Abstract
Purpose
ω3 and ω6 fatty acids (FA) may have divergent effects on the development of obesity. We examined the association of baseline erythrocyte ω3 and ω6 FA composition with body weight change and the risk of becoming overweight or obese in the Women’s Health Study (WHS) participants.
Methods
We identified 534 women who had baseline erythrocyte FA measured and a baseline body mass index (BMI) of 18.5–<25 kg/m2. Body weight was updated at a total of six time points during follow-up.
Results
Weight gain during a mean of 10.4-year follow-up increased with increasing quartiles of baseline erythrocyte cis ω6 FA, ω6/ω3 ratio, and trans FA while decreased with increasing cis ω3 FA. After multivariable adjustment including total energy intake and physical activity, the weight gain (kg) in the highest versus the lowest quartile was 3.08 versus 2.32 for erythrocyte cis ω6 FA (p trend 0.04), 2.07 versus 2.92 for cis ω3 FA (p trend 0.08), 2.93 versus 2.05 for ω6/ω3 ratio (p trend 0.046), and 3.03 versus 2.27 for trans FA (p trend 0.06). Among individual FA, the associations were significant for 18:2ω6, 18:3ω6, and trans 18:1 and marginally significant for 20:3ω6 and trans 18:2. The risk of becoming overweight or obese (defined as BMI ≥25 kg/m2 at any follow-up time point) increased across increasing ω6/ω3 ratio (multivariable model p trend 0.04).
Conclusions
In this prospective study, we found suggestive evidence that erythrocyte cis ω6 FA may be positively associated, and cis ω3 FA inversely associated with weight gain in initially normal-weight women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High fat intake has been implicated in the development of obesity [1, 2]. However, evidence from prospective cohort studies [3–6] and randomized trials [7–10] linking total fat intake to body weight gain remains weak and inconsistent. In the past decades, total fat and saturated fat intake (as % of calories) in the typical Western diet has continuously fallen [11, 12], while the intake of ω6 fatty acid (FA) increased and ω3 FA decreased, resulting in a large increase in the ω6/ω3 ratio from 1:1 [13, 14] to 10:1 [14] or even higher [15]. This change in dietary FA composition parallels an alarming increase in the prevalence of overweight and obesity [15].
ω3 and ω6 classes of polyunsaturated FA (PUFA) are distinguished based on location of the first double bond. Parent FA of ω3 and ω6 subclasses, α-linolenic acid (ALA, 18:3ω3) and linoleic acid (LA, 18:2ω6), respectively, are essential for humans because they cannot be synthesized and must be obtained from diet. Longer-chain FA can be desaturated and elongated, to a very low extent, from parent FA of the same class. No conversion can occur between ω3 and ω6 subclasses of PUFA, making them metabolically distinct. Due to their similar chemical structure, ω3 and ω6 FA compete for incorporation into target tissues and metabolism by common enzymes, which may lead to opposing health effects [16]. Intake of ω3 FA, particularly long-chain ω3 FA such as eicosapentaenoic acid (EPA, C20:5ω3) and docosahexaenoic acid (DHA, C22:6ω3), has demonstrated beneficial effects on multiple cardiometabolic outcomes including hypertension, diabetes, dyslipidemia, and cardiovascular disease (CVD) [17]. The effect of ω6 FA is less clear, with evidence suggesting possible harm [18].
Experimental studies have suggested that ω3 and ω6 FA may elicit divergent effects on body fat gain through mechanisms of adipogenesis [19], lipid homeostasis [20, 21], brain–gut–adipose axis [22], and systemic inflammation [23]. Epidemiologic studies on PUFA intake and either weight gain or the development of obesity are limited, mainly using self-reports from food frequency questionnaires (FFQs) to assess PUFA intake without separate analysis on ω3 and ω6 subclasses [24, 25]. We identified a subgroup of Women’s Health Study (WHS) participants who had erythrocyte FA measured as a biomarker of dietary FA and conducted prospective analyses to examine the association of baseline erythrocyte ω3 FA, ω6 FA, ω6/ω3 ratio, and trans FA with the longitudinal changes in body weight and the risk of becoming overweight or obese during a mean of 10.4-year follow-up.
Subjects and methods
Study population
The WHS was a randomized, double-blind, placebo-controlled, 2 × 2 factorial trial evaluating the risks and benefits of low-dose aspirin and vitamin E in the primary prevention of CVD and cancer [26, 27]. A third component, β-carotene, was initially included in the trial but terminated after a median treatment of 2.1 years [28]. From 1992 to 1995, 39,876 female US health professionals, aged ≥39 years and free from CVD and cancer (except non-melanoma skin cancer), were randomized into the WHS. Baseline blood samples were collected from 28,345 participants and stored in liquid nitrogen freezers. During the course of the trial, the participants received study agents and follow-up questionnaires by mail and reported the occurrence of major cardiovascular and cancer end points and risk factor information every 6 months for the first year and annually thereafter. Blinded treatment of aspirin and vitamin E ended as scheduled on March 31, 2004, after which the cohort follow-up continued for willing women as an observational study. Written informed consent was obtained from all participants. The study was approved by the institutional review board at Brigham and Women’s Hospital, Boston, MA.
We previously conducted a study of 516 incident hypertension cases and 516 matched controls nested within the WHS. Hypertension was defined by meeting any of the following four criteria: a physician diagnosis of hypertension, self-reported systolic blood pressure (BP) ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive treatment. Incident hypertension was identified as women who had no hypertension at baseline but reported newly developed hypertension during follow-up. For each incident hypertension case, one control was randomly selected from women who remained free of hypertension until the case was identified. Each case and the respective control were matched on age (±1 year) and follow-up time (±3 months). The FA composition in baseline erythrocyte membrane was measured in all cases and controls.
For the current study, we included 551 women in this nested case–control study who reported a baseline body mass index (BMI) ranging from 18.5 to <25 kg/m2. We then excluded 14 women who insufficiently completed the FFQ, defined as >70 items left blank, or an implausible mean energy intake of <600 or ≥3500 kcal/d. We also excluded two women with baseline diabetes or pre-randomization CVD or cancer, and one woman who did not update her weight during follow-up. As a result, 534 women remained for analysis.
Blood assays of erythrocyte fatty acid composition
The FA profile in erythrocyte membrane was measured at the Department of Laboratory Medicine and Pathology, University of Minnesota, using the method by Cao et al. [29]. Previous studies have shown that long-term storage of frozen blood samples did not influence FA profiles [30, 31]. After thawing and adding 50 μL of 17:0 internal standard, FA were extracted from erythrocyte membranes with a mixture of chloroform and methanol (2:1, v/v), dissolved in heptane, and injected onto a capillary Varian CP7420 100-m column with a Hewlett Packard 5890 gas chromatograph (GC) equipped with a HP6890A autosampler. The GC was configured for a single capillary column with a flame ionization detector and interfaced with HP chemstation software. Adequate separation of FA methyl esters was obtained over a 50-min period with an initial temperature of 190 °C followed by subsequent temperature gradually increased to 240 °C. FA from 12:0 through 24:1ω9 were separated, identified, and expressed as percent of total FA. FA subtypes, including ω3 FA, ω6 FA, and trans FA, were calculated as sum of the respective individual FA. The ratio of ω6 to ω3 FA was calculated. The coefficients of variation on 51 blind triplicates from 17 individual samples were 5.1 % for ω3 FA, 3.0 % for ω6 FA, and 3.6 % for trans FA.
Ascertainment of body weight change and incident case of becoming overweight or obese
On the baseline questionnaire, WHS participants reported height and weight. Every 6 months during the first year and annually thereafter, participants completed mailed follow-up questionnaires, with weight updated in the 2-, 3-, 5-, 6-, 9-year, and at the end of intervention. BMI was calculated as weight (kg) divided by the square of height (m2) at a total of seven time points (baseline plus six follow-up), and then categorized as <25 kg/m2 (normal weight) and ≥25 kg/m2 (overweight or obese). Women who had normal BMI at baseline but subsequently reported a BMI ≥25 kg/m2 at any follow-up time point were defined as incident cases that became overweight or obese. For each case, the ‘time-of-event’ was estimated as the time point when BMI crossed the cutoff for overweight or obese (i.e., 25 kg/m2) through a regression line from the last-reported BMI of <25 kg/m2 to the first-reported BMI of ≥25 kg/m2 over time. Women who did not become overweight or obese were censored on the last day when a BMI <25 kg/m2 was reported. Women who developed intermediate diabetes, the management of which often involves weight control, were censored on the day of diabetes diagnosis. In a similar population of female health professionals, self-reported weights were highly correlated with clinic measured weights (Pearson r = 0.97) [32]. Studies across different populations also have found that self-reported overweight and obesity status is accurate [33, 34].
Other baseline covariates
On the baseline questionnaire, women also provided self-reports of age, smoking status, alcohol use, recreational exercise, menopausal status, postmenopausal hormone use, multivitamin use, history of diabetes, and history of hypercholesterolemia. Diet was assessed from a 131-item validated semiquantitative FFQ. A commonly used unit or portion size was specified for each food item, and participants reported how often they had consumed that amount, on average, during the previous year. Nutrient intake including FA was computed by multiplying the intake frequency of each unit of food by the nutrient content of the specified portion size according to food composition tables from the US Department of Agriculture and Harvard School of Public Health database sources. The FFQ used in the WHS has demonstrated reasonable validity and reproducibility as a measure of long-term dietary intake [35]. We also used the FFQ to calculate the Alternative Healthy Eating Index-2010 (AHEI-2010) scores. The original HEI was based on the Dietary Guidelines for Americans [36]. The AHEI-2010 further incorporates new knowledge on foods and nutrients predictive of risk of chronic disease [37] and includes greater intake of vegetables (excluding potatoes), fruits (excluding juices), whole grains, nuts, legumes, vegetable proteins, long-chain ω3 FA, other PUFA (excluding long-chain ω3 FA); lower intake of sugar-sweetened beverages or fruit juices, red or processed meats, trans fats, and sodium; and moderate intake of alcohol. Each AHEI-2010 component was scored from 0 (worst) to 10 (best) according to component-specific criteria reflecting either the current dietary guidelines or associations reported in the literature. Total AHEI-2010 scores range from 0 (non-adherence) to 110 (perfect adherence). The rationale for component selection and methodology to derive the AHEI-2010 score has been described previously [37].
Statistical analysis
Statistical analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC, USA). All statistical tests were two-sided, with p < 0.05 considered statistically significant and p > 0.05 but <0.10 marginally significant. The correlation of erythrocyte FA with dietary FA was assessed by Spearman r 2. Erythrocyte PUFA composition was first compared between women who became overweight or obese and those who maintained normal weight, along with major lifestyle and dietary factors. We then divided erythrocyte FA into quartiles based on their distribution in controls. We calculated body weight change from baseline to each follow-up time point and used PROC MIXED models with an unstructured covariance matrix for repeated measures to compare the longitudinal changes in body weight across the quartiles of erythrocyte FA. For women who reported a BMI ≥25 kg/m2 at any follow-up time point, we assigned the body weight at subsequent follow-up as missing, due to the concern that lifestyle and diet may change in response to the weight gain. Basic models controlled for age, race, randomized treatment, and hypertension case–control status. Multivariable models additionally adjusted for total energy intake, physical activity, smoking, alcohol use, menopausal status, postmenopausal hormone use, multivitamin use, history of hypercholesterolemia, AHEI, and energy-adjusted intake of protein, carbohydrates, and cholesterol. Adjustment for baseline BMI attenuated the magnitude, but did not change direction, of the associations. Because adjustment for baseline status may induce biased statistical association in analysis of change [38], we did not show the results with baseline BMI adjustment. We further used Cox regression to calculate hazard ratios (HRs) and 95 % confidence intervals (CIs) of becoming overweight or obese according to the quartiles of erythrocyte FA. Linear trend across the increasing quartiles was tested using the median value in each quartile as an ordinal variable. To evaluate the independent associations for ω3 and ω6 FA, we included ω3 and ω6 FA in the same model and also examined the joint categories of ω3 and ω6 FA that were each dichotomized at the median. Finally, we stratified all analyses by baseline age (<55 vs. ≥55 years) and race/ethnicity (whites vs. non-whites) because of the variations in body composition, and by baseline BMI (18.5–<23, 23–<25 kg/m2) out of concern for misclassification of borderline overweight. Interactions were tested using Wald Chi-square tests.
Results
Women included in the current study had a mean ± SD baseline age of 53.8 ± 6.4 years, BMI of 22.4 ± 1.6 kg/m2, 27.5 % of non-whites (8.9 % of African Americans and 17.7 % of Asian Americans), and were free of CVD, cancer, diabetes, and hypertension at baseline. The overall mean of erythrocyte FA (presented as percent of total FA) was 6.2 % for cis ω3 FA, 27.0 % for cis ω6 FA, and 2.0 % for trans FA. The corresponding mean dietary FA (presented as percent of total fat intake) were 2.7 % for cis ω3 FA, 19.2 % for cis ω6 FA, and 3.7 % for trans FA. Spearman correlation coefficients between erythrocyte and dietary FA ranged from 0.073 (p = 0.09, for 22:5ω3) to 0.41 (p < 0.0001, for 22:6ω3). Among 534 women who initially had normal BMI, 186 women became overweight or obese during a mean of 10.4-year follow-up. Compared with women who maintained normal BMI, those who became overweight or obese were younger and had higher baseline BMI (Table 1). Smoking status, alcohol use, total energy intake, exercise, postmenopausal hormone use, and multivitamin use did not differ according to whether or not the woman became overweight or obese. When comparing erythrocyte FA in women who became overweight or obese with those who did not, no significant difference in cis ω3 FA, cis ω6 FA, ω6/ω3 ratio, and trans FA was found (Table 1).
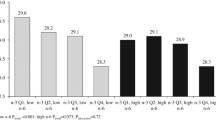
The mean ± SD of body weight change from baseline to 2, 3, 5, 6, and ≥9 years of follow-up in the 534 women was 1.23 ± 3.10, 1.17 ± 2.92, 1.34 ± 3.41, 1.52 ± 3.29, and 1.88 ± 4.21 kg, respectively. In the model that adjusted only for age, race, and randomized treatment, longitudinal weight gain during the overall follow-up across increasing quartiles of baseline erythrocyte PUFA was 2.59, 2.14, 2.29, and 1.62 kg (p trend 0.04) for cis ω3 FA; 1.95, 1.51, 2.44, and 2.63 kg (p trend 0.06) for cis ω6 FA; and 1.58, 2.20, 2.39, and 2.58 kg (p trend 0.02) for ω6/ω3 ratio (Table 2). After additional adjustment for lifestyle and dietary factors including total energy intake and exercise, the associations for cis ω6 FA and ω6/ω3 ratio were statistically significant, while the association for cis ω3 FA was marginally significant. For individual FA, LA (18:2ω6), γ-linoleic acid (GLA, 18:3ω6), and dihomo-γ-linolenic acid (DGLA, 20:3ω6) were each positively associated with weight gain in the basic model. In the multivariable model, the association remained significant for LA and GLA and was marginally significant for DGLA. Total trans FA, trans 18:1, and trans 18:2 were all significantly and positively associated with weight gain in the basic model, but only the association for trans 18:1 remained significant after multivariable adjustment.
The risk of becoming overweight or obese did not significantly differ by quartiles of baseline erythrocyte cis ω3 FA and cis ω6 FA, but significantly increased across increasing quartiles of ω6/ω3 ratio (HR [95 % CI] 1.00, 1.37 [0.84, 2.22], 1.57 [1.01, 2.43], and 1.58 [1.01, 2.46], respectively; p trend 0.046) (Table 3). Additional adjustment for lifestyle and dietary factors in the multivariable model did not attenuate this association. The associations for ω3 and ω6 FA did not change when both were simultaneously included in the same model. There was also no interaction between ω3 and ω6 FA dichotomized at the median in association with weight gain or the risk of becoming overweight or obese. Erythrocyte trans FA was not associated with the risk of becoming overweight or obese. None of the associations differed by age, race/ethnicity, or baseline BMI categories (data not shown).
Discussion
In this prospective analysis, we found suggestive evidence that baseline erythrocyte cis ω3 FA is inversely associated and cis ω6 FA is positively associated with longitudinal weight gain in initially normal-weight women. The associations remained borderline significant after controlling for potential confounding factors, including total energy intake and physical activity. Erythrocyte trans FA also tended to be positively associated with weight gain. The associations of erythrocyte cis ω3 and ω6 FA and trans FA with the risk of becoming overweight or obese followed similar patterns, with a lower magnitude of effect.
Our study is the first to prospectively examine PUFA and trans FA in erythrocyte membrane in relation to the weight gain and the risk of becoming overweight or obese. In epidemiologic studies, FA in plasma lipid (reflecting intake in weeks) [39, 40] and erythrocyte membrane (reflecting intake in months) [41] have been measured as biomarkers of dietary fat. Our study findings suggest that dietary ω3 and ω6 FA may have divergent effects in the development of obesity, and FA composition, in addition to absolute amount of intake, may be important for the prevention of obesity. ω3 and ω6 FA compete for common metabolic enzymes and incorporation into plasma lipids and cell membranes. In the past half century, ω6/ω3 ratio in US diet has substantially increased [14]. Some research groups recommend a reduction in ω6 FA intake to lower ω6/ω3 ratio [42, 43]. However, American Heart Association suggests ω6 FA intake comprising at least 5–10 % of total energy [44]. The optimal intake of ω3 and ω6 FA and the target ω6/ω3 ratio remain to be determined.
ω3 and ω6 FA may elicit contrasting effects in adipogenesis [19] and lipid homeostasis [20, 21]. Metabolites of arachidonic acid (AA, 20:4ω6) play important roles in the terminal differentiation of pre-adipocyte to mature adipocyte [45]. Such effect can be inhibited by ω3 FA at multiple steps [46–49]. ω6 FA also increase cellular triglyceride content by increasing membrane permeability [50], while ω3 FA reduce fat deposition in adipose tissues by suppressing lipogenic enzymes and increasing β-oxidation [51]. In addition, ω3 and ω6 FA differentially modulate the brain–gut–adipose axis [22] and the inflammatory properties of downstream eicosanoids, which ultimately affect pre-adipocyte differentiation and fat mass growth [52]. Epidemiologic studies on dietary FA and changes in body weight and body fat remain limited, with only two known prospective studies [24, 25]. Both studies used self-reported FFQs to assess dietary fat intake and did not examine subtype or individual PUFA. Some intervention studies showed that ω3 FA supplementation reduced body weight and obesity in lean [53], overweight [54, 55], and obese [56] individuals. Comparable data on ω6 FA are lacking. One small trial in 17 healthy, normal-weight men and women found that a 10-week diet intervention to improve ω6/ω3 ratio, with no change in intake of total energy and other macronutrients, did not change body weight, waist/hip ratio, and fat mass but significantly increased plasma adiponectin and decreased plasma inflammatory markers [57]. Large-scale, controlled trials with longer duration are needed to further elucidate the effects of dietary PUFA composition change on obesity and obesity-related morbidities.
trans FA are unsaturated FA with at least one double bond in trans configuration [58]. The trans bonds alter not only the physical properties but also the biological effects of the unsaturated FA. Studies in rats showed that trans FA consumption raised hepatic fat contents [59]. In monkeys, trans FA diet resulted in larger weight gain compared with cis monounsaturated FA diet, and the differential weight gain was largely attributed to higher visceral fat accumulation [60]. The association of trans FA intake with weight gain in epidemiologic studies has been weak to date [24]. The similarly weak associations observed in the current study may be partly due to the fact that WHS participants were health professionals with largely favorable dietary and behavior patterns including relatively low trans fat intake.
In our study, the direction of associations for individual FA was generally consistent with their respective classes, but the magnitude of associations varied. After multivariable adjustment, significant relations with weight gain were found only for DGLA (20:3ω6), LA (18:2ω6), and GLA (18:3ω6) among ω6 FA; EPA (20:5ω3) among ω3 FA; and trans 18:1 among trans FA. The variations by individual FA may be due to unknown and uncontrolled factors involved in the conversion and metabolism of each FA, and should be interpreted cautiously given the multiple comparisons. Moreover, the current study included only women who had normal BMI at baseline to minimize potential confounding and address the risk of becoming overweight or obese. To further evaluate the impact of baseline BMI on the results, we stratified analyses by baseline BMI levels (18.5–≤23, 23–≤25 kg/m2) and also included women who were already overweight or obese at baseline (baseline BMI ≥ 25 kg/m2) in sensitivity analyses. Similar patterns of associations were found in these additional analyses (data not shown).
Several limitations of the current study deserve comments. First, self-reported body weight, though showing excellent validity in health professionals [32], remains subject to random misclassification and may lead to underestimation of the true associations. Second, our study is limited in a single baseline measurement of erythrocyte FA without assessment of any change over time. Third, our study used a convenient sample from a previous study, but was not a priori designed, to test our hypotheses. Nevertheless, we do not anticipate substantial bias to our reported associations due to how this study population was selected. Fourth, although we have adjusted for a broad range of dietary, lifestyle, and clinical factors in analysis, residual confounding cannot be ruled out as in all observational studies. Since adjustment for baseline levels of body weight to control for residual confounding may also induce biased statistical association with the change in body weight [38], we have examined the associations with and without adjustment for baseline BMI. Fifth, because the WHS did not collect data on waist and hip circumference at baseline, we cannot assess abdominal obesity. Finally, WHS participants were predominantly white female health professionals, which limited the generalizability of our study results to other populations.
In conclusion, this prospective study provided suggestive evidence that erythrocyte cis ω3 FA may be inversely associated, while cis ω6 FA, ω6/ω3 ratio, and trans FA positively associated, with longitudinal weight gain. Future studies are needed to further elucidate the role of dietary FA composition in the development of obesity and the underlying mechanisms.
Abbreviations
- FA:
-
Fatty acids
- PUFA:
-
Polyunsaturated fatty acids
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- WHS:
-
Women’s Health Study
- BP:
-
Blood pressure
- FFQ:
-
Food frequency questionnaire
- GC:
-
Gas chromatograph
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- AA:
-
Arachidonic acid
- PPAR:
-
Peroxisome proliferators-activated receptor
- NHS:
-
Nurses’ Health Study
- WHI:
-
Women’s Health Initiative
- AHA:
-
American Heart Association
References
Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD (2012) Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ 345:e7666. doi:10.1136/bmj.e7666
Tremblay A (2004) Dietary fat and body weight set point. Nutr Rev 62:S75–S77
Carmichael HE, Swinburn BA, Wilson MR (1998) Lower fat intake as a predictor of initial and sustained weight loss in obese subjects consuming an otherwise ad libitum diet. J Am Diet Assoc 98:35–39
Heitmann BL, Lissner L, Sorensen TI, Bengtsson C (1995) Dietary fat intake and weight gain in women genetically predisposed for obesity. Am J Clin Nutr 61:1213–1217
Lissner L, Heitmann BL, Bengtsson C (1997) Low-fat diets may prevent weight gain in sedentary women: prospective observations from the population study of women in Gothenburg, Sweden. Obes Res 5:43–48
Sherwood NE, Jeffery RW, French SA, Hannan PJ, Murray DM (2000) Predictors of weight gain in the pound of prevention study. Int J Obes Relat Metab Disord 24:395–403
Sheppard L, Kristal AR, Kushi LH (1991) Weight loss in women participating in a randomized trial of low-fat diets. Am J Clin Nutr 54:821–828
Westerterp-Plantenga MS, Wijckmans-Duijsens NE, Verboeket-van de Venne WP, de Graaf K, van het Hof KH, Weststrate JA (1998) Energy intake and body weight effects of six months reduced or full fat diets, as a function of dietary restraint. Int J Obes Relat Metab Disord 22:14–22
Rock CL, Thomson C, Caan BJ, Flatt SW, Newman V, Ritenbaugh C, Marshall JR, Hollenbach KA, Stefanick ML, Pierce JP (2001) Reduction in fat intake is not associated with weight loss in most women after breast cancer diagnosis: evidence from a randomized controlled trial. Cancer 91:25–34
Brehm BJ, Spang SE, Lattin BL, Seeley RJ, Daniels SR, D’Alessio DA (2005) The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets. J Clin Endocrinol Metab 90:1475–1482
Troiano RP, Briefel RR, Carroll MD, Bialostosky K (2000) Energy and fat intakes of children and adolescents in the united states: data from the national health and nutrition examination surveys. Am J Clinical Nutr 72:1343S–1353S
Swan G (2004) Findings from the latest national diet and nutrition survey. Proc Nutr Soc 63:505–512
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR (2011) Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 93:950–962. doi:10.3945/ajcn.110.006643
Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P (2006) Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res 45:203–236. doi:10.1016/j.plipres.2006.01.003
Simopoulos AP (1999) Essential fatty acids in health and chronic disease. Am Clin Nutr 70:560S–569S
Mozaffarian D, Wu JH (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58:2047–2067. doi:10.1016/j.jacc.2011.06.063
Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR (2013) Use of dietary linoleic acid for secondary prevention of coronary heart disease and death evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 346:e8707
Amri EZ, Ailhaud G, Grimaldi PA (1994) Fatty acids as signal transducing molecules: involvement in the differentiation of preadipose to adipose cells. J Lipid Res 35:930–937
Jump DB, Clarke SD, Thelen A, Liimatta M (1994) Coordinate regulation of glycolytic and lipogenic gene expression by polyunsaturated fatty acids. J Lipid Res 35:1076–1084
Clarke SD, Jump D (1997) Polyunsaturated fatty acids regulate lipogenic and peroxisomal gene expression by independent mechanisms. Prostaglandins Leukot Essent Fatty Acids 57:65–69
Schwinkendorf DR, Tsatsos NG, Gosnell BA, Mashek DG (2011) Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int J Obes 35:336–344. doi:10.1038/ijo.2010.159
James MJ, Gibson RA, Cleland LG (2000) Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 71:343S–348S
Field AE, Willett WC, Lissner L, Colditz GA (2007) Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity 15:967–976
Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz G, Willett W, Rimm E (2003) Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr 78:719–727
Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE (2005) Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA 294:47–55
Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE (2005) Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA 294:56–65
Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH (1999) Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst 91:2102–2106
Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY (2006) Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem 52:2265–2272
Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH (2010) Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res 51:2826–2832. doi:10.1194/jlr.D007534
Zeleniuch-Jacquotte A, Chajes V, Van Kappel AL, Riboli E, Toniolo P (2000) Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr 54:367–372
Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC (1990) Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1:466–473
Nyholm M, Gullberg B, Merlo J, Lundqvist-Persson C, Rastam L, Lindblad U (2007) The validity of obesity based on self-reported weight and height: implications for population studies. Obesity 15:197–208. doi:10.1038/oby.2007.536
Gillum RF, Sempos CT (2005) Ethnic variation in validity of classification of overweight and obesity using self-reported weight and height in American women and men: the Third National Health and Nutrition Examination Survey. Nutr J 4:27
Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE (1985) Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122:51–65
Guenther PM, Reedy J, Krebs-Smith SM (2008) Development of the healthy eating index-2005. J Am Diet Assoc 108:1896–1901. doi:10.1016/j.jada.2008.08.016
Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC (2012) Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142:1009–1018. doi:10.3945/jn.111.157222
Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM (2005) When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 162:267–278. doi:10.1093/aje/kwi187
Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M (1997) Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38:2012–2022
Subbaiah PV, Kaufman D, Bagdade JD (1993) Incorporation of dietary n-3 fatty acids into molecular species of phosphatidyl choline and cholesteryl ester in normal human plasma. Am J Clin Nutr 58:360–368
Skeaff CM, Hodson L, McKenzie JE (2006) Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr 136:565–569
Simopoulos AP, Leaf A, Salem N Jr (1999) Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab 43:127–130
Hamazaki T, Okuyama H (2003) The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet 92:109–132
Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F (2009) Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 119:902–907. doi:10.1161/CIRCULATIONAHA.108.191627
Gaillard D, Negrel R, Lagarde M, Ailhaud G (1989) Requirement and role of arachidonic acid in the differentiation of pre-adipose cells. Biochem J 257:389–397
Corey EJ, Shih C, Cashman JR (1983) Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesis. Proc Natl Acad Sci USA 80:3581–3584
Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, Bourcier T, Weksler BB, De Caterina R (2006) The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A 103:15184–15189
Ringbom T, Huss U, Stenholm A, Flock S, Skattebol L, Perera P, Bohlin L (2001) Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod 64:745–749
Mirnikjoo B, Brown SE, Kim HF, Marangell LB, Sweatt JD, Weeber EJ (2001) Protein kinase inhibition by omega-3 fatty acids. J Biol Chem 276:10888–10896
Hennig B, Watkins BA (1989) Linoleic acid and linolenic acid: effect on permeability properties of cultured endothelial cell monolayers. Am J Clin Nutr 49:301–305
Ukropec J, Reseland JE, Gasperikova D, Demcakova E, Madsen L, Berge RK, Rustan AC, Klimes I, Drevon CA, Sebokova E (2003) The hypotriglyceridemic effect of dietary n-3 FA is associated with increased beta-oxidation and reduced leptin expression. Lipids 38:1023–1029
Lepperdinger G (2011) Inflammation and mesenchymal stem cell aging. Curr Opin Immunol 23:518–524. doi:10.1016/j.coi.2011.05.007
Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F (1997) Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord 21:637–643
Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA (2006) Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes 30:1535–1544
Thorsdottir I, Tomasson H, Gunnarsdottir I, Gisladottir E, Kiely M, Parra MD, Bandarra NM, Schaafsma G, Martinez JA (2007) Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int J Obes 31:1560–1566. doi:10.1038/sj.ijo.0803643
Kunesova M, Braunerova R, Hlavaty P, Tvrzicka E, Stankova B, Skrha J, Hilgertova J, Hill M, Kopecky J, Wagenknecht M, Hainer V, Matoulek M, Parizkova J, Zak A, Svacina S (2006) The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol Res 55:63–72
Guebre-Egziabher F, Rabasa-Lhoret R, Bonnet F, Bastard JP, Desage M, Skilton MR, Vidal H, Laville M (2008) Nutritional intervention to reduce the n-6/n-3 fatty acid ratio increases adiponectin concentration and fatty acid oxidation in healthy subjects. Eur J Clin Nutr 62:1287–1293. doi:10.1038/sj.ejcn.1602857
Teegala SM, Willett WC, Mozaffarian D (2009) Consumption and health effects of trans fatty acids: a review. J AOAC Int 92:1250–1257
Axen KV, Dikeakos A, Sclafani A (2003) High dietary fat promotes syndrome X in nonobese rats. J Nutr 133:2244–2249
Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, Rudel LL (2007) Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity 15:1675–1684
Acknowledgments
We are indebted to the 39,876 participants in the Women’s Health Study for their dedicated and conscientious collaboration, and to the entire staff of the Women’s Health Study for their assistance in designing and conducting the trial. This study was supported by a national scientist development grant funded by American Heart Association (0735390N) and research grants CA047988, HL043851, and HL080467 from the National Institutes of Health, Bethesda, MD. Dr. Wang was supported by Grant HL095649 from the National Heart, Lung, and Blood Institutes. These grants provided funding for study conduction, data collection, data analysis, and manuscript writing.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Manson, J.E., Rautiainen, S. et al. A prospective study of erythrocyte polyunsaturated fatty acid, weight gain, and risk of becoming overweight or obese in middle-aged and older women. Eur J Nutr 55, 687–697 (2016). https://doi.org/10.1007/s00394-015-0889-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0889-y