Abstract
Purpose
To further inform the debate on the possible cognitive benefits of antioxidant nutrients in the elderly, we systematically reviewed available prospective studies while paying a special attention to their methodological quality.
Methods
This is a systematic review of studies involving major antioxidant nutrients and change in cognitive performance. Abstracts were independently reviewed; studies were selected based on prespecified criteria. Methodological quality of primary studies was assessed using a methodological checklist for cohort studies. Findings were presented using a narrative synthesis and tabulation of results.
Results
Eight-hundred and fifty potentially eligible studies were identified; 10 met the inclusion criteria and were retained for data extraction and appraisal. The main supportive evidence came from two studies, both judged to be of high quality: The first observed an accelerated decline in global cognition, attention, and psychomotor speed over 9 years, concomitant to a decrease in plasma selenium levels over the same period; the second study reported a slower rate of global cognitive decline over 3 years in persons in the highest quartile of intake of vitamins C, E, and carotenes. All associations persisted after adjustment for confounding factors. Evidence in favor of beneficial associations of higher dietary intake of vitamin E and flavonoids, as well as higher serum beta carotene levels, came from further studies of only adequate quality.
Conclusions
There is a possibility for protective effects of antioxidant nutrients against decline in cognition in older people although the supportive evidence is still limited in number. This association deserves further examination in additional quality investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intact cognitive function is critical to the health and well-being of older people [1]. As people age, many individuals experience a decline in one or more areas of cognitive function, such as memory and processing speed [2]. Whereas subtle cognitive deficits may prevent a person from performing at the highest possible level of ability, impaired cognitive function, which manifests itself in mild to severe changes across different cognitive domains, is a major determinant of long-term institutionalization and dependency among older people [2, 3].
As more individuals reach old age, impaired cognition is predicted to become a major public health challenge. For example, it is thought that between 5 and 10 % of persons aged 65 years and older and 30 % of those over 80 years of age suffer from dementia, a clinical syndrome characterized by progressive brain deterioration that results in overt cognitive dysfunction [4–7]. By mid-twenty-first century, the number of older people with dementia may more than triple [8]. Many will have experienced mild cognitive deficits for several years before converting to full-blown dementia [9]. Since effective treatment for dementia is currently unavailable, these projections underscore the importance of efforts directed at preventing or delaying its onset, including the identification and correction of risk factors for early cognitive changes in non-demented older people [10, 11].
Oxidation is one potentially important component of the physiologic substrate underlying cognitive impairment that has received considerable attention in studies into the causes, treatment, and prevention of age-related cognitive decline and dementia [12]. Specifically, oxidative stress, which denotes a shift toward the pro-oxidant in the pro-oxidant/antioxidant balance following increase in oxidative metabolism, has been suggested as an etiological factor in human aging and several major age-related diseases, including cancer, cardiovascular diseases, and neurodegenerative diseases such as Alzheimer’s disease (AD), dementia, and Parkinson’s disease [13–16]. It is possible that the high lipid and iron contents of the central nervous system, coupled with its high aerobic metabolic activity, make it particularly susceptible to oxidative damage by free reactive oxygen species, especially when antioxidant defenses against oxidative stress become insufficient or depleted. In fact, considerable effort has gone into determining the effects of antioxidant nutrients on the risk and progression of dementia in susceptible individuals, although the results of several randomized antioxidant vitamin trials have been disappointing [12, 17]. For example, a recent systematic review of clinical trials undertaken by the Cochrane Dementia and Cognitive Improvement Group concluded that there is currently no evidence of efficacy of vitamin E in the prevention of or treatment for mild to severe dementia [18]. It is possible, however, that a number of methodological issues limited the ability of these trials to detect any effects of vitamin E on cognition [17].
In contrast, findings from epidemiological research on the benefits of antioxidant nutrients for cognitive function have been somewhat more consistent despite substantial methodological differences among studies [17]. As an example, a recent, broad-focused, systematic review of cohort studies of major lifestyle risk factors and cognitive function noted protective cognitive effects of higher vitamin E levels in a few primary studies included in the review but not in several others [19]. None of the studies reviewed, however, reported beneficial effects of vitamin C, carotenes, or flavonoids on cognition. Beyond these results, the review offered no evaluation of the underlying reasons for the observed discrepancy in findings, including the potential influence of between-study variability in antioxidant nutrient type and measurement, control for important confounding factors, and definition and assessment of cognitive function.
The need for a systematic evaluation of the available evidence for antioxidants and cognitive function has been emphasized [20]. To further inform the debate on the potential cognitive benefits of antioxidants, the aim of the present study was to systematically review findings from population-based cohort studies regarding the association between antioxidant nutrients and cognitive function in older people, while paying a special attention to the methodological quality of individual studies.
Methods
Study eligibility criteria
We followed published guidelines produced by the meta-analysis of observational studies in epidemiology (MOOSE) group on reporting systematic reviews of observational epidemiological studies although the present review was restricted to English language–reported studies [21]. Other eligibility criteria further limited the primary studies included in the review to original, population-based, cohort studies that provided at least a single baseline measurement of one or more major antioxidant nutrients [22, 23], including carotene, flavonoids, antioxidant vitamins C and E, and selenium, in addition to assessment of cognitive function with standardized tests on at least two different occasions. The following types of studies were considered ineligible for inclusion in the review: non-original studies, those based on non-human samples, those using other types of study designs (e.g., cross-sectional or case–control epidemiological study designs), studies based on hospital inpatients or other selected patient samples, those without appropriate antioxidant nutrient data (e.g., studies lacking data on individual nutrients), and studies without information on cognitive performance assessed on more than one occasion (or those only having diagnostic information pertinent to cognition, for example, presence or absence of cognitive impairment or dementia).
Bibliographic databases, literature search strategy, and data extraction
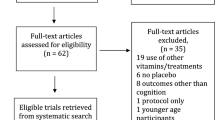
In order to identify relevant studies, the following bibliographic databases were searched from inception: MEDLINE (1950–October week 3 2010); EMBASE (1980–week 43 2010); Global Health (1973–October 2010); the Commonwealth Agricultural Bureau (CAB) abstracts (1973–week 42 2010); and PsychINFO (1806–October week 4 2010). Reference lists of all full-text articles retrieved for further evaluation were also inspected (see step 4 in Fig. 1).
The literature search strategy was devised using a combination of text words and medical subject headings (MeSH) in order to maximize retrieval [24]. The following common and scientific terms were used for the exposure of interest [antioxidants, ascorbic acid, vitamin C, vitamin E, tocopherols, tocotrienols, carotenoids, beta carotene, flavonoids, selenium, and phenols], the study outcome [cognitive function, cognitive decline, cognitive change, mental decline, general cognition, mental state, neuropsychology, intelligence, attention, executive function, memory, problem solving, perception, psychomotor speed, visuospatial, language, and learning], and the study design [cohort study, follow-up study/studies, and prospective study]. A similar search string was adopted for each of the five databases.
All data searches were performed by S.B.R. Titles and abstracts of identified studies were screened independently by two reviewers (S.B.R. and V.D.) for eligibility according to the previously described inclusion criteria. Based on calculations [25], the overall agreement between the two reviewers was 94 %. Relevant full-text articles were subsequently retrieved and further assessed for suitability for inclusion in the review. Any disagreement arising was resolved by discussion. A third reviewer (A.T.) was available for consultation where discrepancies remained. The following information was extracted by the two reviewers from each study using a predesigned evaluation form: study design and population [e.g., date of fieldwork, length of follow-up, population size, sex, and age], antioxidant nutrients [e.g., type of nutrient assessed, assessment method, and date of assessment], evaluation of cognitive function [e.g., names of individual cognitive tests, length of cognitive follow-up, and date of cognitive assessment], data analysis [e.g., confounding factors adjusted for in multivariate analysis], and study results [e.g., direction and size of associations of each antioxidant nutrient with performance on each cognitive test].
Study quality assessment
The primary studies included in the review were assessed for methodological quality using a pro forma for prospective cohort studies [26]. The quality appraisal focused on assessing the internal and external validity of the primary studies by evaluating specific methodological components of the study design, and the conduct and analysis of each study. Few quality criteria were met if studies scored <4 (out of 12); studies met some criteria if they scored between 5 and 8; studies scoring more than 8 were considered to meet most of the quality criteria. The results of the quality appraisal were used to inform the overall evaluation of the studies included in the review.
We evaluated between-study heterogeneity with respect to the type and assessment of antioxidant nutrients (e.g., studies variably used biomarker data, data on antioxidant nutrient supplement use, and dietary methods to determine availability of these nutrients), the definitions of thresholds for effects of individual nutrients, and the types of statistical comparisons made (e.g., whether effects were examined across tertiles or quartiles). We also evaluated whether there was variability between studies regarding how cognitive function was assessed (e.g., what cognitive tests were administered, what the length of time between individual cognitive assessments was, and how the cognitive outcome data were analyzed). Other sources of heterogeneity considered were between-study differences regarding the study sample characteristics, the length of time that passed between assessments of study exposures and outcomes, and the way the data were analyzed (e.g., what potential confounding factors were controlled for in a multivariate analysis). We used narrative synthesis and tabulation of results to present the findings instead of a meta-analysis [27].
Results
Study characteristics
Of the 850 potentially eligible studies that we identified, 10 met the inclusion criteria and were retained for data extraction and quality assessment (Fig. 1). Details of the study populations and methods are shown in Table 1. The included studies came from five different countries: Four studies were USA-based [28–31], two were from the Netherlands [32, 33], two were from France [34, 35], one study came from Canada [36], and there was one from Portugal [37]. Nine studies used a conventional, prospective, cohort design where the participants were followed from baseline to the point they underwent cognitive testing (or alternatively until they were lost to follow-up). One study used a case–control design nested within a prospective cohort study [33]. In this study, case status was based on a predetermined definition of change in cognitive test performance over an average of 6.5 years. All study populations consisted of community-residing older persons. Eight studies included both men and women although their proportion in each study varied. One study included only older men [32], whereas another was based on older female registered nurses [31].
The number of individual antioxidant nutrients assessed in different studies ranged from one to six. Information on antioxidant vitamins C and E and tocopherols was available in seven studies [28, 30–33, 36, 37]. Four studies collected information on carotenes [29–32], and two on flavonoids [32, 35] and selenium [34, 37] (Table 1). Other antioxidant nutrients were measured less frequently. Therefore, in order to be able to compare findings across studies, we further restricted this review to those nutrients that were present in at least two of the investigations.
In five studies, levels of individual antioxidant nutrients were estimated from food intake data [28, 30, 32, 35, 37], four studies directly determined antioxidant levels in the blood [29, 31, 33, 34], and in one study [36], information on antioxidant nutrient levels was based on self-reported use of vitamin supplements. Furthermore, and in line with the eligibility criteria of this review, all studies assessed change in cognitive test performance over at least two separate time points (Table 1). The reported time between the two assessments (or the first and the most recent from those studies from which more than two such assessments were carried out) ranged from a mean of 8.5 months [37] to a mean of 10 years [35]. Where information on more than one cognitive follow-up period is available in a study, our discussion focuses on the longest one reported (or alternatively the one showing the strongest association for a given antioxidant exposure—see e.g., ref. [30]). All 10 studies administered tests of overall (global) cognitive function, most commonly the mini-mental status examination (MMSE); four studies also used a variety of other tests for assessing domain-specific function, including memory performance [28, 31, 35], executive function [28], attention [34, 35], and psychomotor speed [34, 35].
Study quality assessment
As shown in Table 2, the 10 studies varied substantially in potential for bias. Five studies met most, and the other five met some, of the quality assessment criteria (i.e., fulfilled more than eight or between five and eight of the 12 criteria, respectively) although they further differed with respect to which specific criteria each met. All studies clearly reported the research hypothesis/question under study and described the source or eligible study population. Participation rates, or proportions, were provided for seven studies [28–33, 35]. Six studies evaluated, and subsequently excluded from the analysis, subjects with overt cognitive impairment or dementia [28–30, 33, 35, 36]. Two studies compared participants lost to follow-up with the full sample, or those remaining in the study, by antioxidant nutrient status [29, 34]. The main study outcomes were clearly described in all studies although in only two studies was the study outcome determined blind with respect to antioxidant exposure status [28, 33]. Four studies provided information on efforts taken to ensure a valid assessment of antioxidant nutrients [28, 31–33]. Two studies assessed antioxidant nutrient levels at two or more occasions [32, 34]. In four studies, the multivariate analysis controlled for potential confounding by one or more conventional cardiovascular risk factors such as smoking, blood pressure level, and blood lipids [29, 32, 34, 36]. Of these studies, three also controlled for prior level (i.e., the baseline performance score) of cognitive function [29, 32, 36] and two also adjusted for prevalent cardiovascular diseases, such as stroke, as well as diabetes [34, 36].
Carotenes and cognitive decline
Table 3 shows that four studies examined the relationship between carotenes and change in cognitive function. One study [29] found that high serum beta carotene levels (≥0.19 μmol/L) were related to smaller decline over 7 years in global cognitive function although the effects were only found in APOE 4-positive individuals. Another study [30] reported that subjects consuming the highest quartile of carotene (from food alone or from food and supplements combined) had slower rates of cognitive decline than those in the lowest quartiles of intake at the 3-year follow-up, although at the 7-year follow-up, the association was no longer observed. In contrast, two other studies did not find any association between carotene levels and cognitive function; the former examined the association between high dietary beta carotene levels and decline in global cognitive function over a 3-year period [32], while the latter studied the relationship between high plasma levels of beta carotene (as well as total carotene levels) and decline in global cognitive function and memory performance over a 4-year follow-up [31].
Flavonoids and cognitive decline
The association between flavonoids and cognitive decline was determined in two studies (Table 3). One observed a slower rate of global cognitive decline over 10 years in subjects with high (3rd and 4th quartiles) total dietary flavonoid intake [35]. In contrast, in the second study, higher dietary levels of flavonoids were not associated with a reduced likelihood of decline over 3 years in global cognitive function [32].
Selenium and cognitive decline
Two studies reported on the association between selenium levels and change in cognitive performance over time, including the apparent effect on cognitive function of declining plasma selenium levels (Table 3). Thus, the first [34] showed that the probability of decline over 9 years of follow-up in global cognitive function, attention, and psychomotor speed was increased in relation to declining plasma selenium levels over the same 9-year period (but not over 2 years). In contrast, the second [37] found no difference in mean selenium dietary intake levels between persons whose global cognitive performance improved over time or not over a follow-up of 8.5 months.
Vitamin C and E and cognitive decline
Information on the association between vitamin C levels and cognitive decline was gathered from four studies (Table 3). One [30] found that participants in the higher quartiles of vitamin C intake from food only (but not food and supplements combined) had slower rates of cognitive decline than those in the lowest quartile of vitamin C intake at the 3-year follow-up (but not at the 7-year follow-up). In contrast, high dietary vitamin C intake was not associated with general cognitive decline over 3 years in another study [32]. Similarly, a third study [36] failed to find an association of vitamin C supplements alone with change in general cognitive function. Lastly, a Portuguese study found no significant difference in average vitamin C dietary intake levels when persons who did and did not show improvement in global cognitive function were compared after a short follow-up of 8.5 months [37].
Seven studies examined the associations of vitamin E (or different tocopherol forms) with the rate of cognitive decline (Table 3). One investigation [30] found that subjects consuming the highest quartile of vitamin E (from food alone or in combination with supplements) had slower rates of general cognitive decline than participants in the lowest quartile at the 3-year follow-up (although at the 7-year follow-up, the previous association was no longer observed). Similarly, a second study [28] showed that high food intake of vitamin E was linearly and negatively associated with the rate of decline in global cognitive function over an average of 6 years. In contrast, five other studies reached a different conclusion. The first of these [32] did not find any association between a high dietary vitamin E intake and decline in general cognitive function over a 3-year period. Similar null results were reported by a second study [33] which examined global cognitive decline in relation to plasma vitamin E levels. A third investigation [36] also failed to find an association between vitamin E supplements alone and the rate of general cognitive decline over 5 years. Another study of female registered nurses [31] further noted no association between total plasma tocopherol levels and 4-year change in global and domain-specific cognitive functions. The final study [37] failed to detect a difference in average vitamin E dietary intake between individuals who did and did not demonstrate improvement in general cognitive function over a follow-up of 8.5 months.
Discussion
Main findings and interpretation
The main aim of this systematic review was to examine the available evidence for an association between major antioxidant nutrients and decline in cognitive function in older people from population-based cohort studies. Although there was substantial between-study methodological heterogeneity, findings in favor of this association were observed in a total of five investigations [28–30, 34, 35].
The main evidence in support of an association came principally from two studies, both judged to be of high methodological quality since each met most of the criteria used for assessing the validity of the primary studies included in the review (e.g., whether the determination of main study exposures and outcomes was reliable, whether important confounding factors were adjusted for in the data analysis, etc.—see Table 2). In the first [34], the investigators reported that declining plasma levels of selenium (over a period of 9 years) were associated with a similar 9-year decline in global cognitive function, as determined by performance on the mini-mental state examination (MMSE). Moreover, the authors also noted that performance on two domain-specific cognitive tests (assessing attention and psychomotor speed) was also negatively affected by declining selenium plasma levels. Importantly, these associations persisted after the authors controlled for potential confounding by multiple sociodemographic and physiologic factors, as well as comorbidity. The second study [30] observed a slower rate of decline in global cognitive function (as determined by performance on the modified mini-mental state examination or 3MS test) over a modest follow-up of 3 years in subjects with the highest levels of vitamin C (from diet only), vitamin E (both from diet and from diet and supplements combined), and carotenes (both from diet and from diet and supplements combined), even after controlling for multiple potential confounding factors, such as sociodemographic and lifestyle factors, other antioxidants, and concomitant chronic diseases. However, at the 7-year follow-up, the protective effects of antioxidants against cognitive decline were no longer observed.
Three further investigations, all rated as being of adequate quality, provided further evidence in support of an association between antioxidant nutrients and cognitive decline. Thus, the first [28] observed a negative, linear relationship between dietary intake of vitamin E and 6-year decline in global cognitive function, which was based on combining four individual cognitive tests. Although the authors controlled for possible confounding by sociodemographic characteristics as well as intake of vitamin C, confounding effects of other lifestyle, physiologic, or comorbid variables cannot be ruled out. Similarly, in another study [29], serum beta carotene levels equal to or >0.19 μmol/L were associated with smaller decline in global cognitive function (as determined by performance on the Short Portable Mental Status Questionnaire, SPMSQ) over a follow-up of 7 years; however, the association was limited to APOE 4-positive persons. In the last of the three studies [35], the authors reported a slower decline in global cognitive function (which was based on combining three individual cognitive tests) over a 10-year period in persons in the two highest quartiles (13.60–17.69 and 17.70–36.94 mg/day, respectively) of total intake of flavonoids. Since these comparisons were only adjusted for age, sex, and education, potential confounding by other antioxidants, multiple lifestyle factors or concurrent morbidity is still possible.
In several studies, however, the majority of the associations between individual antioxidants and cognitive outcomes we reviewed were reported as null. More specifically, no beneficial cognitive effects of beta carotene were observed in three studies [30–32]; of flavonoids in one study [32]; of selenium in one study [37]; of vitamin C in three studies [32, 36, 37]; or of vitamin E in a total of six studies [30–33, 36, 37]. Although the exact reasons for this discrepancy may be hard to determine, this review suggests that comparisons based on a modest sample size as well as those involving performance on general cognitive status rather than domain-specific tests (the latter may be more sensitive to mild or subtle cognitive deficits and changes in performance over time), and those in which the period of cognitive change was limited due to a relatively short cognitive follow-up, were more likely to be nonsignificant. Differences and possible errors in the exposure assessment (e.g., of antioxidants from diet), as well as residual confounding by either unmeasured or uncontrolled confounding factors in some of the analyses, could also partly explain the variable results across studies.
Despite this discrepancy, a relationship between antioxidants and cognitive function may still be biologically plausible. Antioxidants might benefit cognitive function by influencing several different metabolic pathways affected by oxidative stress. For example, although it is still unclear whether the types of lesions that characterize AD pathology (B-amyloid and tau proteins) induce, occur consequent to, or are associated statistically but non-causally with oxidative stress, growing body of evidence implicates oxidative stress and generation of free radicals in at least the propagation of cellular injury and death that leads to neurodegenerative disease [38.39]. As a result, the inhibition of oxidative stress therapeutically (e.g., by antioxidant nutrients) might help break the cycle of cell death [39]. In AD, oxidative stress is not only high but also chronic and is superimposed upon an age-related vulnerable environment [38]. In addition to its involvement in AD pathology, oxidative stress has also been identified as critical component of many of the steps in the pathophysiology of atherosclerosis and acute thrombotic events, including dyslipidemia leading to the formation of atheroma, the oxidation of low-density lipoproteins, endothelial damage, plaque rupture, myocardial ischemic injury, and recurrent thrombosis, all pathways that may be associated with the development and progression of vascular cognitive impairment and dementia [40, 41]. Hence, this association deserves further investigation.
Strengths and limitations
We consider the strengths of this systematic review to be grounded in the following attributes: the use of a search strategy which included several bibliographic databases, each of which was searched from inception over an extended time period; the use of multiple search terms for the main study exposures and outcomes; and the use of published guidelines for conducting and reporting systematic reviews of observational epidemiological studies [21]. Moreover, the selection, data extraction, and the study quality appraisal were performed independently by two reviewers, resulting in high overall agreement. The methodological aspects of each included study were further assessed using previously published criteria designed specifically for appraising cohort studies [26]. As a potential downside, however, we included only published, English language–reported studies. Although unlikely, it is still possible that we failed to identify either studies published in other languages or non-published material.
Our objective was to identify studies with information on those antioxidant nutrients that have received the most attention in epidemiological studies [22, 23], and in order to be able to compare the results across studies, we restricted the reporting to those antioxidants that were available in at least two studies. However, in a few other investigations, the study authors reported potentially beneficial cognitive effects of additional nutrients with potential antioxidant properties, including vitamin A [33] and different forms of tocopherols [28], although we did not include these in our main discussion.
In addition, we limited the review to studies that assessed performance on one or more cognitive tests on at least two occasions. Other studies have focused on assessment of cognitive function at a single point in time. Change in cognitive function is, however, considered to be less prone to error than cognitive performance on a single occasion and is also likely to be more relevant as a study outcome since the pathological process of interest (i.e., cognitive decline) is characterized by change [42]. Still, other studies have defined cognitive function (or impairment) based on diagnostic criteria or specific cut-off levels. Differences in available criteria and assessment procedures have been described [43], and the inclusion of these studies might have added further methodological heterogeneity to our review. Also, we excluded studies using dementia as the main study outcome, since examining the influence of potentially modifiable risk factors early in the process of cognitive decline, and before the onset of impaired cognition, is likely to be more relevant for informing strategies aimed at preventing or delaying the onset of cognitive impairment and dementia.
Furthermore, the present review was restricted to the inclusion of epidemiological studies using a cohort design although we are aware of other published investigations examining the association under review here using different study designs (e.g., cross-sectional and case–control studies). When we want to infer about the potential causal effects of a particular exposure, the total relevant evidence needs to be assessed (including from randomized controlled trials). The purpose of this review was, however, to examine data on the association of antioxidant nutrients with cognitive decline in neurologically intact older people in general population cohort studies. These studies are less prone to selection and information bias than other types of observational epidemiological studies and provide results that are frequently more generalizable than those from randomized intervention studies.
Finally, we used a narrative rather than a statistical approach to synthesizing the results from the individual studies included in the review. We are, of course, aware that a meta-analysis is frequently undertaken as a part of systematic reviews of observational epidemiological studies despite being a contentious issue [44]. In our opinion, performing a meta-analysis of the results from these cohort studies (and in the absence of individual patient data) would have been unhelpful, given the heterogeneity of exposures and outcomes among the individual studies.
Future directions
A systematic review offering critical assessment of available studies can be useful for informing planning of future studies. The results we report here occur in the context of substantial methodological heterogeneity across available investigations. Future studies need to be large enough to allow adequate statistical power and should ensure ample follow-up time with respect to the study of change across multiple domains of cognitive function in neurologically intact older people. Antioxidant nutrients need to be assessed comprehensively; biomarkers should be examined whenever possible since dietary recall in older people may be affected by impaired cognitive function. The results from this review do not allow us to recommend a particular antioxidant nutrient for study in future investigations. In fact, focusing on a single nutrient may be inadequate, and a group or a panel of antioxidant nutrients (or an antioxidant capacity index) may need to be considered. Moreover, repeat measurements of antioxidant biomarkers should be attempted since single biomarker measurements may be prone to substantial random temporal fluctuations. Such temporal variation may deflate the association between antioxidant biomarkers and cognitive outcomes. Also, more thought needs to be given to the issue of confounding in future investigations. Specifically, which potential confounding factors are important, and how they are measured, needs to be considered since this may reduce the likelihood of residual confounding (for all of the studies we reviewed, this cannot be excluded as a potential explanation for the observed findings). As a final point, more life-course studies are needed for examining the association between antioxidant nutrients and age-related cognitive decline, given the likelihood of reverse causation in such investigations, and the possibility that effects of antioxidant nutrients against cognitive deterioration may be observed over the life span rather than only in old age.
Conclusions
This systematic review examined the available evidence for the potential beneficial effects of major antioxidant nutrients against age-related cognitive decline in population-based cohort studies. Relatively few, relevant, investigations were identified based on the inclusion criteria used. Substantial methodological heterogeneity was observed across studies which precluded a quantitative meta-analysis of the results. Overall, there is some evidence, albeit from a limited number of high-quality investigations, in support of beneficial cognitive effects of antioxidant nutrients, highlighting the need for additional and longer investigations.
References
Fried LP (2000) Epidemiology of aging. Epidemiol Rev 22:95–106
Waldstein SR, Elias MF (2003) Introduction to the special section on health and cognitive function. Health Psychol 22:555–558
Melzer D, Ely M, Brayne C (1997) Cognitive impairment in elderly people: population based estimate of the future in England, Scotland, and Wales. Brit Med J 315:462
Haan MN, Wallace R (2004) Can dementia be prevented? Brain aging in a population-based context. Annu Rev Public Health 25:1–24
Ritchie K, Lovestone S (2002) The dementias. Lancet 360:1759–1766
Seshadri S, Wolf PA (2007) Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol 6:1106–1114
van der Flier WM, Scheltens P (2005) Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry 76:v2–v7
Alzheimer’s Disease International (2010) World Alzheimer report 2010. Alzheimer’s Disease International, London
Bruscoli M, Lovestone S (2004) Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr 16:129–140
Purandare N, Ballard C, Burns A (2005) Preventing dementia. Adv Psychiatr Treat 11:176–183
Román GC (2003) Stroke, cognitive decline and vascular dementia: the silent epidemic of the 21st century. Neuroepidemiology 22:161–164
Haan MN (2003) Can vitamin supplements prevent cognitive decline and dementia in old age? Am J Clin Nutr 77:762–763
Christen Y (2000) Oxidative stress and Alzheimer diseases. Am J Clin Nutr 71:621S–629S
Langseth L (1995) Oxidants, antioxidants, and disease prevention. International Life Sciences Institute (ILSI Europe), Brussels
Singh RP, Sharad S, Kapur S (2004) Free radicals and oxidative stress in neurodegenerative diseases: relevance of dietary antioxidants. J Ind Acad Clin Med 5:218–225
Willcox BJ, Curb JD, Rodriguez BL (2008) Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol 101:75D–86D
Morris MC, Christine TC (2011) A potential design flaw of randomized trials of vitamin supplements. J Am Med Assoc 305:1348–1349
Isaac M, Gad El Kareem N, Quinn R, Tabet N (2008) Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev 3. doi:10.1002/14651858.CD002854.pub2
Lee Y, Back JH, Kim J, Kim S-H, Na DL, Cheong H-K, Hong CH, Kim YG (2010) Systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr 22:174–187
Serra-Majem L, Roman B, Estruch R (2006) Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2:S27–S47
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. J Am Med Assoc 283:2008–2012
Mayne ST (2003) Antioxidant nutrients and chronic disease: use of biomarkers of exposure and of oxidative stress status in epidemiologic research. J Nutr 133:933S–940S
Mayne ST, Wright ME, Cartmel B (2004) Assessment of antioxidant nutrient intake and status for epidemiologic research. J Nutr 134:3199S–3200S
Sahu DR, Ramakantan RR, Bavdekar SB (2000) The art and science of web-based literature search: the MEDLINE. J Postgrad Med 46:123
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308
The Scottish Intercollegiate Guidelines Network (2001–2012) http://www.sign.ac.uk. Accessed 14 Dec 2011
Centre for Reviews and Dissemination (CRD) (2009) Systematic reviews: CRD’s guidance for undertaking reviews in health care. http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf. Accessed 3 Apr 2012
Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, Scherr PA (2005) Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr 81:508–514
Hu P, Bretsky P, Crimmins EM, Guralnik JM, Reuben DB, Seeman TE (2006) Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with and without apolipoprotein E 4 alleles: MacArthur studies of successful aging. J Gerontol (A Biol Sci Med Sci) 61A:616–620
Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, Skoog I, Norton MC, Tschanz J, Breitner JCS, Welsh-Bohmer KA (2007) Antioxidant intake and cognitive function of elderly men and women: the Cache County study. J Nutr Health Aging 11:230–237
Kang JH, Grodstein F (2008) Plasma carotenoids and tocopherols and cognitive function: a prospective study. Neurobiol Aging 29:1394–1403
Kalmijn S, Feskens EJM, Launer LJ, Kromhout D (1997) Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol 145:33–41
Engelhart MJ, Ruitenberg M, Meijer J, Kiliaan A, van Swieten JC, Hofman A, Witteman JCM, Breteler MMB (2005) Plasma levels of antioxidants are not associated with Alzheimer’s disease or cognitive decline. A population-based study. Dement Geriatr Cogn 19:134–139
Akbaraly NT, Hininger-Favier I, Carrière I, Arnaud J, Gourlet V, Roussel A-M, Berr C (2007) Plasma selenium over time and cognitive decline in the elderly. Epidemiology 18:52–58
Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P (2007) Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. doi:10.1093/aje/kwm036
Maxwell CJ, Hicks MS, Hogan DB, Basran J, Ebly EM (2005) Supplemental use of antioxidant vitamins and subsequent risk of cognitive decline and dementia. Dement Geriatr Cogn 20:45–51
Velho S, Marques-Vidal P, Baptista F, Camilo ME (2008) Dietary intake adequacy and cognitive function in free-living elderly: a cross-sectional and short-term prospective study. Clin Nutr 27:77–86
Castellani RJ, Lee H, Perry G, Smith MA (2006) Antioxidant protection and neurodegenerative disease: the role of amyloid-β and tau. Am J Alzheimers Dis Other Demen 21:126–130
Anderson JK (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Rev Neurosci 5:S18–S25
O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST (2003) Vascular cognitive impairment. Lancet 2:89–98
Pashkow FJ (2011) Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? Int J Inflamm. doi:10.4061/2011/514623
Morris MC, Evans DA, Hebert LE, Bienias JL (1999) Methodological issues in the study of cognitive decline. Am J Epidemiol 149:789–793
Bischkopf J, Busse A, Angermeyer MC (2002) Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 106:403–414
Egger M, Schneider M, Smith GD (1998) Meta-analysis spurious precision? Meta-analysis of observational studies. Brit Med J 316:140–144
Lezak M (1995) Neuropsychological assessment, 3rd edn. Oxford University Press, New York
Acknowledgments
This work was supported by the CHANCES (Consortium on Health and Ageing: Network of Cohorts in Europe and the United States) project (Grant Agreement number: 242244) funded by the European Commission under the FP7 framework programme. The authors would like to thank Professor Dimitrios Trichopoulos, Harvard School of Public Health, and Dr. Roger Humphry, Epidemiology Research Unit, Scottish Agricultural College, for providing valuable comments on earlier versions of this paper. Also, we thank the referees assigned to this paper for their constructive comments and suggestions which considerably helped improve the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rafnsson, S.B., Dilis, V. & Trichopoulou, A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr 52, 1553–1567 (2013). https://doi.org/10.1007/s00394-013-0541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0541-7