Abstract
Purpose
We tested the hypothesis that maternal low-protein (LP) diet during gestation and lactation can program myostatin (MSTN) signaling and protein synthesis in skeletal muscle of offspring at weaning stage (35 days).
Methods
Fourteen Meishan sows were fed either LP or standard-protein diets throughout gestation and lactation, male offspring piglets were killed at weaning stage and longissimus dorsi (LD) muscles were taken. The cross-sectional areas (CSA) of LD muscles were measured by hematoxylin and eosin staining. The levels of free amino acids in plasma were measured by amino acid auto-analyzer. Proteins and mRNA were determined by Western blot and RT-qPCR, respectively.
Results
Body weight, LD muscle weight and the myofiber CSA were significantly decreased (P < 0.05) in LP piglets; meanwhile, the concentration of branched-chain amino acids was also significantly decreased (P < 0.001). MSTN protein content tended to be higher (P = 0.098) in LP piglets, while the expression of MSTN receptors, activin type II receptor-beta and transforming growth factor type-beta type I receptor kinase, was significantly up-regulated (P < 0.05). Furthermore, p38 mitogen-activated protein kinase, the downstream signaling factor of MSTN, was also enhanced significantly (P < 0.05). In addition, key factors of translation initiation, phosphorylated eukaryotic initiation factor 4E and the 70 kDa ribosomal protein S6 kinase, were significantly decreased (P < 0.05) in LP piglets.
Conclusions
Our results suggest that maternal LP diet during gestation and lactation affects MSTN signaling and protein synthesis in skeletal muscle of offspring at weaning stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, a large body of experimental evidence from animals has demonstrated the programming effects of maternal nutrition during gestation and lactation on offspring growth and development [1–4]. Skeletal muscle is one of the most susceptible organs to prenatal and neonatal malnutrition. Maternal high-protein or low-protein (LP) diet during gestation affected the body composition and the cellularity of the skeletal muscle in newborn and weanling piglets [5, 6]. Furthermore, maternal LP diet elicited pronounced changes on the expression of regulatory and metabolic genes in the skeletal muscle [7–9].
Myostatin (MSTN), a member of the transforming growth factor type β (TGF-β) super-family, normally acts as a negative regulator of skeletal muscle development and growth. MSTN and TGF-β share the same signaling pathway mediated by activin receptors (ActR). MSTN binds initially to the two activin type II receptors, mainly ActRIIB and, to a lesser extent, ActRIIA, and then to a TGF-β type I receptor kinase (ALK5). The activated ALK5 phosphorylates the intracellular mediators of signaling, Smad2 and/or Smad3, to inhibit muscle differentiation and growth [10]. Additionally, MSTN also signals through the mitogen-activated protein kinase (MAPK) pathway [11, 12]. The binding of MSTN to ActRIIB activates c-Jun N-terminal kinase (JNK) signaling pathway in both proliferating and differentiating C2C12 cells [12]. Moreover, MSTN can activate the p38 MAPK, which is independent of Smad signaling, through the TGF-β-activated kinase 1 (TAK1) in C2C12 cells to inhibit the proliferation of myoblasts [11].
Recent studies have shown that MSTN is responsive to the nutritional status. Muscle MSTN can be programmed by neonatal underfeeding [13] or can serve as a key mediator of inadequate prenatal nutrition [14] in rats. Feeding LP diet to sows throughout gestation and lactation produced offspring pigs with altered skeletal muscle MSTN expression distinctively at weaning and finishing stages [15]. However, it remains unclear whether alterations in MSTN expression may influence the MSTN signaling and thereby cause MSTN-regulated metabolic changes in skeletal muscle of offspring piglets.
It has been reported that MSTN regulates skeletal muscle protein metabolism possibly through protein kinase B (PKB)/mammalian target of rapamycin (mTOR) signaling pathway in mice [16]. The inhibition of the MSTN downstream signaling Smad2/3 was reported to cause muscle hypertrophy which was partially dependent on mTOR signaling in adult mice [17]. mTOR signaling is well known to mediate the effects of maternal amino acids deficiency on protein metabolism in various fetal and placental tissues [18, 19]. The branched-chain amino acids (BCAA), especially leucine, are shown to be key regulators of skeletal muscle protein synthesis [20, 21]. Leucine increases skeletal muscle protein synthesis in neonatal piglets by enhancing the mammalian target of rapamycin complex 1 (mTORC1) activation and stimulating mTOR-dependent translation initiation [22–24]. The mTOR protein induces the phosphorylation of eukaryotic initiation factor 4E binding protein 1 (4E-BP1), which releases eukaryotic initiation factor 4E (eIF4E) to promote the initiation of translation. In addition, mTOR phosphorylates and activates S6 kinase (S6K, formerly known as p70S6K) which phosphorylates and activates ribosomal protein S6 to facilitate the recruitment and translation of specific mRNAs [25]. Leucine supplementation in a LP diet increases the phosphorylation of S6K and 4E-BP1 in weaning piglets, resulting in enhanced skeletal muscle protein synthesis [26]. However, it remains unknown whether maternal LP diet during pregnancy and lactation affects muscle protein synthesis through translation initiation pathway in weaning piglets.
Therefore, the present study was aimed to investigate, first, whether maternal LP diet affects MSTN signaling in skeletal muscle of offspring piglets; second, whether maternal LP diet affects serum amino acids concentration in offspring piglets; and third, whether maternal LP diet affects offspring muscle protein synthesis through translation initiation pathway.
Materials and methods
Animals and samples
The animal experiment was carried out in the National Meishan Pig Preservation and Breeding Farm at Jiangsu Polytechnic College of Agriculture and Forestry, Jurong, Jiangsu Province, P.R. China. Fourteen primiparous purebred Meishan gilts, with the body weight of 36.1 ± 1.8 kg in average, were assigned randomly into standard-protein (SP) and LP groups. It is reported that sows are very efficient at protein energy utilization and can tolerate very low dietary protein level without affecting fetal growth and development. DuPriest et al. [27] found that dietary protein level higher than 3 % did not induce growth restriction but lower than 0.5 % failed to support pregnancy. However, the extent of protein deficiency the sows can tolerate is dependent on the timing and the duration of dietary intervention, as well as the breed and physiological state of the sow. In the study of DuPriest et al., Yucatan microswine was used and the dietary treatments were applied during the last fourth of gestation plus 2 weeks of postnatal life. In this study, we used primiparous purebred Meishan sows, and the dietary treatments were applied from conception to weaning. Based on the consideration that Meishan pigs, being raised, over the last 30 years, with commercial diets composed following NRC standards, were traditionally raised on LP diets containing approximately half of the protein in the modern commercial diets, we fed the sows in SP group with diets containing 12 and 14 % crude protein, while those in LP group with diets containing 6 and 7 % crude protein to mimic the protein levels in traditional diets during gestation and lactation, respectively (Table 1). The dietary treatment started before artificial insemination at the first observation of estrus. Sows were fed twice daily (0800 and 1400 hours) with the rations of 1.8 and 2.6 kg/day during gestation and lactation, respectively. Litter size was adjusted to 7–8 pigs per litter at 24 h post-parturition in the same group. Newborn piglets were allowed free access to their mothers, and 14 male pigs (one per litter, seven per group) were killed at weaning stage (35 days). Longissimus dorsi (LD) muscles (removing visible fat and connective tissues) were taken from the right half of the carcass within 20-min postmortem, snap-frozen in liquid nitrogen and stored at −80 °C until further analysis.
The experiment was undertaken following the guidelines of Animal Ethics Committee of Nanjing Agricultural University.
Growth performances
Pigs were weighed before slaughter at the age of 35 days. The weight of LD muscle was also recorded after dissection.
Classic hematoxylin and eosin (H & E) staining was employed to measure the cross-sectional area (CSA) of myofibers in LD muscle of weaning piglets. Briefly, muscle blocks were excised perpendicularly to the direction of the myofibers, and serial tissue sections of 10 μm were cut using a cryostat, air-dried at room temperature for 15 min and followed by H & E staining. The serial sections were viewed on a light microscope (Olympus, Tokyo, Japan), and five areas were selected randomly for each animal for the measurement of myofibers CSA within one complete muscle bundle (fasciculus).
Branch amino acids (BCAAs) concentration in the plasma
Blood was collected into heparinized tubes, and then centrifuged at 3,000×g, 4 °C, for 10 min to isolate plasma. One milliliter plasma was used to measure the concentration of free amino acids. The protein in the plasma was removed and neutralized by using 1 mL 1.5 mol/L HClO4 and 0.5 mL 2 mol/L K2CO3. The concentration of amino acids was analyzed by HPLC, as described previously [28].
RNA isolation and qPCR
Total RNA was isolated from LD muscle using Trizol Reagent (TIANGEN Biotech Co, Ltd., China) according to manufacturer’s instructions. Concentration and quality of the extracted RNA were measured using a NanoDrop ND-1000 spectrophotometer. The iScriptTM cDNA synthesis kit (Promega, Madison, WI, USA) was used to synthesize cDNA from 2 μg of total RNA from each sample according to manufacturer’s instructions. Two microliters of diluted cDNA (1:20) was used in each real-time PCR. All primers (Table 2) were synthesized by Invitrogen (Invitrogen Life Technologies, Carlsbad, CA, USA). Real-time PCR was performed with Mx3000P (Agilent Technologies, CA, USA). In a pilot experiment, we detected the expression of three endogenous reference genes, 18S, GAPDH and peptidylprolyl isomerase A (PPIA). Among these three genes, 18S was not affected by the maternal dietary protein level and was thus chosen as a reference gene in this study. The specificity of amplification was determined by melting curve analysis and agarose gel electrophoresis. The PCR products were sequenced to validate the identity of the amplicons.
Tissue protein extraction and Western blot analysis
Total protein was extracted from frozen LD muscle using basic lysis buffer (150 mmol/L NaCl, 10 mmol/L Tris–HCl, 5 mmol/L EDTA, 1 % Triton X-100, 0.1 % SDS). The protease inhibitor cocktail (Roche Applied Science) was added according to the manufacturer’s instruction. The protein concentration was measured by Pierce BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Western blot analysis for MSTN, Smad2, phosphorylated Smad2, Smad3, phosphorylated Smad3, Smad4, JNK, phosphorylated JNK, extracellular signal-regulated kinase (ERK), phosphorylated ERK, p38 MAPK, phosphorylated p38 MAPK, p70S6K1, phosphorylated p70S6K1, eIF4E, phosphorylated eIF4E were carried out according to a previous publication [29]. The content of MSTN protein was normalized with GAPDH and presented as the fold change relative to SP. The antibodies used in this study were shown in Table 3.
Statistical analysis
All data are presented as mean ± SEM and were analyzed using independent samples T test with SPSS 13.0 for Windows. The method of 2−ΔΔCt was used to analyze the real-time PCR data, which are expressed as the fold change relative to the SP group. Differences were considered significant when P < 0.05.
Results
Growth performance and myofiber CSA
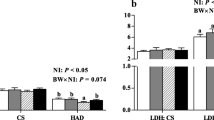
Body weight and LD muscle weight were both significantly lower in LP piglets than in SP piglets (Fig. 1a, b), and the ratio of LD muscle weight to body weight was significantly decreased (P < 0.001) in LP piglets (Fig. 1c). Furthermore, maternal LP diet significantly decreased (P < 0.05) the myofiber CSA in LD muscles (Fig. 1d).
Effect of maternal LP diet during gestation and lactation on body weight (a), LD muscle weight (b), the ratio of LD muscle weight and body weight (c) and myofiber CSA in LD muscles (d) of offspring piglets at weaning stage. Scale bar 40 μm. SP maternal standard-protein diet. LP maternal low-protein diet. The values shown represent the mean ± SEM, n = 7; *P < 0.05 compared with the SP group; ***P < 0.001 compared with the SP group
The plasma concentration of BCAAs
Maternal LP diet during gestation and lactation significantly decreased plasma concentration of BCAAs. Among 15 amino acids detected in the plasma, eight amino acids (leucine, isoleucine, valine, proline, methionine, glycine, serine, and alanine) were significantly lower (P < 0.05) in LP piglets when compared with SP piglets (Table 4). All the three BCAAs (leucine, isoleucine and valine) were significantly lower (P < 0.001) in the LP offspring piglets.
MSTN signaling
MSTN protein content tended to be up-regulated (P = 0.098) in LD muscle of LP piglets (Fig. 2a). In addition, the expression of MSTN type II receptor ActRIIB (Fig. 2b) and its type I receptor ALK5 (Fig. 2c) were both significantly increased (P < 0.05) in LP piglets. The downstream Smad pathway signals, including phosphorylated Smad2, phosphorylated Smad3 and total Smad4, were not different between LP and SP piglets (Fig. 2d, e). The phosphorylated p38 MAPK was significantly higher (P < 0.05) in LP piglets, yet no significant difference was detected for the phosphorylations of JNK and ERK between LP and SP piglets (Fig. 2f, g).
Effect of maternal LP diet during gestation and lactation on MSTN protein expression (a), MSTN receptor ActRIIB (b) and ALK5 (c) mRNA expression and the phosphorylation of MSTN downstream signaling Smads (D&E) and MAPK (F&G) in LD muscle of offspring piglets at weaning stage. SP maternal standard-protein diet. LP maternal low-protein diet. The values shown represent the mean ± SEM, n = 7; *P < 0.05 compared with the SP group
Muscle protein synthesis
The expression of p70S6K showed a tendency of decrease (P = 0.06) in LP piglets (Fig. 3a). In addition, maternal LP diet significantly (P < 0.05) down-regulated the phosphorylation of eIF4E (Fig. 3b).
Discussion
In the present study, maternal LP diet during gestation and lactation reduced body weight, LD muscle weight and myofiber CSA in the weaning piglets, which was consistent with previous observations in German Landrace pigs subjected to maternal protein restriction [5, 30]. The phenotypic changes in muscle characteristics are often associated with metabolic alterations. For instance, maternal protein restriction during pregnancy altered glucose metabolism in skeletal muscle of rat offspring [31]. Maternal high-protein/low-carbohydrate diet during lactation retarded offspring skeletal muscle growth in mice [32]. Rat offspring derived from protein restricted dams demonstrated disturbed gene expression in growth-related pathways in skeletal muscles [6]. In this study, maternal LP diet increased the expression of MSTN in the skeletal muscle of weaning piglets, which supports a previous report that maternal undernutrition during gestation resulted in the aberrant regulation of MSTN in the skeletal muscle of rat offspring [33]. It is noted that the expression of some house keeping genes is affected be by maternal low-protein diet, in a sex-, organ- and age-dependent manner [34]. Therefore, although the present study focused only on one sex (male), one organ (skeletal muscle) and one age (weaning stage, 35 days), cautions have been taken to select a suitable endogenous reference gene which is not affected by the experimental factor, the maternal dietary protein level.
In the present study, the up-regulation of MSTN expression in the skeletal muscle of LP offspring was associated with increased expression of MSTN receptors, ActRIIB and ALK5. The post-receptor signaling involves ALK-Smad signaling pathway [17, 35] and MAPK pathways including p38 [11], ERK1/2 [36] and JNK [12]. In the present study, no significant alterations were detected in the phosphorylation of Smad signaling molecules, or the phosphorylation of ERK1/2 or JNK. However, the phosphorylation of p38 MAPK was found to be significantly increased in the skeletal muscle of LP piglets, suggesting that MSTN-induced muscle growth retardation in this study is mediated predominantly by p38 MAPK pathway.
Amino acids are known to play important roles in skeletal muscle protein synthesis. We detected significant decrease in the plasma concentration of free amino acids in the offspring piglets born to sows fed LP diet throughout pregnancy and lactation. Among 15 amino acids detected in the plasma, eight amino acids (leucine, isoleucine, valine, proline, methionine, glycine, serine and alanine) were found to be significantly decreased. It is noted that all the three BCAAs (leucine, isoleucine and valine) were significantly lower in the LP offspring piglets. Similar observations were reported in sheep that nutritional restriction markedly reduced fetal plasma concentrations of BCAAs [37].
It is well known that amino acids, especially BCAAs, participate in the regulation of skeletal muscle protein synthesis through mTOR/S6K signaling pathway [38, 39]. Infusion of physiological levels of leucine significantly enhanced protein synthesis in the skeletal muscle of neonatal piglets [40]. Interestingly, mTOR/S6K signaling also mediates the effect of MSTN on skeletal muscle protein metabolism [17]. Genetic loss of MSTN leads to enhanced muscle expression and elevated activity of mTOR/S6K signaling components [16]. Therefore, mTOR/S6K signaling might be a common pathway to mediate the effects of MSTN and BCAAs on offspring muscle protein metabolism in the present study.
The pathway of eukaryotic translation initiation is known to be the downstream effector of mTOR signaling [41–43]. p70S6K and 4E-BP1 activity are regarded to be the markers for the activation of translation initiation pathway [44]. In this study, we failed to detect the phosphorylated 4E-BP1, due to poor specificity and affinity of the antibody. Nevertheless, the phosphorylated eIF4E, the partner of 4E-BP1, as well as phosphorylated p70S6K, a key factor in protein synthesis, were significantly reduced in the skeletal muscle of LP offspring piglets, suggesting a down-regulation of the global protein synthesis rate in skeletal muscle.
Taken together, we provide evidences that maternal LP diet throughout gestation and lactation causes retardation in muscle hypertrophy and protein synthesis, which appears to be mediated, at least partly, by the activation of MSTN signaling and the inhibition of BCAAs-activated mTOR pathway. The disrupted eukaryotic translation initiation pathway seems to serve as a common downstream effector and to contribute to the phenotypic alterations in the skeletal muscle of LP piglets at weaning stage. These findings may shed new light on the understanding of molecular mechanism underlying the fetal and neonatal nutritional programming of skeletal muscle growth.
References
DelCurto H, Wu G, Satterfield MC (2013) Nutrition and reproduction: links to epigenetics and metabolic syndrome in offspring. Curr Opin Clin Nutr Metab Care 16(4):385–391
Wood-Bradley RJ, Henry SL, Vrselja A, Newman V et al (2013) Maternal dietary intake during pregnancy has longstanding consequences for the health of her offspring. Can J Physiol Pharmacol 91(6):412–420
Lukaszewski MA, Eberlé D, Vieau D, Breton C (2013) Nutritional manipulations in the perinatal period program adipose tissue in offspring. Am J Physiol Endocrinol Metab 305(10):E1195–E1207
Rao KR, Padmavathi IJ, Raghunath M (2012) Maternal micronutrient restriction programs the body adiposity, adipocyte function and lipid metabolism in offspring: a review. Rev Endocr Metab Disord 13(2):103–108
Rehfeldt C, Lefaucheur L, Block J, Stabenow B et al (2012) Limited and excess protein intake of pregnant gilts differently affects body composition and cellularity of skeletal muscle and subcutaneous adipose tissue of newborn and weanling piglets. Eur J Nutr 51(2):151–165
Cabeço LC, Budri PE, Baroni M, Castan EP et al (2012) Maternal protein restriction induce skeletal muscle changes without altering the MRFs MyoD and myogenin expression in offspring. J Mol Histol 43(5):461–471
Mortensen OH, Olsen HL, Frandsen L, Nielsen PE et al (2010) Gestational protein restriction in mice has pronounced effects on gene expression in newborn offspring’s liver and skeletal muscle; protective effect of taurine. Pediatr Res 67(1):47–53
Mortensen OH, Olsen HL, Frandsen L, Nielsen PE et al (2010) A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J Biomed Sci 17(Suppl 1):S38
Zheng S, Rollet M, Pan YX (2011) Maternal protein restriction during pregnancy induces CCAAT/enhancer-binding protein (C/EBPbeta) expression through the regulation of histone modification at its promoter region in female offspring rat skeletal muscle. Epigenetics 6(2):161–170
Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ et al (2003) Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 23(20):7230–7242
Philip B, Lu Z, Gao Y (2005) Regulation of GDF-8 signaling by the p38 MAPK. Cell Signal 17(3):365–375
Huang Z, Chen D, Zhang K, Yu B et al (2007) Regulation of myostatin signaling by c-Jun N-terminal kinase in C2C12 cells. Cell Signal 19(11):2286–2295
Carneiro I, González T, López M, Señarís R et al (2013) Myostatin expression is regulated by underfeeding and neonatal programming in rats. J Physiol Biochem 69(1):15–23
Peiris HN, Ponnampalam AP, Mitchell MD, Green MP (2010) Brief Communication: sexual dimorphic expression of myostatin and follistatin like-3 in a rat trans-generational under-nutrition model. Nutr Metab (Lond) 7:44
Liu X, Wang J, Li R, Yang X et al (2011) Maternal dietary protein affects transcriptional regulation of myostatin gene distinctively at weaning and finishing stages in skeletal muscle of Meishan pigs. Epigenetics 6(7):899–907
Lipina C, Kendall H, McPherron AC, Taylor PM et al (2010) Mechanisms involved in the enhancement of mammalian target of rapamycin signalling and hypertrophy in skeletal muscle of myostatin-deficient mice. FEBS Lett 584(11):2403–2408
Sartori R, Milan G, Patron M, Mammucari C et al (2009) Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol 296(6):C1248–C1257
Malandro MS, Beveridge MJ, Kilberg MS, Novak DA (1996) Effect of low-protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol 271(1 Pt 1):C295–C303
Regnault TR, Friedman JE, Wilkening RB, Anthony RV et al (2005) Fetoplacental transport and utilization of amino acids in IUGR—a review. Placenta 26(Suppl A):S52–S62
Glynn EL, Fry CS, Drummond MJ, Timmerman KL et al (2010) Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 140(11):1970–1976
Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D (2012) Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr 31(4):512–519
Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA et al (2008) Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295(4):E868–E875
Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA et al (2010) Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140(12):2145–2152
Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA et al (2012) Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71(4 Pt 1):324–331
Carrera AC (2004) TOR signaling in mammals. J Cell Sci 117(Pt 20):4615–4616
Yin Y, Yao K, Liu Z, Gong M et al (2010) Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39(5):1477–1486
Dupriest EA, Kupfer P, Lin B, Sekiguchi K et al (2012) Accelerated growth without prepubertal obesity in nutritionally programmed microswine offspring[J]. J Dev Orig Health Dis 3(2):92–102
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424
Li LA, Xia D, Wei S, Li X et al (2008) Diminished expression of acth signaling proteins and steroidogenic limiting factors in adrenocortical cells isolated from halothane nn pigs. Domest Anim Endocrinol 35:1–7
Altmann S, Murani E, Schwerin M, Metges CC et al (2012) Maternal dietary protein restriction and excess affects offspring gene expression and methylation of non-SMC subunits of condensin I in liver and skeletal muscle. Epigenetics 7(3):239–252
Zheng S, Rollet M, Pan YX (2012) Protein restriction during gestation alters histone modifications at the glucose transporter 4 (GLUT4) promoter region and induces GLUT4 expression in skeletal muscle of female rat offspring. J Nutr Biochem 23(9):1064–1071
Rehfeldt C, Langhammer M, Kucia M, Nürnberg G et al (2013) Enhanced sensitivity of skeletal muscle growth in offspring of mice long-term selected for high body mass in response to a maternal high-protein/low-carbohydrate diet during lactation. Eur J Nutr 52(3):1201–1213
Peiris HN, Ponnampalam AP, Osepchook CC, Mitchell MD et al (2010) Placental expression of myostatin and follistatin-like-3 protein in a model of developmental programming. Am J Physiol Endocrinol Metab 298(4):E854–E861
DuBois B, Pearson J, Hastings B, Mahmood T et al (2013) Maternal low-protein diet alters the expression of real-time quantitative polymerase chain reaction reference genes in an age-, sex-, and organ-dependent manner in rat offspring. Nutr Res 33(3):235–241
Miyake M, Hayashi S, Taketa Y, Iwasaki S et al (2010) Myostatin down-regulates the IGF-2 expression via ALK-Smad signaling during myogenesis in cattle. Anim Sci J 81(2):223–229
Yang W, Chen Y, Zhang Y, Wang X et al (2006) Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res 66(3):1320–1326
Kwon H, Ford SP, Bazer FW, Spencer TE et al (2004) Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids. Biol Reprod 71(3):901–908
Drummond MJ, Bell JA, Fujita S, Dreyer HC et al (2008) Amino acids are necessary for the insulin-induced activation of mTOR/S6K1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin Nutr 27(3):447–456
Pruznak AM, Kazi AA, Frost RA, Vary TC et al (2008) Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr 138(10):1887–1894
Davis TA, Fiorotto ML (2009) Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12(1):78–85
Mayer S, Wrenzycki C, Tomek W (2014) Inactivation of mTor arrests bovine oocytes in the metaphase-I stage, despite reversible inhibition of 4E-BP1 phosphorylation. Mol Reprod Dev 81(4):363–375
Martineau Y, Wang X, Alain T, Petroulakis E et al (2014) Control of Paip1-eukayotic translation initiation factor 3 interaction by amino acids through S6 kinase. Mol Cell Biol 34(6):1046–1053
Fingar DC, Richardson CJ, Tee AR, Cheatham L et al (2004) mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24(1):200–216
Avruch J, Long X, Ortiz-Vega S, Rapley J et al (2009) Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 296(4):E592–E602
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB124703, 2004CB117505), the Major National Science and Technology Program (2009ZX08009-138B), the Special Fund for Agro-scientific Research in the Public Interest (201003011), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of interest
Xiujuan Liu, Shifeng Pan, Xiao Li, Qinwei Sun, Xiaojing Yang, and Ruqian Zhao declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiujuan Liu and Shifeng Pan have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, X., Pan, S., Li, X. et al. Maternal low-protein diet affects myostatin signaling and protein synthesis in skeletal muscle of offspring piglets at weaning stage. Eur J Nutr 54, 971–979 (2015). https://doi.org/10.1007/s00394-014-0773-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0773-1