Abstract
Purpose
Non-alcoholic fatty liver disease (NAFLD), known to be related to insulin resistance, has been the focus of intensive research efforts due to its increasing prevalence and clinical significance. Rapid eating behavior is another emerging health issue associated with insulin resistance. We aimed to clarify the correlation between self-reported eating speed and NAFLD, both known to be related to insulin resistance.
Methods
A cross-sectional study was conducted during routine medical checkups on 7,917 consecutively enrolled participants. Anthropometric, biochemical, nutritional, and social parameters were checked. The self-reported eating speed per their usual meal (<5, 5–10, 10–15, and more than 15 min) was recorded by a registered dietitian.
Results
The faster eating groups had a higher proportion of NAFLD, and the grade of NAFLD was advanced. After controlling for anthropometric, cardiometabolic, social, and nutritional parameters, the fastest eating group (<5 min) showed an increased risk of NAFLD compared with the lowest eating speed group (≥15 min) both in total [odds ratio (OR) 1.81, 95 % confidence interval (CI) 1.24–2.63] and the participants with BMI < 25 kg/m2 (OR 1.79, 95 % CI 1.22–2.61). As the self-reported eating speed increased, the risk of NAFLD also increased in total and those with BMI < 25 kg/m2 (P for trend <0.001).
Conclusions
Fast eating is associated with an increased risk of the presence and grade of NAFLD in Korean adults, especially those with BMI < 25 kg/m2, since presence of overweight or obesity may be overwhelming the effect on NAFLD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD), a condition of hepatic steatosis without excessive alcohol drinking, is one of the most common liver diseases, and its prevalence is increasing [1]. Moreover, patients with NAFLD are at risk of cirrhosis and hepatocellular carcinoma in addition to the increased incidence and mortality from cardiovascular disease [2–4]. Obesity, hypertension, type 2 diabetes mellitus, and dyslipidemia are associated with higher rates of NAFLD [2, 5]. Reducing body weight, which also includes the reduction of hepatic fat, dramatically decreases the risk of diabetes in obese people [6]. The underlying mechanism connecting these cardiometabolic diseases has been thought to be the effect of insulin resistance [7].
Insulin resistance can also be associated with faster eating speed, since it has been shown in many articles that rapid eating speed contributes to metabolic conditions including obesity, type 2 diabetes mellitus, dyslipidemia, and increased cardiometabolic risks [8–11].
Anorexigenic gut peptide hormones such as cholecystokinin, glucagon-like peptide 1 (GLP-1), and peptide YY (PYY) are secreted after meals, which decrease appetite and prevent overeating [9, 12, 13]. However, eating fast showed a weaker anorexigenic gut hormone response than eating at a moderate pace [12]. Additionally, some studies illustrated the effects of GLP-1 on the hepatocytes in patients with non-alcoholic steatohepatitis, by activating genes involved in fatty acid β-oxidation and insulin sensitivity [14, 15]. Therefore, these gut hormones may have a role between eating speed and the pathogenesis of NAFLD, which needs further investigation.
Although there is both convincing rationale and prior reports suggesting on the correlation between NAFLD and eating speed, it has not been clearly illustrated to date [8, 10]. There has been a study revealing that increased eating speed is correlated with liver fat; however, this study was only restricted to a small sample of severely obese individuals [8]. In this cross-sectional study, we tested the hypothesis that a fast eating speed is associated with high prevalence and advanced grade of NAFLD in adults with a large sample size encompassing lean, normal weight, and overweight/obese groups. We also checked that the association, if any, between self-reported eating speed and NAFLD remains significant, independently from potential confounders, such as total energy intake or body mass index (BMI).
Methods
Study participants
Study participants underwent routine medical checkups at the Center for Health Promotion at the Korea University Anam Hospital located in Seoul, Korea, between March 1, 2007 and March 31, 2009. We examined a total of 12,593 consecutively enrolled individuals during this period. We excluded from this study subjects with medical history of being HBs Ag positive, HCV Ab positive, liver cirrhosis, or hepatocellular carcinoma, elevated liver enzyme [aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥200 IU/l], and elevated gamma-glucronyl transpeptidase (GGT) (≥1,000 IU/l), participants who had been taking medicine due to a liver disease or persons who reported drinking more than 20 g of alcohol per day. Subjects under the age of 20 and those who did not comply with ultrasonography and questionnaires were also excluded from this study. The final study sample included 7,917 subjects. The Institutional Review Board at the Korea University Anam Hospital approved this study, and written consent forms were signed by the participants.
Anthropometric and laboratory measurements
During the anthropometric measurement, all participants wore light clothing without shoes. Height was measured to the nearest 0.1 cm, and weight was measured to the nearest 0.1 kg. BMI was obtained using the following calculation: weight (kg) divided by the square of height (m). Blood pressure was measured on one of upper arms after at least 10 min of rest with an automated blood pressure measuring device (MP800, MEKICS, Chuncheon, Korea). Fasting blood samples (over 10 h) were obtained between 0800 and 0900 hours. An automated analyzer (TB200FR; Toshiba Co., Otawara, Japan) was utilized to measure serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), uric acid, AST, ALT, and GGT. Fasting insulin levels were measured using an immunoradiometric assay with the INS-IRMA kit (Diasourse, Nivelles, Belgium). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following equation: HOMA-IR = fasting glucose (mg/dl) × fasting insulin (μU/ml)/405 [16].
Questionnaires
Self-reported questionnaires were administered to participants regarding their medical history including hypertension, diabetes mellitus, dyslipidemia, thyroid disease, gout, and medication history. Also, lifestyles such as alcohol drinking habits (frequency, amount, and kind), current smoking status, and physical activity were checked.
Nutritional assessment
The food intake and eating behaviors were assessed by a registered dietitian. The amount and frequency of food items were assessed by using a food frequency questionnaire. Total energy intake was obtained by using CAN-Pro 3.0 (Computer Aided Nutritional Analysis program, version 3.0; The Korean Nutrition Society, Seoul, Korea), which is based on the Dietary Reference Intakes for Koreans in 2005 [17]. Each subject’s eating speed per usual meal was obtained by choosing one among the following statements:
-
Less than 5 min to finish a meal.
-
Between 5 and 10 min to finish a meal.
-
Between 10 and 15 min to finish a meal.
-
15 min or more to finish a meal.
Sonographic examinations
After fasting for at least 10 h, participants underwent an abdominal sonogram (1.0–5.0-MHz (C5-1) transducer, iU22, Philips, WA, USA) by one of the two radiologists at Korea University Anam Hospital, and the results were confirmed by a single highly experienced radiologist (S.M.B.). A diffuse increase in echogenecity (a bright liver) and vascular blurring (more than renal cortex or spleen) was sonographically defined as a fatty liver [18]. Grades of NAFLD were determined according to the brightness of liver and visualization of intrahepatic vessels and diaphragm [19].
Statistical analysis
SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The t test or Chi-square test was used for comparison of the difference of anthropometric, laboratory, nutritional, and social variables according to the presence or absence of NAFLD. The Chi-square test was used to compare the prevalence and grade of NAFLD according to self-reported eating speed, and the linear trend was calculated by using the linear-by-linear association. Multiple logistic regression analyses were used to evaluate self- reported eating speed as an independent variable for the presence of NAFLD. The reference for eating speed was ≥15 min/meal. Variables that had a significant association (P < 0.05) with the dependent variable (NAFLD) in univariate analysis or known risk factors for both eating speed and NAFLD were included in the model as covariates. First, the relationship between self-reported eating speed and NAFLD (Model 1) was analyzed. Then, the sex, age, BMI, systolic blood pressure (SBP), smoking, physical activity, total energy intake, and the number of meals per day were adjusted (Model 2). Lastly, covariates in Model 2 plus TC, HDL-C, TG, ALT, FBG, uric acid, and HOMA-IR were adjusted (Model 3). The interaction term between self-reported eating speed and BMI groups (<25 and ≥25 kg/m2) was included in Model 2 and 3. The prevalence and the odds ratios (ORs) for NAFLD were further stratified by BMI groups because of a significant interaction between the self-reported eating speed and BMI category in the logistic regression model. A two-sided P value lower than 0.05 was regarded to be statistically significant.
Results
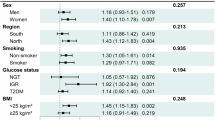
Table 1 shows the general characteristics of the research participants according to the presence of NAFLD. Subjects with NAFLD had higher male proportion, age, BMI, waist–hip ratio, blood pressure, TC, TG, LDL-C, FBG, uric acid, ALT, GGT, HOMA-IR, total energy intake, and the higher proportion of current smoker and regular exerciser, whereas HDL-C was lower in that group.
Figure 1 shows the prevalence of NAFLD according to the self-reported eating speed and BMI. Overall, the percentage of NAFLD showed 60.5, 51.1, 40.9, and 33.7 % for participants who ate their meal within 5, 5–10, 10–15, and ≥15 min, respectively (P for trend <0.001). In the participants with BMI < 25 kg/m2, the prevalence was 41.5, 32.7, 26.7, and 23.5 % for those who ate within 5, 5–10, 10–15, and ≥15 min (P for trend <0.001), while in group with BMI ≥ 25 kg/m2, the prevalence of NAFLD showed 79.8, 82.7, 79.7, and 79.8 %, respectively (P for trend = 0.407).
The prevalence of NAFLD according to self-reported eating speed and BMI. Left shows an increasing trend of the percentage of NAFLD as self-reported eating speed is increased in total participants. Middle and right represent the prevalence of NAFLD according to BMI levels. NAFLD, non-alcoholic fatty liver disease; BMI, body mass index
Figure 2 reveals the grade of NAFLD according to self-reported eating speed. Faster eating groups had higher proportion of advanced grade of the disease (P for trend <0.001).
Table 2 shows the ORs for NAFLD across the four categories of self-reported eating speed (≥15, 10–15, 5–10, and <5 min) in total and two BMI groups. Participants with high eating speed had an elevated risk of NAFLD before and after adjusting for total energy intake, BMI, and other anthropometric, social, and nutritional variables [OR 1.64, 95 % confidence interval (CI) 1.30–2.06 for group with 5–10 min; OR 2.32, 95 % CI 1.63–3.31 for group with <5 min; P for trend <0.001 in Model 2]. After further adjustments for biochemical variables, participants whose eating speed was <5 min had an increased risk of NAFLD (OR 1.81, 95 % CI 1.24–2.63) compared to those with a speed of ≥15 min. As self-reported eating speed increased, the risk of NAFLD also increased (P for trend <0.001 in Model 3). In the participants with BMI < 25 kg/m2, those who ate faster had a high risk of NAFLD after adjusting for anthropometric, social, nutritional, biochemical variables, and total energy intake (OR 1.41, 95 % CI 1.10–1.81 for group with 5–10 min; OR 1.79, 95 % CI 1.22–2.61 for group with <5 min; P for trend <0.001 in Model 3). However, in those with BMI ≥ 25 kg/m2, there was no relationship between self-reported eating speed and NAFLD.
Discussion
Fast eating speed is related to high prevalence and the advanced grade of NAFLD, especially in lean participants. This is the first study that has shown the association between self-reported eating speed and NAFLD in a large sample with all BMI groups. The association lasted even after adjusting for total energy intake and BMI as well as other anthropometric, cardiometabolic, social, and nutritional parameters.
NAFLD accounted for 75 % of chronic liver diseases in 2005–2008 and showed an increasing trend along with steady increase in obesity, visceral obesity, type 2 diabetes mellitus, and insulin resistance since 1988–1994 National Health and Nutrition Examination Survey in the US [20]. The prevalence of NAFLD reached 30 % among the Western adults population and was observed as high as 98 % in non-diabetic obese individuals [21, 22]. Excessive calorie intake compared to energy consumption, which also results in obesity, affects liver fat accumulation. Insulin resistance is a well-known risk factor for NAFLD, acting as an enhancer for the efflux of free fatty acid from adipose tissue, which in turn induces hepatic steatosis [23]. Obesity is characterized by the increased amount of non-esterified fatty acids, glycerol, and hormones such as leptin, pro-inflammatory cytokines, and other factors, all of which are involved in the development of insulin resistance [24]. Therefore, both NAFLD and obesity share insulin resistance as a pathophysiology or a mechanism.
Several studies have been focused on the relationship between obesity and fast eating speed. An epidemiological study reported that current BMI is associated positively with the self-reported speed of eating independently of energy intake [10]. Japanese studies have shown that both eating quickly and eating until full have an impact on being overweight, with additive interaction of both eating behaviors and the speed of eating is associated with the rate of weight gain in all age groups [25, 26]. Eating fast induces more consumption of energy, which may act as a major role in overweight and/or obesity [27]. However, evidence of association between the speed of eating and NAFLD, which is closely related to obesity, is rare [8]; though slow eating behavior is recommended during nutritional education for NAFLD patients to prevent overeating.
We found that a fast eating speed was associated with the prevalence and the grade of NAFLD. Insulin resistance may have a role between them although the exact mechanism remains to be elucidated. Moreover, the association between the self-reported eating speed and the prevalence of NAFLD remained significant after adjusting for total energy intake and BMI, suggesting that this relationship is not fully explained by these factors. This effect was more pronounced in lean subjects, whereas in obese group (BMI ≥ 25 kg/m2), the effect of obesity may be overwhelming any effect of self-reported eating speed on NAFLD. Therefore, the role of gut peptide hormones between eating speed and NAFLD should be underscored. Gut-originated anorexigenic hormones including PYY, GLP-1, and cholecystokinin act on the nucleus of the solitary tract located in the hindbrain, inducing satiety with consequent meal termination, whereas orexigenic peptides, such as ghrelin, increase appetite, and food intake [13]. Physiological moderate speed of eating drew out more pronounced response of anorexigenic hormones and led to early termination of meal, which in turn may act favorably on weight control. Fast eating, on the other side, induces less responsiveness of PYY and GLP-1, failing to provoke satiety signals with increased energy intake and obesity [12]. According to other report, slow eating had more exacerbated response of PYY and GLP-1 at the end of meal [28]. Not only are gut hormones associated with appetite signals, but their levels may be related to insulin resistance. A study of postprandial response to fat load revealed that insulin resistance impairs the GLP-1 and PYY secretion in morbidly obese individuals [29]. This relationship of the gut hormone with insulin resistance may elucidate the connection between eating speed and insulin resistance [30], which is directly associated with NAFLD.
In this study, the percentage of NAFLD did not differ among the self-reported eating speed groups with BMI ≥ 25 kg/m2, but there was an increasing trend of NAFLD prevalence as the self-reported eating speed increased in subjects with BMI < 25 kg/m2. We further analyzed the association between self-reported eating speed and HOMA-IR according to BMI category. In subjects with BMI ≥ 25 kg/m2, the proportion of high HOMA-IR (defined as ≥1.95 [31]) was not different among the self-reported eating speed groups (P for trend =0.638), while the percentage of high HOMA-IR increased as the self-reported eating speed increased in those with BMI < 25 kg/m2 (P for trend =0.001). Waist–hip ratio was substituted for HOMA-IR, and results were similar to the BMI category (data not shown).
To our knowledge, this is the first investigation that reveals the relationship between self-reported eating speed and NAFLD in a large sample from all BMI groups. One of the limitations to this research is that the subjects were participants of routine medical checkup; thus, they cannot represent the general Korean population. Also, a causal relationship could not be established because this research was a cross-sectional study. Another limitation is that we did not check the participants’ eating speed directly and depended on answers provided by the participants. Although magnetic resonance imaging (MRI) scans are able to assess the different types of hepatic fat better than ultrasonography [32], we were not able to gain access to MRI scans due to the large number of participants in this study, and this would also be prohibitively costly. Future, population-based larger studies need to further explore this association prospectively and/or with direct measurement of eating speed. Observational studies cannot explore mechanisms, and thus, future studies on underlying mechanisms are also needed. Uncontrolled confounding or misclassification remains as possibilities, but the latter would be expected to be random and thus would have suppressed effect estimates and thus could not have influenced the significant results demonstrated herein. The findings of this study suggests, if confirmed prospectively, an interventional strategy to reduce hepatic fat by slowing the speed of eating. This may also influence healthy dietary habits, which can prevent diabetes and other cardiovascular diseases [33].
In conclusion, those with a fast eating speed are at higher risk of NAFLD after adjusting for anthropometric, cardiometabolic, and social parameters in Korean adults. Given the potential this observation has in the prevention of NAFLD, further investigations regarding mechanisms between eating speed and NAFLD as well as large prospective studies are warranted.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- FBG:
-
Fasting blood glucose
- GGT:
-
Gamma-glucronyl transpeptidase
- GLP-1:
-
Glucagon-like peptide 1
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- MRI:
-
Magnetic resonance imaging
- NAFLD:
-
Non-alcoholic fatty liver disease
- OR:
-
Odds ratio
- PYY:
-
Peptide YY
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
References
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M et al (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140:124–131
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K et al (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55:2005–2023
Targher G, Day CP, Bonora E (2010) Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363:1341–1350
Karagozian R, Derdak Z, Baffy G (2014) Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism 63:607–617
Smith BW, Adams LA (2011) Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol 7:456–465
Paulweber B, Valensi P, Lindström J, Lalic NM, Greaves CJ, McKee M et al (2010) A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res 42(Suppl. 1):S3–S36
Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A (2013) Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 5:1544–1560
Kral JG, Buckley MC, Kissileff HR, Schaffner F (2001) Metabolic correlates of eating behavior in severe obesity. Int J Obes Relat Metab Disord 25:258–264
Lee KS, Kim DH, Jang JS, Nam GE, Shin YN, Bok AR et al (2013) Eating rate is associated with cardiometabolic risk factors in Korean adults. Nutr Metab Cardiovasc Dis 23:635–641
Otsuka R, Tamakoshi K, Yatsuya H, Murata C, Sekiya A, Wada K et al (2006) Eating fast leads to obesity: findings based on self-administered questionnaires among middle-aged Japanese men and women. J Epidemiol 16:117–124
Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Nagasawa SY et al (2012) Self-reported speed of eating and 7-year risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism 61:1566–1571
Kokkinos A, le Roux CW, Alexiadou K, Tentolouris N, Vincent RP, Kyriaki D et al (2010) Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon-like peptide-1. J Clin Endocrinol Metab 95:333–337
Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG (2013) The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism 62:457–478
Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C et al (2011) Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 31:1285–1297
Wang XC, Gusdon AM, Liu H, Qu S (2014) Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J Gastroenterol 20:14821–14830
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
The Korean Nutrition Society (2005) Dietary reference intakes for Koreans (KDRIs). The Korean Nutrition Society, Seoul
Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB (2006) Fatty liver: imaging patterns and pitfalls. Radiographics 26:1637–1653
Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M et al (2002) The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123:745–750
Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H et al (2011) Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 9:524–530 e521; quiz e560
Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G (2010) A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 53:372–384
Machado M, Marques-Vidal P, Cortez-Pinto H (2006) Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol 45:600–606
Tsatsoulis A, Mantzaris MD, Bellou S, Andrikoula M (2013) Insulin resistance: an adaptive mechanism becomes maladaptive in the current environment—an evolutionary perspective. Metabolism 62:622–633
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Maruyama K, Sato S, Ohira T, Maeda K, Noda H, Kubota Y et al (2008) The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: cross sectional survey. BMJ 337:a2002
Tanihara S, Imatoh T, Miyazaki M, Babazono A, Momose Y, Baba M et al (2011) Retrospective longitudinal study on the relationship between 8-year weight change and current eating speed. Appetite 57:179–183
Viskaal-van Dongen M, Kok FJ, de Graaf C (2011) Eating rate of commonly consumed foods promotes food and energy intake. Appetite 56:25–31
Karl JP, Young AJ, Rood JC, Montain SJ (2013) Independent and combined effects of eating rate and energy density on energy intake, appetite, and gut hormones. Obesity (Silver Spring) 21:E244–E252
Fernandez-Garcia JC, Murri M, Coin-Araguez L, Alcaide J, El Bekay R, Tinahones FJ (2014) GLP-1 and peptide YY secretory response after fat load is impaired by insulin resistance, impaired fasting glucose and type 2 diabetes in morbidly obese subjects. Clin Endocrinol (Oxf) 80:671–676
Otsuka R, Tamakoshi K, Yatsuya H, Wada K, Matsushita K, OuYang P et al (2008) Eating fast leads to insulin resistance: findings in middle-aged Japanese men and women. Prev Med 46:154–159
Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH (2006) Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 30:1529–1534
Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T et al (2005) Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol 39:619–625
Schwarz PE, Greaves CJ, Lindström J, Yates T, Davies MJ (2012) Nonpharmacological interventions for the prevention of type 2 diabetes mellitus. Nat Rev Endocrinol 8:363–373
Acknowledgments
There is no funding source for this study to disclose. S.L., B.J.K., and C.S.M. designed the study; S.L., Y.G., and B.D.H. collected data; B.J.K. and K.H. analyzed and interpreted the data; S.L. and B.J.K. wrote the first draft of the manuscript; C.S.M., Y.J.Y., S.P., A.L., and J.H.K. provided critical revision of the manuscript and contributed to the discussion. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Saehyun Lee and Byung-Joon Ko have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, S., Ko, BJ., Gong, Y. et al. Self-reported eating speed in relation to non-alcoholic fatty liver disease in adults. Eur J Nutr 55, 327–333 (2016). https://doi.org/10.1007/s00394-015-0851-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0851-z