bio-Tek) according to the manufacturer’s instructions. Primers and probes (Table 1) were designed with Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA) and followed 16S rRNA sequences of maximum species of each genus homology downloaded from GenBank database, European Molecular Biology Laboratory and DNA Data Bank of Japan to obtain specific amplification, and the sequences of all the genera taken from the database were submitted to DNAStar (MegAlign) program (DNASTAR, Inc., Madison, WI), as described by Zhang et al. [22]. Next, these sequences were submitted to alignment, in which the maximum number of species belonging to one genus was gathered and the regions showing conservations were picked up as genus-specific primers and probes. All the primers and probes used in this experiment were commercially synthesized by Invitrogen (Shanghai, China).
Quantitative real-time PCR was conducted with CFX96 Real-Time PCR System (Bio-Rad Laboratories, Inc., Hercules, CA) with optical-grade 96-well plates. For the quantification of total bacteria, the reaction mixture (25 μL) contained 1 μL forward and 1 μL reverse primers (100 nM), 12.5 μL SYBR Premix EX Taq (Takara, Dalian, China), 1 μL template DNA and 9.5 μL nuclease-free water. The thermal cycling conditions were an initial predenaturation step at 95 °C for 10 s, 40 cycles of denaturation at 95 °C for 5 s, annealing at 64.5 °C for 25 s and extension at 72 °C for 60 s. For the quantification of Lactobacillus, Escherichia coli, Bifidobacterium and Bacillus, real-time PCR was conducted in a reaction volume of 20 μL with 1 μL probe enhancer solution, 0.3 μL probe (100 nM), 1 μL forward and 1 μL reverse primers (100 nM), 8 μL RealMasterMix (Tiangen, Beijing, China), 1 μL template DNA and 7.7 μL nuclease-free water. The PCR conditions involved 10 s at 95 °C and 50 cycles for 5 s at 95 °C, 25 s at annealing temperature (Table 1) and 60 s at 72 °C. The threshold cycle (CT) values and baseline settings were determined by automatic analysis settings, and the copy numbers of the target group for each reaction were calculated from the standard curves.
For the quantification of bacteria in the test samples, specific standard curves were generated by constructing standard plasmids, as presented by Han et al. [15]. Deoxyribonucleic acid concentrations of standard plasmids were detected using a spectrophotometer (Beckman Coulter DU 800; Beckman Coulter, Fullerton, CA). A series of tenfold dilution (1 × 109 to 1 × 101 copies/μL) of plasmid DNA were used to construct their respective standard curves. Each standard curve was generated by a linear regression of the plotted points with the logarithm of template copy numbers as the abscissa and the CT values as the ordinate. The gene copy numbers were calculated by the following formula: (6.0233 × 1023 copies/mol × DNA concentration (μg/μL))/(660 × 106 × DNA size (bp)).
Total RNA extraction and real-time RT-PCR
Total RNA was extracted from frozen ileal samples using TRIzol reagent (catalogue no. 15596-026; Invitrogen) according to the manufacturer’s instructions, and the quality and purity of RNA samples were assessed by electrophoresis on 1.0 % agarose gels (Egel; Invitrogen, Carlsbad, CA, USA) and nucleic acid analyser (A260/A280, Beckman DU-800; Beckman Coulter, Inc.), respectively. Subsequently, the RNA was performed at 37 °C for 15 min, followed by RT inactivation at 85 °C for 5 s using PrimeScript™ RT reagent kit (catalogue no. RR047A, Takara). A portion of the RT products (1μL) was used directly for real-time PCR. Real-time PCR assays were performed on complementary DNA samples in 384-well optical plates on a 7900HT ABI Prism Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using the SYBR green system (catalogue no. RR820A, Takara). Primers for individual genes were designed using Primer Express 3.0 (Applied Biosystems) and are given in Table 2. The reaction mixture (10 μL) contained 5 μL of freshly SYBR® Premix Ex Taq II (Tli RNaseH Plus) and 0.2 μL ROX Reference Dye II (50×), 0.8 μL of the primers, 1 μL of RT products and 3 μL diethylpyrocarbonate-treated water. The PCR protocol was used as following: 1 cycle (95 °C 30 s), 40 cycles (95 °C 5 s, 60 °C 31 s) and 1 cycle (95 °C 15 s, 60 °C 1 min and 95 °C 15 s). The standard curve of each gene was run in duplicate and three times for obtaining reliable amplification efficiency values as described previously [23]. The correlation coefficients (r) of all the standard curves were more than 0.99, and the amplification efficiency values were between 90 and 110 %. At the end of amplification, dissociation analyses of the PCR product were performed to confirm the specificity of PCR products. The relative mRNA abundance of analysed genes was calculated using the method of 2−ΔΔCt, as described previously [24]. The most stable housekeeping genes (β-actin and GADPH) were chosen for normalization. Finally, the mRNA level of each target gene for IUGR-FORM group was set to 1.0.
Western blotting
Protein extracts were obtained by homogenizing ileal tissues with a total protein extraction kit (Beyotime Biotechnology, Jiangsu, China), according to the manufacturer’s guide. The protein content was measured using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA). The antibody was used in our experiment: goat polyclonal anti-ZO-1 (sc-8146, Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat polyclonal anti-claudin-1 (sc-17658, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse monoclonal anti-β-actin (sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Western blot analysis was performed as previously described [25]. Chemiluminescence detection was performed using the ECL Plus™ Western Blotting Detection System (Amersham, Arlington Heights, IL, USA), according to the manufacturer’s instructions. The relative expression of target protein was normalized using β-actin as the internal protein, and then, the normalized values were used for the comparison of the expression of target protein in groups.
Statistical analysis
The data were analysed by Duncan’s multiple comparisons for the 2 × 2 factorial experimental design using the general linear model (GLM) procedure of SPSS statistical software (Ver. 20.0 for Windows, SPSS, Chicago, IL, USA) in the following model: y ijk = μ + a i + b j + (ab) ij + e ijk (i = 1, 2, j = 1, 2, k = 1, 2, …, n ij ), where y ijk represents the dependent variable, μ is the mean, a i is the effect of BW (IUGR, NBW), b j is the effect of diet (FORM, FORM-BsPB6), (ab) ij is the interaction between BW and diet, and e ijk is the error term. Results are presented as means with their standard errors (SEM). Differences were considered as significant when P < 0.05, and a tendency was recognized when P < 0.10.
Results
Growth performance
Regardless of diet, both ADG (−28 %, P < 0.001) and ADMI (−31 %, P < 0.001) were significantly decreased in IUGR piglets compared with NBW piglets, while the FCR had no significant difference, and accordingly, the final BW and net BW gain of IUGR piglets were lower (−28 to 31 %, P < 0.001) than those of NBW piglets (Table 3). Regardless of BW, FORM-BsPB6 had no significant effects on the ADG, ADMI, final BW and net BW gain, but the FCR was markedly decreased (−10 %, P = 0.043) in piglets fed FORM-BsPB6. During the whole experimental period, furthermore, IUGR piglets fed FORM-BsPB6 had significantly lower FCR compared with piglets fed FORM (−17 %, P < 0.050).
Organ indices
As shown in Table 4, regardless of diet, IUGR significantly decreased (−11 to 35 %, P < 0.010) the weights of internal organs such as heart, liver, spleen, kidney, pancreas, intestine and the BMI on day 28; moreover, the intestinal length and 28 CRL of IUGR piglets were shorter (−13 to 18 %, P < 0.010) than those of NBW piglets. However, the relative intestinal length to intestinal weight and the relative intestinal length to BW in IUGR piglets were higher (+18 to 22 %, P < 0.050) than NBW piglets. FORM-BsPB6 had no significant influence on the organ indices of piglets.
Plasma immunoglobulin and cytokines
As shown in Table 5, irrespective of diet, IUGR markedly decreased the plasma concentrations of IgA (P < 0.001) and IL-1β (P = 0.006) and the ratio of IL-1β to IL-10 (P < 0.001). However, FORM-BsPB6 had no significant influence on plasma immunoglobulin subset and cytokines. Furthermore, IL-1β concentration in IUGR piglets fed FORM-BsPB6 had no significant difference with NBW piglets.
Composition of peripheral leucocytes and lymphocyte percentages
IUGR significantly decreased the count (P = 0.021) and percentage (P = 0.025) of lymphocytes but markedly increased the percentage of neutrophils (P = 0.023) and CD8+ (P = 0.009); moreover, the counts of leucocyte (P = 0.057) and monocytes (P = 0.063) and the percentage of monocytes (P = 0.097) had a tendency to decrease in IUGR piglets (Tables 6, 7). However, FORM-BsPB6 had no significant influence on composition of peripheral leucocytes and lymphocyte percentages in piglets. Furthermore, the counts of monocytes, the percentages of neutrophils, lymphocytes and monocytes in IUGR piglets fed FORM-BsPB6 had no significant difference with NBW piglets.
Intestinal morphology and goblet cell density
As shown in Table 8, regardless of diet, IUGR significantly decreased (P = 0.039) villous height in the duodenum of piglets while increased goblet cell number per villous in the duodenum (P < 0.001) and jejunum (P = 0.019). Regardless of BW, FORM-BsPB6 significantly increased villous height (P = 0.023) and the VCR (P < 0.001) while decreased the crypt depth (P = 0.012) in the ileum.
Digestive enzyme activities
In the jejunum, IUGR significantly decreased the activity of maltase (P = 0.011) while increased the activity of lactase (P = 0.030). Regardless of BW, piglets fed FORM-BsPB6 had a tendency to increase the activities of maltase (P = 0.082) and sucrase (P = 0.095) compared to piglet fed FORM. Furthermore, the activity of maltase in IUGR piglets fed FORM-BsPB6 had no significant difference with NBW piglets (Table 9).
Gene expression in the ileum
As shown in Table 10, IUGR markedly decreased the mRNA abundance of TLR-9 (P = 0.020) and TOLLIP (P = 0.001); moreover, the mRNA abundance of MyD88 (P = 0.098) and TLR2 (P = 0.065) had a tendency to decrease, while the mRNA abundance of SIGIRR (P = 0.081) had a tendency to increase in the ileum of piglets with IUGR. FORM-BsPB6 markedly increased the mRNA abundance of ZO-1 (P = 0.001) in the ileum of piglets. In addition, the significant interaction between BW and diet was observed for the mRNA abundance of TOLLIP (P = 0.046) in the ileum.
Gut microbial population
Regardless of diet, there were no significant differences in microbial populations in colonic digesta between IUGR and NBW piglets (Table 11). FORM-BsPB6 significantly increased the copy number of Bacillus (P = 0.010) in colonic digesta of piglets.
Protein abundances of ZO-1 and claudin-1
Regardless of diet, IUGR had no significant influences on the protein abundances of ZO-1 and claudin-1 in the ileum of piglets. However, FORM-BsPB6 markedly increased the protein abundances of ZO-1 (Fig. 1a) and claudin-1 (Fig. 1b) in the ileum of piglets (P < 0.050).
Relative protein expression levels of ZO-1 (a) and claudin-1 (b) in the ileum of IUGR and NBW piglets fed FORM or FORM-BsPB6. Values are means, with standard errors represented by vertical bars, n = 7. The value of protein expression = densitometry units of selected protein/densitometry units of β-actin detected by Western blotting. IUGR intrauterine growth retardation, NBW normal birth weight, FORM formula milk, FORM-BsPB6 formula milk supplemented with B. subtilis PB6, ZO-1 zonula occludens 1. a,bMean values with unlike letters were significantly different (P < 0.05). *P < 0.05 for the respective sources of variation (the diet)
Discussion
IUGR is associated with increased neonatal mortality and morbidity in both animals and humans [26, 27]. Numerous studies using porcine models have shown that IUGR delayed postnatal growth and gut development [4, 7, 11]. Meanwhile, increased bacterial adhesion in the intestine due to IUGR is a predisposing factor to intestinal pathologies [10, 11]. B. subtilis, as a well-tolerated facultative anaerobe, may facilitate growth performance and intestinal homoeostasis in mammals [28]. In this study, we investigated the effects of B. subtilis PB6 supplementation in formula milk on growth performance, intestinal development and immune function of IUGR piglets during the early postnatal period.
First, the ADMI and ADG were reduced in IUGR piglets, as compared to NBW piglets. As a result, the net weight gain and final BW were much lower in IUGR than in NBW piglets. These findings indicate that IUGR negatively affects birth weight and postnatal growth of neonates, consistent with the results of previous studies [29–31]. Dietary supplementation with B. subtilis PB6 had no significant influences on the ADMI and ADG, but significantly improved feed efficiency, as indicated by the lower FCR value. Similarly, previous studies indicated that B. subtilis improves nutrient digestibility and utilization in both weaning and finishing pigs via modulation of microbial populations [13, 32, 33]. The results of the current study also showed that dietary supplementation of B. subtilis PB6 markedly increased Bacillus populations in colonic digesta, indicating the crucial role of B. subtilis PB6 on microbial composition.
It was reported that maternal nutrition can be selectively allocated for the growth of internal organs [34]. In this study, the relative weights of internal organs of IUGR piglets were similar to those of NBW piglets, although the absolute weights of the internal organs were lower. In accordance with the findings of a previous study [35], CRL and BMI were lower in piglets with IUGR than NBW piglets. BMI could be used as an indicator of survival, with a higher BMI value associated with greater survival [36]. In this study, the lower BMI in IUGR piglets may partially explain the greater mortality in IUGR neonates [37].
Generally, intestinal morphology is an important factor reflecting intestinal development [38]. Previous findings showed that IUGR impaired cell proliferation, as well as absorptive and digestive function in the small intestine [8, 39]. Similarly, our results indicated that IUGR significantly decreased villous height in the duodenum of piglets. However, dietary supplementation with B. subtilis PB6 markedly increased villous height and decreased crypt depth in the ileum, resulting in a significant increase in the VCR. Lee et al. [33] reported that increased villus height and VCR in weaning piglets fed a diet supplemented with B. subtilis LS 1-2. The VCR is an important parameter to evaluate nutrient digestion and absorption capacity [40]. Therefore, an increase in villus height and VCR of piglets receiving B. subtilis PB6 suggests better intestinal development.
Several studies have reported that IUGR decreased the activity of digestive enzymes in the intestine of neonates [10, 21]. In the present study, IUGR was found to inhibit maltase activity, but enhanced lactase activity in the jejunum of piglets. The relatively higher lactase activity in IUGR piglets may be a compensatory response to enteral nutrition, which is consistent with the findings of our previous study [7]. However, dietary B. subtilis PB6 supplementation tended to increase the activities of maltase and sucrase in the jejunum of piglets. In particular, maltase activity in IUGR piglets receiving B. subtilis PB6 was normalized to similar levels as NBW piglets. The increasing activities of disaccharidases by B. subtilis PB6 indicate improved digestive capability of piglets.
IUGR neonates suffer from persistent immunological impairment throughout childhood [41]. The immunotype of blood is an important tool in the diagnosis of immunological disorders. In the present study, concentrations of IgA and IL-1β, numbers or percentages of lymphocytes and monocytes and the IL-1β: IL-10 ratio in the peripheral blood were lower in IUGR than in NBW piglets. Previous studies have demonstrated that IUGR alters cytokine profiles in the placenta and foetus [42, 43]. Meanwhile, decreased proliferation of lymphocytes in the thymus and cytokine levels in the peripheral blood were observed in rat and sheep models of IUGR [44, 45]. In this study, the increase in the number of CD8+ T cells indicates impaired T cell development in IUGR neonates. It has been shown that dietary probiotics supplementation could enhance cellular and humoral immune function in weaned piglets [46]. In this study, the levels of peripheral IL-1β, neutrophils, lymphocytes and monocytes in IUGR piglets receiving B. subtilis PB6 were consistently similar to those of NBW piglets, suggesting that the impairment of cellular immune function by IUGR can be alleviated by supplementation with probiotics.
Generally, intestinal epithelial cells provide immunological regulation to microbial invasion through both innate and adaptive immune responses [47]. It has been demonstrated that Toll-like receptors (TLRs) are typical pattern recognition receptors that mediate the innate host defence to maintain mucosal and commensal homoeostasis [48]. In this study, immune function was impaired in the intestine of IUGR piglets, as indicated by the decreased expression levels of TLR-9 and TOLLIP in the ileum of IUGR compared to NBW piglets. However, these gene expressions had not been markedly affected by dietary supplementation with B. subtilis PB6. The doses and strains of B. subtilis may affect the modulating effects of the animal immune function, as reported previously [49]. In addition, the gastrointestinal tract plays a central role as a physiological barrier between the outer environment and the body [50]. The paracellular barrier function of intestinal epithelia is thought to be regulated by tight junction proteins [51, 52]. The results of the current study indicate that dietary supplementation with B. subtilis PB6 increased expressions of ZO-1 and claudin-1 in the ileum of piglets. Both ZO-1 and claudin-1 are essential structural and functional components of tight junctions [25]. Hence, increased expression of tight junction proteins suggests improved intestinal barrier function in response to dietary supplementation of B. subtilis PB6.
This study provides new insights into the effects of dietary probiotics supplementation on growth performance, intestinal development and immune function of neonates. The results showed that IUGR delayed postnatal growth, intestinal development and impaired immune function in piglets. However, dietary B. subtilis PB6 supplementation improves growth rate, intestinal development and immune function, as indicated by the better digestive capability, barrier function and blood immunotype of piglets. These findings suggest that probiotics treatment could play an important role in the intestinal development and immune function of neonates during the early postnatal period.
References
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84(9):2316–2337. doi:10.2527/jas.2006-156
Pallotto EK, Kilbride HW (2006) Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol 49(2):257–269
Wang J, Chen L, Li D, Yin Y, Wang X, Li P, Dangott LJ, Hu W, Wu G (2008) Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr 138(1):60–66
Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ (2005) Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate 88(1):66–72. doi:10.1159/000084645
Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M (2013) Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med 26(3):222–225. doi:10.3109/14767058.2012.715006
Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, He S, Zhang K, Che L (2013) Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr 110(10):1819–1827. doi:10.1017/S0007114513001232
Hu L, Liu Y, Yan C, Peng X, Xu Q, Xuan Y, Han F, Tian G, Fang Z, Lin Y, Xu S, Zhang K, Chen D, Wu D, Che L (2015) Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br J Nutr 114(1):53–62. doi:10.1017/S0007114515001579
Zhong X, Wang T, Zhang X, Li W (2010) Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones 15(3):335–342. doi:10.1007/s12192-009-0148-3
Wang M, Radlowski EC, Monaco MH, Fahey GC Jr, Gaskins HR, Donovan SM (2013) Mode of delivery and early nutrition modulate microbial colonization and fermentation products in neonatal piglets. J Nutr 143(6):795–803. doi:10.3945/jn.112.173096
Fanca-Berthon P, Hoebler C, Mouzet E, David A, Michel C (2010) Intrauterine growth restriction not only modifies the cecocolonic microbiota in neonatal rats but also affects its activity in young adult rats. J Pediatr Gastroenterol Nutr 51(4):402–413. doi:10.1097/MPG.0b013e3181d75d52
D’Inca R, Kloareg M, Gras-Le Guen C, Le Huerou-Luron I (2010) Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr 140(5):925–931. doi:10.3945/jn.109.116822
Lin H-C, Su B-H, Chen A-C, Lin T-W, Tsai C-H, Yeh T-F, Oh W (2005) Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115(1):1–4
Alexopoulos C, Georgoulakis IE, Tzivara A, Kyriakis CS, Govaris A, Kyriakis SC (2004) Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J Vet Med A Physiol Pathol Clin Med 51(6):306–312. doi:10.1111/j.1439-0442.2004.00637.x
Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M (2011) Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 93(1):81–86. doi:10.3945/ajcn.2010.29799
Han GQ, Xiang ZT, Yu B, Chen DW, Qi HW, Mao XB, Chen H, Mao Q, Huang ZQ (2012) Effects of different starch sources on Bacillus spp. in intestinal tract and expression of intestinal development related genes of weanling piglets. Mol Biol Rep 39(2):1869–1876. doi:10.1007/s11033-011-0932-x
Hong HA, le Duc H, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29(4):813–835. doi:10.1016/j.femsre.2004.12.001
Hu Y, Dun Y, Li S, Zhao S, Peng N, Liang Y (2014) Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian Australas J Anim Sci 27(8):1131
Macfarlane GT, Cummings JH (1999) Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ 318(7189):999–1003. doi:10.1136/bmj.318.7189.999
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130(2S Suppl):396S–402S
Sangild PT (2006) Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med 231(11):1695–1711
Che L, Thymann T, Bering SB, LEH-L I, D’Inca R, Zhang K, Sangild PT (2010) IUGR does not predispose to necrotizing enterocolitis or compromise postnatal intestinal adaptation in preterm pigs. Pediatr Res 67(1):54–59. doi:10.1203/PDR.0b013e3181c1b15e
Zhang Y, Chen DW, Yu B, He J, Yu J, Mao XB, Wang JX, Luo JQ, Huang ZQ, Cheng GX, Zheng P (2015) Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J Anim Sci 93(6):2967–2976. doi:10.2527/jas.2014-8820
Chen Y, Chen D, Tian G, He J, Mao X, Mao Q, Yu B (2012) Dietary arginine supplementation alleviates immune challenge induced by Salmonella enterica serovar Choleraesuis bacterin potentially through the Toll-like receptor 4-myeloid differentiation factor 88 signalling pathway in weaned piglets. Br J Nutr 108(6):1069–1076. doi:10.1017/S0007114511006350
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Zhang B, Guo Y (2009) Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr 102(5):687–693. doi:10.1017/S0007114509289033
Garite TJ, Clark R, Thorp JA (2004) Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol 191(2):481–487
Mandruzzato G, Antsaklis A, Botet F, Chervenak FA, Figueras F, Grunebaum A, Puerto B, Skupski D, Stanojevic M, WAPM (2008) Intrauterine restriction (IUGR). J Perinat Med 36(4):277–281. doi:10.1515/JPM.2008.050
Musa H, Wu S, Zhu C, Seri H, Zhu G (2009) The potential benefits of probiotics in animal production and health. J Anim Vet Adv 8(2):313–321
Rehfeldt C, Kuhn G (2006) Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J Anim Sci 84(Suppl):E113–E123
Morise A, Seve B, Mace K, Magliola C, Le Huerou-Luron I, Louveau I (2011) Growth, body composition and hormonal status of growing pigs exhibiting a normal or small weight at birth and exposed to a neonatal diet enriched in proteins. Br J Nutr 105(10):1471–1479. doi:10.1017/S0007114510005386
Alvarenga AL, Chiarini-Garcia H, Cardeal PC, Moreira LP, Foxcroft GR, Fontes DO, Almeida FR (2013) Intra-uterine growth retardation affects birthweight and postnatal development in pigs, impairing muscle accretion, duodenal mucosa morphology and carcass traits. Reprod Fertil Dev 25(2):387–395. doi:10.1071/RD12021
Chen Y, Min B, Cho J, Kwon O, Son K, Kim H, Kim I (2006) Effects of dietary Bacillus-based probiotic on growth performance, nutrients digestibility, blood characteristics and fecal noxious gas content in finishing pigs. Asian Australas J Anim Sci 19(4):587
Lee SH, Ingale SL, Kim JS, Kim KH, Lokhande A, Kim EK, Kwon IK, Kim YH, Chae BJ (2014) Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim Feed Sci Technol 188:102–110. doi:10.1016/j.anifeedsci.2013.12.001
Fanca-Berthon P, Michel C, Pagniez A, Rival M, Van Seuningen I, Darmaun D, Hoebler C (2009) Intrauterine growth restriction alters postnatal colonic barrier maturation in rats. Pediatr Res 66(1):47–52. doi:10.1203/PDR.0b013e3181a2047e
Amdi C, Krogh U, Flummer C, Oksbjerg N, Hansen CF, Theil PK (2013) Intrauterine growth restricted piglets defined by their head shape ingest insufficient amounts of colostrum. J Anim Sci 91(12):5605–5613. doi:10.2527/jas.2013-6824
Hales J, Moustsen VA, Nielsen MB, Hansen CF (2013) Individual physical characteristics of neonatal piglets affect preweaning survival of piglets born in a noncrated system. J Anim Sci 91(10):4991–5003. doi:10.2527/jas.2012-5740
Garite TJ, Clark R, Thorp JA (2004) Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol 191(2):481–487. doi:10.1016/j.ajog.2004.01.036
Dong L, Zhong X, He J, Zhang L, Bai K, Xu W, Wang T, Huang X (2015) Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin Nutr. doi:10.1016/j.clnu.2015.03.002
Xu RJ, Mellor DJ, Birtles MJ, Reynolds GW, Simpson HV (1994) Impact of intrauterine growth retardation on the gastrointestinal tract and the pancreas in newborn pigs. J Pediatr Gastroenterol Nutr 18(2):231–240
Montagne L, Pluske J, Hampson D (2003) A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol 108(1):95–117
Kelly D, Coutts AG (2000) Early nutrition and the development of immune function in the neonate. Proc Nutr Soc 59(2):177–185
Amu S, Hahn-Zoric M, Malik A, Ashraf R, Zaman S, Kjellmer I, Hagberg H, Padyukov L, Hanson LÅ (2006) Cytokines in the placenta of Pakistani newborns with and without intrauterine growth retardation. Pediatr Res 59(2):254–258
Briana DD, Liosi S, Gourgiotis D, Boutsikou M, Marmarinos A, Baka S, Hassiakos D, Malamitsi-Puchner A (2012) Fetal concentrations of the growth factors TGF-alpha and TGF-beta1 in relation to normal and restricted fetal growth at term. Cytokine 60(1):157–161. doi:10.1016/j.cyto.2012.06.005
Contreras YM, Yu X, Hale MA, Callaway CW, Bareyan D, McKnight RA, Joss-Moore LA, Enioutina EY, Lane RH (2011) Intrauterine growth restriction alters T-lymphocyte cell number and dual specificity phosphatase 1 levels in the thymus of newborn and juvenile rats. Pediatr Res 70(2):123–129. doi:10.1203/PDR.0b013e31821f6e75
Morrison JL (2008) Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35(7):730–743. doi:10.1111/j.1440-1681.2008.04975.x
Wang S-P, Yang L, Tang X-S, Cai L-C, Liu G, Kong X-F, Blachier F, Yin Y-L (2011) Dietary supplementation with high-dose Bacillus subtilis or Lactobacillus reuteri modulates cellular and humoral immunities and improves performance in weaned piglets. J Food Agric Environ 9(2):181–187
Suarez-Souto MA, Lara-Padilla E, Reyna-Garfias H, Viloria M, Lopez-Sanchez P, Rivera-Aguilar V, Miliar-Garcia A, Kormanovski A, Dominguez-Lopez ML, Campos-Rodriguez R (2012) Caloric restriction modifies both innate and adaptive immunity in the mouse small intestine. J Physiol Biochem 68(2):163–173. doi:10.1007/s13105-011-0128-9
Newburg DS, Walker WA (2007) Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 61(1):2–8. doi:10.1203/01.pdr.0000250274.68571.18
Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44(1):26–46. doi:10.1007/s00535-008-2296-0
Lalles JP (2012) Long term effects of pre- and early postnatal nutrition and environment on the gut. J Anim Sci 90(Suppl 4):421–429. doi:10.2527/jas.53904
Elkouby-Naor L, Ben-Yosef T (2010) Functions of claudin tight junction proteins and their complex interactions in various physiological systems. Int Rev Cell Mol Biol 279:1–32. doi:10.1016/S1937-6448(10)79001-8
Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF (2011) Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal 15(5):1195–1219. doi:10.1089/ars.2010.3542
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (31101727); the International Cooperation in Science and Technology Project of Sichuan Province (2014HH0034); the Program for Changjiang Scholars and Innovative Research Team in University (IRT13083); and the Natural Science Foundation of Sichuan Province (12ZA110).
Author contributions
The authors’ contributions are as follows: L. H. and L. C. designed the study; L. H., X. P., C. Y., Y. L. and Q. X. carried out the study; L. H., L. Q., Z. F., Y. L., S. X., B. F., J. L. and D. W. performed the analysis and analysed the data; L. H. wrote the paper; and L. C. and H. C. made some modifications in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hu, L., Peng, X., Chen, H. et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur J Nutr 56, 1753–1765 (2017). https://doi.org/10.1007/s00394-016-1223-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1223-z