Abstract
Purpose
Proper iodine intake is important during pregnancy for both fetal neurodevelopment and maternal thyroid function. Korea is known as a high iodine intake area. However, there are no data regarding iodine status in pregnant Korean women. Therefore, we evaluated the iodine status of pregnant women in Korea by measuring urine iodine concentration.
Methods
This study had an observational, prospective design. We enrolled 344 healthy pregnant women who visited Samsung Medical Center in Korea for a routine antenatal checkup between April 2012 and September 2013. We measured iodine and creatinine concentration (Cr) in spot urine samples and TSH level in serum at the time of enrollment.
Results
The median urinary iodine concentration (UIC) and UIC adjusted by Cr were 427.3 μg/L and 447.9 μg/gCr, respectively. There was no difference in median UIC according to trimester of pregnancy (P value = 0.953). Serum TSH level was not different according to UIC level when subjects were grouped according to WHO iodine recommendations (P value = 0.401).
Conclusions
The median UIC of healthy pregnant women in Korea was 427.3 μg/L and 447.9 μg/gCr, which are more than adequate according to WHO criteria. Considering the wide range of UIC, we recommend active education about adequate iodine intake during pregnancy in areas where iodine intake is more than adequate according to WHO criteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iodine is an essential component of thyroid hormones. Intake of an appropriate level of iodine during pregnancy is important for fetal brain development and maternal thyroid function. It is well established that iodine deficiency during pregnancy is linked to maternal goiter and fetal intellectual dysfunction [1–3].
During pregnancy, iodine requirement increases due to change in maternal thyroid hormone metabolism and renal clearance. The main physiologic changes in thyroid hormone level are caused by an increase in serum thyroxine-binding globulin (TBG) and stimulation of the thyrotropin receptor by human chorionic gonadotropin (hCG). Transfer of iodine from the mother to the fetus also increases the need for maternal iodine. Moreover, renal iodine excretion increases because glomerular filtration rate (GFR) is enhanced by up to 50 % in normal pregnant women [4–7].
In 2007, the World Health Organization (WHO) proposed epidemiologic criteria for assessing iodine nutrition based on the median urinary iodine concentration (UIC) in pregnant women [8]. A median UIC of 150–249 μg/L is considered as an adequate intake of iodine. A UIC between 250 and 499 μg/L is more than adequate, and 500 μg/L or more is defined as excessive iodine intake.
Regarding the safe upper limit of iodine intake during pregnancy, data are limited and current guidelines suggest variable cutoffs of tolerable daily iodine intake. According to the guidelines recently published by the Endocrine Society [9] and the European Thyroid Association [10], iodine intake of pregnant women should not exceed 500 μg/day, which is twice the daily recommended iodine intake during pregnancy (250 μg/day), whereas the American Thyroid Association guidelines recommend <500–1100 μg/day of sustained iodine intake during pregnancy [11].
Korea is known as a high iodine intake area. A previous study reported that the average iodine intake of healthy adults in Korea was 479 μg/day according to a food questionnaire, and that the average UIC was 674 μg/g creatinine (Cr) [12]. Recently, Lee et al. [13] reported excessive iodine status in Korean preschool children (2–7 years of age), reporting a median UIC of 438.8 μg/L. However, the iodine status of pregnant women in Korea, who are also exposed to iodine-rich diet environment, has not yet been reported.
In 2010, the total population of Korea was 48.6 million, with approximately 44 % in Seoul, the capital city, and its suburbs, which is the present study was conducted [14].
Therefore, our aim in this study was to report for the first time the iodine status of pregnant Korean women in Seoul and its suburbs. In addition, we investigated whether iodine status affected thyroid function in healthy pregnant women in Korea.
Methods
Subjects
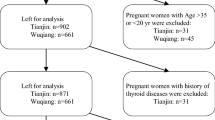
We consecutively and prospectively enrolled 344 healthy pregnant women who visited the Obstetrics Department at Samsung Medical Center for a routine antenatal checkup. We used an observational, prospective design, and the study period was between April 2012 and September 2013. Samsung Medical Center is located in the southern part of Seoul, and most pregnant women visiting its Obstetrics Department live in Seoul or its surrounding suburbs. According to a census report performed every 5 years by the Korean Statistical Information Service (KOSIS), Korea had a population of 48.6 million in 2010, and 44 % of Koreans resided in metropolitan Seoul (9.8 million) or its suburbs (11.4 million), where this study was performed [14].
All trimesters were represented in our sample (78 in the first trimester, 133 in the second trimester, and 133 in the third trimester). Exclusion criteria were a visible or palpable diffuse or nodular goiter, a history of thyroid disease or medication usage, multi-fetal gestation, high-risk pregnancy, pregnancy by any artificial reproductive technology, and pregnancy-related complications of any type. Informed consent was obtained from all participants. The research protocol was approved by the institutional review board of Samsung Medical Center.
Laboratory methods
Urine and blood samples were obtained from all subjects during obstetrical visits. We simultaneously measured urinary iodine and creatinine (Cr) from single-voided spot urine at the time of enrollment. UIC is presented in two ways in this study: simple iodine concentration (μg/L) and iodine concentration relative to Cr (μg/gCr). Creatinine concentration adjusts for the adequacy of urine sample collection. The iodine to Cr ratio in a random single-voided urine sample of a well-nourished person may be a more reliable measure than simple iodine concentration because of the large day-to-day variability in iodine intake and hydration status of each individual [4]. Urinary iodine was measured by inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent 7500 series instrument (Agilent Technologies, Inc., Tokyo, Japan) as described in a previous study [15]. Urine Cr was measured using a Cobas Integra 800 instrument (Roche Diagnostics, Basel, Switzerland). Blood samples were obtained in order to determine serum TSH, which was measured using an immunoradiometric assay kit (Immunotech, Marseille, France). Serum titer of anti-TPO antibody was determined using a radioimmunoassay kit (BRAHMS AG, Hennigsdorf, Germany). Serum anti-Tg antibody level was measured using an anti-Tg RIA kit (BRAHMS AG, Hennigsdorf, Germany). Values of anti-TPO antibody and anti-Tg antibody less than 60 U/mL were considered negative. We used trimester-specific reference ranges for serum TSH of 0.1–2.5 mIU/L in the first trimester, 0.2–3.0 mIU/L in the second trimester, and 0.3–3.0 mIU/L in the third trimester based on the current guidelines of the Endocrine Society and American Thyroid Association [9, 11].
Statistical analysis
Statistical analyses were performed using SPSS Statistics 18 (SPSS Inc., Chicago, IL, USA). Descriptive statistics (mean, SD, median, interquartile ranges) were tabulated for baseline characteristics and UIC. Clinical variables that did not show a normal distribution, including UIC and serum TSH, were analyzed using nonparametric statistics, such as the Kruskall–Wallis test and Mann–Whitney test, as appropriate. For variables with a normal distribution, values are presented as mean ± SD. Independent t test was used for comparison among patients in the three trimesters.
Results
Baseline characteristics
The mean (±SD) age of 344 participants was 33 (±4) years. About 63 % of women (n = 217) were primiparous, and 68 % (n = 234) lived in Seoul, with the remaining residing in its suburbs. Approximately 5 % of subjects were positive for anti-TPO antibody (14/312), and 2 % showed positive results for anti-Tg antibody (7/312). The baseline characteristics of study participants are described in Table 1.
Urinary iodine concentration
The median of simple UIC and adjusted by Cr were 427.3 μg/L and 447.9 μg/gCr, respectively. The median UICs according to trimester were 449.0 μg/L in the first trimester, 392.6 μg/L in the second trimester, and 446.5 μg/L in the third trimester, and there was no difference in UIC among the three trimesters (P value = 0.953). Median, interquartile ranges and minimum and maximum values of UIC (μg/L and μg/g Cr) according to pregnancy trimester are detailed in Table 2. When we compared UIC according to region of residence, we found no difference between Seoul and its suburbs (Table 3).
Distribution of urinary iodine concentrations
When we divided all subjects by UIC level according to WHO criteria [8], only 13 % (46/344) were in the adequate range (i.e., UIC between 150 and 249 μg/L). Approximately two-thirds of the patients (224/344, 65 %) showed a UIC 250 μg/L or greater. On the contrary, 21 % (73/344) of participants had a UIC <150 ug/L (Table 4). However, interpretation and extrapolation of these UIC results to individual patients should be performed with caution because dietary iodine intake and UIC are highly variable from day to day. Thus, even in populations with high iodine intake, there are always individuals with a low UIC on the day of the survey; however, this does not necessarily imply iodine deficiency.
Maternal thyroid function according to urinary iodine concentration
Median serum TSH was 1.18, 1.50, and 1.63 mIU/L in the first, second, and third trimesters, respectively. Maternal serum TSH was not statistically different according to UIC group determined by WHO criteria (serum TSH 1.52 mIU/L in UIC ≤150 μg/L, 1.44 mIU/L in UIC 150–249 μg/L, and 1.48 mIU/L in UIC 250–499 μg/L) (Table 4).
Discussion
Here, we report the iodine status of 344 healthy pregnant women in Korea, and found that the median UIC was 427.3 μg/L and 447.9 μg/gCr. There was no association between thyroid function and UIC in these study subjects. To the best of our knowledge, this is a first report of iodine status in pregnant women in Korea, where iodine intake is considered high.
Korea is an iodine-sufficient country. Kim et al. [12] reported that the mean iodine intake of healthy adults in Korea was 479 μg/day according to a food questionnaire, and that the mean UIC was 674 μg/gCr. A recent study of 540 healthy adults living in an urban area in Korea found that the median UIC was 268 μg/L [16]. Another study for healthy preschool children aged between 2 and 7 years reported that the median UIC was 439 μg/L, and that two-thirds of the children had excessive iodine intake. As the dietary habits of children are determined by those of parents, this result suggests that the Korean population as a whole consumes iodine-rich foods [13]. In the present study, the median UIC was comparable to the figure reported for preschool children, which indicates that iodine intake in Korea is excessive according to WHO criteria.
The median UIC of pregnant women in Korea in this study was higher than that reported in previous studies in populations with high iodine intake in adjacent regions in Asia. Fuse et al. [17] reported a sufficient iodine status in 934 pregnant Japanese women, with a median UIC of 219.0 μg/L. Another Japanese group reported a median UIC of 328 μg/L in 514 women in early pregnancy [18]. Iodine status is highly variable according to province in China [19]; a recent study of pregnant Chinese women reported a median UIC of 1240 μg/L in subjects from areas where tap water is supplemented with iodine [20].
In the present study, the median UIC was consistently high across all trimesters. Fuse et al. [17] reported that the median UIC in Japan tended to decrease in late pregnancy (221 μg/L in the first trimester, 193 μg/L in the third trimester), although the difference was not significant. A similar tendency has been observed in several previous studies conducted in iodine-deficient areas such as Australia, Ireland, Iran, and Switzerland [21–24]. Iodine transfer from mother to fetus in late pregnancy might cause decreased maternal renal excretion of iodine [25], although an insufficient UIC does not always indicate real depletion of the iodine pool. However, the passage of iodine from mother to fetus does not seem to cause much change in UIC during late pregnancy in areas where iodine intake is excessive.
UIC value determined as micrograms per liter and adjusted according to Cr from spot urine was used in this study. All iodine is obtained by oral intake; because more than 90 % of dietary iodine is excreted through urine [4, 26], UIC is considered the best indicator of iodine status in epidemiologic studies. There are several methods to assess iodine status, and measuring spot urine is preferred to 24-h urine sampling in epidemiologic studies due to the simplicity and practicality of spot urine sampling [4, 27]. As UIC in each individual can vary widely according to diet, time of measurement, and urine volume, individual-level iodine status should not be determined from the value of UIC in single spot urine sample. However, this variation tends to be negligible when analyzing population-level data [8]; therefore, spot urine iodine concentration is accepted as an international criterion for assessing and monitoring the iodine status of population. During pregnancy, GFR begins to rise within 4 weeks after conception and increases through the end of the first trimester [7, 28]. As renal hyper-filtration leads to an increase in urinary iodine excretion, several studies have suggested that UIC calculated as μg/gCr may be a more reliable tool for measurement of iodine status in pregnant women than simple UIC expressed as μg/L [4, 17, 29]. However, due to the additional expense of measuring Cr, a single measurement of UIC is recommended by various health organizations as an useful indicator of iodine status in a large number of pregnant women [8]. In our study, simple urine iodine concentration (μg/L) and urine iodine to creatinine ratio (μg/gCr) did not differ significantly across the trimester.
We did not find an association between UIC and maternal serum TSH in this study population. Consistent with our results, no significant correlation was observed between UIC and serum TSH in a Japanese study [17]. However, several studies have reported a positive correlation between UIC and serum TSH. Orito et al. [18] reported a positive relationship between UIC and serum TSH (r = 0.1326; P < 0.005) in early pregnant women, although the correlation was weak. Sang et al. [20] also suggested that excessive iodine intake during the late period of pregnancy may cause mild thyroid dysfunction in mothers, which mainly presents as subclinical hypothyroidism. Teng et al. [30] demonstrated that excessive iodine intake may trigger autoimmunity of thyroid glands, which leads to either subclinical or overt hypothyroidism in non-pregnant adults. Possible explanation for the lack of a relationship between UIC and serum TSH in our study population was that we enrolled only healthy pregnant women without goiter or history of thyroid disease. The prevalence of thyroid autoantibody was <5 % in our study group, which is far less than that reported in the normal population [31, 32]. In addition, subjects with low UIC might not actually have iodine deficiency because day-to-day variation in UIC can be quite high in individuals.
There are some limitations to this study. First, this study was conducted at a tertiary referral hospital and not in a primary hospital setting, and it was not a cohort-based study. However, the Obstetrics Department of Samsung Medical Center functions not only as a tertiary referral hospital, but also as a primary care center for pregnant women. This is because the hospital is located in a populated area containing few delivery hospitals due to poor reimbursement for childbirth from the national health insurance system. Thus, we are confident that the participants in this study reflect normal pregnancy in Korea because we excluded all high-risk pregnancies and all conditions that could affect thyroid functional status. Furthermore, there were no differences in UIC according to habitation region. Secondly, information about iodine intake was not collected during this study. Calculation of iodine intake and iodine contents in food is quite difficult because of the complexity of Korean foods. The major sources of dietary iodine in Korean healthy adults are seaweeds and dairy products [12]. However, a positive strong relationship between iodine intake and UIC was demonstrated in a previous study of Korean adults [12]. Finally, we did not evaluate the relationship between maternal UIC and neonatal outcome, especially neonatal thyroid function, because it was beyond the scope of the study. In most adults, thyroid homeostasis is maintained in spite of chronic excessive iodine status [33, 34]. However, avoidance of the acute Wolff–Chaikoff effect, which is one of processes by which thyroid hormone production recovers, may not occur in fetuses younger than 36-week gestation [35, 36]. Thus, in the setting of iodine excess, the fetus may be susceptible to hypothyroidism [35, 36]. However, the few studies that have studied iodine intake and fetal outcomes in iodine-excessive areas have reported favorable outcomes even in iodine-excessive areas [17, 18], although the longest duration for observation was 1 year after birth [18]. Further investigations are warranted to clarify the long-term, neurodevelopmental outcomes of children born to mothers with high iodine intake, and to determine the safe upper limits of UIC in pregnant women.
In conclusion, this is a first report of iodine status in normal pregnant women in Korea. The median UIC and UIC adjusted by Cr were 427.3 μg/L and 447.9 μg/gCr, respectively, which were more than adequate according to WHO criteria. Considering the wide range of UIC, we recommend active education about adequate iodine intake for pregnant women even in areas where the UIC is more than adequate according to WHO criteria. Further investigations about the effect of iodine status in pregnancy on fetal outcome are warranted.
References
Glinoer D, De Nayer P, Delange F, Lemone M, Toppet V, Spehl M, Grun JP, Kinthaert J, Lejeune B (1995) A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab 80:258–269
Dunn JT, Delange F (2001) Damaged reproduction: the most important consequence of iodine deficiency. J Clin Endocrinol Metab 86:2360–2363
Qian M, Wang D, Watkins WE, Gebski V, Yan YQ, Li M, Chen ZP (2005) The effects of iodine on intelligence in children: a meta-analysis of studies conducted in China. Asia Pac J Clin Nutr 14:32–42
Soldin OP (2002) Controversies in urinary iodine determinations. Clin Biochem 35:575–579
Glinoer D (2004) The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab 18:133–152
Koutras DA (2000) Thyroidopathies. Ann N Y Acad Sci 900:77–88
Davison JM, Dunlop W (1980) Renal hemodynamics and tubular function normal human pregnancy. Kidney Int 18:152–161
World Health Organization (2007) International council for the control of iodine deficiency disorders 2007 Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. World Health Organization, Geneva
De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S (2012) Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:2543–2565
Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B (2014) 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 3:76–94
Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W (2011) Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125
Kim JY, Moon SJ, Kim KR, Sohn CY, Oh JJ (1998) Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J 39:355–362
Lee J, Kim JH, Lee SY, Lee JH (2013) Iodine status in Korean preschool children as determined by urinary iodine excretion. Eur J Nutr 53:683–688
Statistics Korea (2010) Population, households and housing units by census in Korea (2010). Statistics Korea Publishing KosisWeb. http://kosis.kr/eng/statisticsList/statisticsList_01List.jsp?vwcd=MT_ETITLE&parmTabId=M_01_01#SubCont. Assessed 7 January 2015
Lee JH, Ji OJ, Song MJ, Park HD, Kim HK, Kim SW, Chung JH, Lee SY (2010) Determination of urinary iodine concentration by inductively coupled plasma-mass spectrometry in thyroid cancer patients on low-iodine diet. Korean J Lab Med 30:351–356
Choi J, Kim HS, Hong DJ, Lim H, Kim JH (2012) Urinary iodine and sodium status of urban Korean subjects: a pilot study. Clin Biochem 45:596–598
Fuse Y, Ohashi T, Yamaguchi S, Yamaguchi M, Shishiba Y, Irie M (2011) Iodine status of pregnant and postpartum Japanese women: effect of iodine intake on maternal and neonatal thyroid function in an iodine-sufficient area. J Clin Endocrinol Metab 96:3846–3854
Orito Y, Oku H, Kubota S, Amino N, Shimogaki K, Hata M, Manki K, Tanaka Y, Sugino S, Ueta M, Kawakita K, Nunotani T, Tatsumi N, Ichihara K, Miyauchi A, Miyake M (2009) Thyroid function in early pregnancy in Japanese healthy women: relation to urinary iodine excretion, emesis, and fetal and child development. J Clin Endocrinol Metab 94:1683–1688
Kim DS (2010) Introduction: health of the health care system in Korea. Soc Work Public Health 25:127–141
Sang Z, Wei W, Zhao N, Zhang G, Chen W, Liu H, Shen J, Liu J, Yan Y, Zhang W (2012) Thyroid dysfunction during late gestation is associated with excessive iodine intake in pregnant women. J Clin Endocrinol Metab 97:E1363–E1369
Stilwell G, Reynolds PJ, Parameswaran V, Blizzard L, Greenaway TM, Burgess JR (2008) The influence of gestational stage on urinary iodine excretion in pregnancy. J Clin Endocrinol Metab 93:1737–1742
Ainy E, Ordookhani A, Hedayati M, Azizi F (2007) Assessment of intertrimester and seasonal variations of urinary iodine concentration during pregnancy in an iodine-replete area. Clin Endocrinol (Oxf) 67:577–581
Brander L, Als C, Buess H, Haldimann F, Harder M, Hanggi W, Herrmann U, Lauber K, Niederer U, Zurcher T, Burgi U, Gerber H (2003) Urinary iodine concentration during pregnancy in an area of unstable dietary iodine intake in Switzerland. J Endocrinol Invest 26:389–396
Smyth PP (1999) Variation in iodine handling during normal pregnancy. Thyroid 9:637–642
Glinoer D, Delange F (2000) The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid 10:871–887
Cardis E, Hatch M (2011) The Chernobyl accident–an epidemiological perspective. Clin Oncol (R Coll Radiol) 23:251–260
Kim DS (2010) Special issue on the national health care system of South Korea. Soc Work Public Health 25:125–126
Dafnis E, Sabatini S (1992) The effect of pregnancy on renal function: physiology and pathophysiology. Am J Med Sci 303:184–205
Andersen SL, Moller M, Laurberg P (2013) Iodine concentrations in milk and in urine during breastfeeding are differently affected by maternal fluid intake. Thyroid 24:764–772
Edmonds CJ (1979) Treatment of thyroid cancer. Clin Endocrinol Metab 8:223–242
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE (2002) Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab 87:489–499
Pedersen IB, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Laurberg P (2003) Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin Endocrinol (Oxf) 58:36–42
Pennington JA (1990) A review of iodine toxicity reports. J Am Diet Assoc 90:1571–1581
Wolff J, Chaikoff IL (1948) Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem 174:555–564
Fisher DA, Klein AH (1981) Thyroid development and disorders of thyroid function in the newborn. N Engl J Med 304:702–712
Theodoropoulos T, Braverman LE, Vagenakis AG (1979) Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science 205:502–503
Conflict of interest
The authors have nothing to declare about this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yoon Young Cho and Hye Jeong Kim have equally contributed to this study as the first authors.
Rights and permissions
About this article
Cite this article
Cho, Y.Y., Kim, H.J., Oh, Sy. et al. Iodine status in healthy pregnant women in Korea: a first report. Eur J Nutr 55, 469–475 (2016). https://doi.org/10.1007/s00394-015-0864-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0864-7