Abstract
Purpose
Glutamine (Gln) is a nutrient with immunomodulatory effects in metabolic stressed conditions. This study investigated the effects of Gln on colonic-inflammatory-mediator expression and mucosal repair in mice with dextran sulfate sodium (DSS)-induced colitis.
Methods
C57BL/6 mice received distilled water containing 3 % DSS for 5 d to induce colitis. One of the DSS-treated groups was intraperitoneally injected with an alanyl (Ala)-Gln solution 3 days before (G-DSS) while the other group was administered Ala-Gln 3 days after colitis (DSS-G) was induced. The Ala-Gln solution provided 0.5 g Gln/kg/d. The saline-DSS group (S-DSS) received an identical amount of saline before and after colitis was induced to serve as a positive control.
Results
The S-DSS group had a shorter colon length, higher plasma haptoglobin level, and more-severe colon inflammation. Also, the toll-like receptor (TLR)4 level, nuclear factor (NF)-κB activation, and inflammatory cytokine gene expression in the colon were higher than those of the normal control group. Gln administration either before or after colitis suppressed TLR4 protein levels, decreased plasma haptoglobin, and reduced colon inflammation. Histological inflammatory scores were also lowered. Compared to the post-colitis Gln group, preventive use of Gln had higher colon length, expressions of mucin 2, trefoil factor 3, and heat shock protein 72 genes were also upregulated in the colon.
Conclusions
These results suggest that Gln administered either before or after the colitis mitigated inflammation of colitis that was not observed in group without Gln injection. Prophylactic treatment with Gln had more-beneficial effects on reducing inflammatory markers and enhancing the recovery of mucosa in DSS-induced colitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel diseases (IBD) are chronic disorders of the intestines characterized by acute exacerbation followed by remission. Although the underlying pathogenic mechanism remains unclear, dysregulation of immune reactions interacting with genetic and environmental factors is commonly discussed [1]. The ability of the immune system to tolerate the commensal microflora contributes to homeostasis in the intestine [2, 3]. This tolerance ability is impaired in patients with IBD who show hyperreactive immune responses to resident intestinal microorganisms [4, 5]. The innate immunity plays a central role in regulating immune responses to intestinal bacteria, and toll-like receptors (TLRs) are important proteins in activating innate immunity.
TLRs are a family of receptors that detect motifs of pathogens and host materials released during injury. TLRs are activated by recognition of commensal- and pathogen-associated molecular patterns, which leads to the production of cytokines, chemokines, and antimicrobial molecules. TLR4 recognizes lipopolysaccharide (LPS) that is a major component of the outer membrane of gram-negative bacteria and triggers signaling cascades mediated by myeloid differentiation factor (MyD)88 to activate the transcription factor, nuclear factor (NF)-κB [6]. TLR4 is expressed at low levels in normal intestines but is upregulated in patients with IBD [7, 8] and in mice with dextran sulfate sodium (DSS)-induced colitis [9, 10], which may contribute to initiation or perpetuation of intestinal inflammation.
Animal models of IBD have significantly contributed to our present understanding of the disease, as they provide a platform through which some of these complex mechanisms can be systemically investigated. DSS-induced colitis is a useful model for investigating the innate response since it does not depend on acquired immunity to induce inflammation [11]. However, T helper (Th) cells are responsible for modulating the inflammatory response in DSS-induced colitis [12]. Mice with acute DSS-induced colitis exhibit similar expression profiles of cytokines and histological changes to those observed in human IBD, particularly ulcerative colitis (UC) [13]. In DSS-induced acute colitis, massive infiltrates appear in inflammatory lesions. A variety of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6, IL-8, IL-12, and IL-17, produced by immune cells are considered to be associated with progression of the disease [13–16].
Recent studies implicate that amino acids play potential therapeutic roles in gut-related diseases [17]. Glutamine (Gln) is the most abundant free amino acid in plasma and tissue pools. Previous study showed that Gln supplementation attenuated proinflammatory cytokine release, protected against organ damage, and decreased mortality in a LPS-treated animal model [18]. Gln is the main respiratory substrate for enterocytes and has long been studied as a promising agent to improve intestinal barrier function [19, 20] and preserve epithelial tight junction integrity [21]. Also, Gln restores gut glutathione stores and villus height in stressed conditions [22]. However, studies concerning the effects of Gln on mucosal inflammation resolution in IBD are rare. We hypothesized that Gln administration would attenuate the inflammatory response and enhance recovery of the mucosa in DSS-induced colonic damage. Therefore, TLR4, MyD88, and NF-κB in colon tissue were analyzed. Also, the gene expression of mucin presented in the mucus lining the colon was measured in the recovery phase. IBD are disorders that flare up with remissions and relapses. Although preventive administration of Gln may be recommended for patients in the acute phase of UC, Gln treatment after acute onset of colitis is more likely to occur in UC patients. Therefore, we administered Gln before or after the colitis in this study to investigate the effects of Gln provided at different stages on inflammatory responses and the mucosal integrity in DSS-induced colitis during the recovery period. In this study, we used C57BL/6 mice to evaluate consequences of DSS exposure on inflammatory mediator expressions, because this mice strain offers a promising animal model for examining pathological inflammatory changes observed in UC [16].
Materials and methods
Animals
Six-week-old male C57BL/6 mice were purchased from the National Laboratory Animal Center (Taipei, Taiwan). Mice weighing 22–25 g at the beginning of the experiment were used in this study. All mice were housed in a temperature- and humidity-controlled room and were allowed free access to a standard chow diet for 1 week before the study. Care of laboratory animals was in full compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), and protocols were approved by the institutional Animal Care and Use Committee of Taipei Medical University.
Study protocols
Thirty-six mice were randomly assigned to 1 normal control (NC) group and 3 DSS-treated groups, with 9 mice in each group. NC group was considered as a normal reference group. Mice in the NC group were healthy and received distilled water. DSS-treated groups were given distilled water containing 3 % (wt/vol) DSS (MW 40 kDa; MP Biomedicals, Solon, OH) for 5 days as previously described [16]. Among the DSS-treated groups, mice in the S-DSS group were injected with saline intraperitoneally (i.p.) daily for 3 days before and after receiving DSS water. Mice in the S-DSS group were considered as colitis reference animals. A Gln solution (Dipeptiven, Fresenius Kabi, Bad Homburg, Germany) was i.p. injected into mice in the G-DSS group for 3 days before receiving DSS water, and saline was injected for 3 days after receiving DSS water. A saline solution was injected into mice in the DSS-G group for 3 days before DSS water, and a Gln solution was injected for 3 days after receiving DSS water. The dosage of Gln was 0.75 g Ala-Gln/kg body weight (BW), which provided 0.5 g Gln/kg BW/d. This amount of Gln was found to have beneficial effects in a mice model of muscular dystrophy [23]. The duration of the experimental period was 11 days. Volumes of saline and the Gln solution administered were identical among the groups. The volume for i.p. injection was ranging from 85 to 95 μl. During the experimental period, BWs were recorded daily. All mice had free access to a standard chow diet and water. At the end of the experiment, mice were anesthetized and killed by cardiac puncture. Blood samples were collected in tubes containing heparin. The colon was cut close to the ileocecal valve, and its length and weight were measured. Sections (1 cm) of the distal and proximal colon were cut. The middle segment of colon tissues was used for RNA analysis and immunoblotting. The distal segment of colon tissues was collected for histopathological assessment.
Measurements of haptoglobin in plasma
Whole blood was centrifuged at 3,000×g for 10 min at 4 °C to obtain plasma. Plasma samples were used to determine the concentration of haptoglobin by an enzyme-linked immunosorbent assay (ELISA) kit (ICL, Newberg, OR). Procedures followed the manufacturer’s instructions.
RNA extraction and quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from colon tissue using the Trizol reagent (Invitrogen, Carlsbad, CA). RNA (1 μg) was reverse-transcribed using oligo(dT)18 primers with a complementary (c)DNA synthesis kit (Fermentas, Glen Burnie, MD) according to standard protocols. A real-time RT-PCR was carried out in optical 96-well plates on an ABI 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Primers used in this study are listed in Table 1. The expression of each gene was assayed in a total volume of 25 μl containing 1 × SYBR green master mix reagent (Applied Biosystems), 100 nM of each primer, and 50 ng of cDNA. Amplification was performed according to the thermocycling protocol recommended by the PCR system, with a final dissociation curve (DC) analysis. No-template controls and a melting curve analysis were used to confirm the specificity of the real-time PCR. All samples were analyzed in triplicate, and multiples of change of messenger (m)RNA were calculated by the equation 2−△△Ct (ΔCt indicates the difference in threshold cycles between the test gene and β-actin, and ΔΔCt indicates the difference of ΔCt between the colitis and normal control groups).
Immunoblotting
Colon tissues were homogenized in lysis buffer (50 mM Tris (pH 7.4), 1 % sodium dodecylsulfate (SDS), and 10 mM EDTA) containing a protease inhibitor cocktail (Sigma) to prepare whole-tissue lysates. The homogenates were centrifuged at 15,000 × g for 15 min, and the supernatants were used for immunoblotting. Protein concentrations of the supernatant were determined using a Bradford Protein Assay Reagent kit (Bio-Rad, Richmond, CA). Thirty micrograms of protein was loaded and separated on 12 % SDS–polyacrylamide gel electrophoresis (PAGE) and then transferred to a polyvinylidene difluoride (PVDF) membrane in a wet-transfer apparatus. Membranes were blocked with 5 % nonfat milk in Tris-buffered saline (TBS) for 30 min and then incubated with antibodies overnight at 4 °C. Polyclonal rabbit antibodies against NF-κB p65 or IkBα were purchased from Millipore (Temecula, CA). A monoclonal mouse antibody against β-actin was purchased from Sigma. After washing 3 times in TBS-T (TBS containing 0.1 % Tween-20), membranes were incubated with horseradish peroxidase (HRP)-conjugated species-specific antibodies for 1 h. Membranes were then washed with TBS-T for 30 min, and blots were developed with the high-sensitivity chemiluminescence substrate, Western Lighting Ultra (PerkinElmer Life Sciences, Waltham, MA), and exposed to X-ray films. The relative intensity was measured to quantify the protein level using Image-Pro Plus software (Media Cybernetics, Bethesda, MD). All blots were normalized against actin to adjust for the amount of protein loaded.
Histopathology
One centimeter of distal segment of colon tissues was used for histopathological assessment. The colon tissues were fixed with buffered 4 % paraformaldehyde. Five-micrometer paraffin-embedded colon sections were stained with periodic acid-Schiff (PAS) reagent (Sigma Chemical, St. Louis, MO), and standard hematoxylin (Sigma) nuclear staining was applied to contrast cell nuclei. PAS technique was used to stain goblet cell mucins. Hematoxylin and eosin staining were also performed. Digital images at 80 × magnification per section were captured with a Zeiss Axiphot light microscope (Carl Zeiss MicroImaging LLC, Thornwood, NY) and a Nikon D1X digital camera (Tokyo, Japan). Five fields per section were examined to determine morphological lesions and changes in the colon mucosa. The degree of IBD was measured by a modified scoring system of Iba et al. [24]. Inflammatory colitis was scored 0–3 for lesions based on loss of epithelium, length of crypts, depletion of goblet cells, and infiltration of leukocytes (Table 2). The total histological score ranged 0–12, which represented the summed scores of histological changes.
Statistical analysis
All data are presented as the mean ± standard error of the meant (SEM). Differences among groups were analyzed by analysis of variance (ANOVA) using Tukey’s test. Two-way ANOVA using the Bonferroni post-test was used to analyze the differences of body weight. A p value of < 0.05 was considered statistically significant.
Results
BW
There were no differences in initial BWs among the 4 groups. Pretreatment with Gln for 3 days did not affect BWs. BW loss reached a peak at 2 days after stopping exposure to DSS. BWs were lower in the S-DSS and DSS-G groups (20.9 ± 0.6 and 21.0 ± 0.5 g, respectively) than in the NC group (24.7 ± 0.5 g) at the end of the study, whereas there were no significant differences between the G-DSS (22.6 ± 0.6 g) and NC groups (Fig. 1).
Colon length and plasma haptoglobin concentration
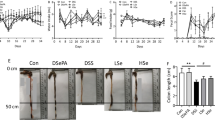
The weight of the colon showed no difference between the 3 DSS-colitis groups and NC group. However, the length of the colon was significantly shorter in S-DSS and DSS-G groups than the NC group, indicating that colonic edema were more severe in these two groups. Plasma haptoglobin concentrations were higher in DSS-colitis groups than the NC group. The S-DSS group had the highest haptoglobin concentrations among the 3 DSS groups (Table 3).
Inflammatory gene and protein expressions in the colon
The G-DSS and DSS-G groups had lower TLR mRNA and protein expression than the S-DSS group (Fig. 2). Compared to the NC group, the S-DSS and DSS-G groups showed significantly higher NF-κB p65 expressions. The IκBα/NF-κB ratio was lower in DSS-treated groups than the NC group. The S-DSS group had the lowest IκBα/NF-κB ratio among the 3 DSS groups (Fig. 3). Expressions of IL-17A, KC, and TNF-α were significantly higher in the S-DSS group than the NC group. The G-DSS and DSS-G groups had lower IL-17A and KC mRNA levels, and the G-DSS group had lower TNF-α mRNA expression than the S-DSS group (Fig. 4).
Expression of toll-like receptor (TLR)4 in mouse colonic tissue. mRNA expressions of TLR4 and MyD88 (a and b). mRNAs were analyzed by a real-time PCR. Quantitation of mRNA changes was calculated by the comparative CT (2−△△Ct) method, and mRNA expression of normal control (NC) mice was used as a calibrator. c Protein expression of TLR4. Whole-tissue lysates were analyzed by immunoblotting, and β-actin was used as a loading control. Results of the densitometric analysis are shown as the mean ± SEM. *Significant difference from the NC group. †Significant difference from the S-DSS group (p < 0.05)
Protein levels of nuclear factor (NF)-κB p65 and inhibitory factor κBα (IκBα) in colon tissues. a Protein expressions of NF-κB p65 and IκBα. Whole-tissue lysates were analyzed by immunoblotting, and β-actin was used as a loading control. b Densitometric analysis of the blot corrected by the protein loading control. c The ratio of IκBα to NF-κB p65. Results are shown as the mean ± SEM. *Significant difference from the normal control (NC) group. †Significant difference from the S-DSS group. ‡Significant difference from the DSS-G group (p < 0.05)
mRNA expressions of inflammatory genes in colon tissues. mRNAs of interleukin (IL)-17A, keratinocyte-derived chemokine (KC), and tumor necrosis factor (TNF)-α were analyzed by a real-time PCR. Quantitation of mRNA changes was calculated by the comparative CT (2−△△Ct) method, and mRNA expression of normal control (NC) mice was used as a calibrator. Data are shown as mean ± SEM. *Significant difference from the NC group. †Significant difference from the S-DSS group (p < 0.05)
Expressions of mucosal repair and anti-apoptotic genes in the colon
Mucin 2 (Muc2) and trefoil factor 3 (Tff3) gene expressions in the mucus lining the colon were significantly higher in the G-DSS group than in the other 3 groups (Fig. 5). The G-DSS group had the highest Hsp72 mRNA expression among the NC and DSS groups. Although lower than that of the G-DSS group, Hsp72 gene expression in the DSS-G group was significantly higher than those of the NC and S-DSS groups. mRNA expressions of Bcl-xL in the S-DSS and G-DSS groups were lower than those in the NC and DSS-G groups (Fig. 5).
mRNA expressions of mucosal recovery-related and antiapoptotic genes in colon tissues. mRNA levels were analyzed by a real-time PCR. a Expression of mucin 2 (Muc2) mRNA. b Expression of trefoil factor 3 (Tff3) mRNA. c Expression of heat shock protein (Hsp)72 mRNA. d Expression of B cell lymphoma-extra large (Bcl-xL) mRNA. Quantitation of mRNA changes was calculated by the comparative CT (2−△△Ct) method, and mRNA expression of normal control (NC) mice was used as a calibrator. Data are shown as the mean ± SEM. *Significant difference from the NC group. †Significant difference from the S-DSS group (p < 0.05)
Histopathological aspects of the colon
Purple PAS-stained areas with blue hematoxylin-stained nuclei (Fig. 6a) were seen in colon tissues with DSS-induced colitis. Morphological changes showed that colon tissues of the NC group had intact epithelium, well-defined gland lengths, and no leukocyte infiltration in the mucosa. DSS-induced colitis resulted in mucosal ulceration, infiltration of leukocytes, crypt distortion, and hyperplastic epithelium (Fig. 6b). Compared to the G-DSS and DSS-G groups, more-severe inflammatory lesions of the colic mucosa were observed in the S-DSS group. A histological evaluation of inflammatory scores is shown in Fig. 6c. The S-DSS group had the highest score among all groups.
Histopathology of colon tissues. a Periodic acid-Schiff (PAS) reagent staining and b hematoxylin and eosin staining of colon tissues from mice in the NC, DSS, G-DSS, and DSS-G groups. Representative histological images are shown at 80 × magnification. Histological scores of colitis c are presented as the mean ± SEM, which were determined as described in “Materials and Methods”. Mucosal inflammation of the S-DSS group was more severe than that of the G-DSS and DSS-G groups.*Significant difference from the other 3 groups (p < 0.05)
Discussion
This investigation showed that Gln administered either before or after the colitis mitigated inflammation of colitis that was not observed in group without Gln injection. Pretreatment with Gln had more favorable effects than post-colitis Gln administration. Hulsewe et al. [25] found that plasma and mucosal Gln concentrations had negative association with the severity of colonic inflammation. A recent study reported that Gln concentrations of serum and colon tissue were significantly lower in the acute phase of colonic inflammation, and Gln supplementation attenuated the degree of microscopic injury induced by DSS [26]. Our study supports the potential therapeutic use of Gln and may imply that prophylactic treatment with Gln can be considered for IBD patients to resolve inflammation in remission period. Report by Isyaeli et al. [27] was consistent with our findings. They also found that Gln exerts a beneficial effect when administered before induction of colitis, by increasing the resistance of the colonic tissue to inflammatory injury.
Previous studies found that in a rodent model of DSS-induced colitis, administration of Gln decreased the diarrhea score, improved the barrier function and prevented the bacterial translocation [28–30]. A study by Kretzmann et al. [31] demonstrated that Gln inhibited the expression of proinflammatory mediators that are regulated by NF-κB signaling pathways in a model of experimental colitis. TLR4 is implicated as the critical receptor mediating the inflammatory response. MyD88 is a central adaptor protein for a majority of TLRs and acts as a link between receptors and the downstream kinase which leads to NF-κB activation [6]. Recent study demonstrated that TLR4 plays as a mediator of intestinal inflammation [32] and TLR4 blockade during the DSS administration period ameliorated the severity [33]. In this study, we found that both DSS groups with Gln had lower TLR4 expressions, which indicated that the subsequent inflammatory cascade may have been attenuated.
The results of this study showed that pretreatment with Gln suppressed the expression of colonic NF-κB p65 protein; in contrast, the NF-κB p65 protein level was highest in the DSS-G group. Since NF-κB p65 overexpression is thought to be correlated with the inflammatory response, this finding is inconsistent with lower mRNA expressions of KC and TNF-α in the DSS-G group. However, we observed that although the NF-κB p65 protein level was high, the IκBα to NF-κB p65 ratio was also higher in the DSS-G group than in the S-DSS group. NF-κB DNA binding is inhibited by IκBα, and the inhibitory effect increases with increasing IκBα to p65 ratio [34]. The higher IκBα/NF-κB p65 ratio in the Gln treated groups may indicate lower NF-κB activation as compared to the colitis reference group. In addition to modulating the inflammatory response, NF-κB also regulates apoptosis by inducing transcription of genes coding for antiapoptotic proteins [35]. Bcl-xL is a potent regulator of antiapoptosis. A previous study found that NF-κB activation proceeds though IKKβ leading to Bcl-xL expression. Activation of IKKβ and the expression of Bcl-xL promote the survival of primary CD4 lymphocytes [36]. We speculated that NF-κB activation observed in colon tissues of the DSS-G group was associated with the anti-apoptotic effects of colonic epithelial cells. The higher colonic Bcl-xL gene expression in the DSS-G group may explain the comparable histological scores between the 2 Gln-administered DSS groups.
Cytokines are principle mediators of the immune response in mucosal inflammation. In DSS-induced acute colitis, expression profiles of cytokines are similar to those observed in patients with UC [3]. In order to understand the effects of Gln on cytokine patterns in an acute-colitis model, expressions of IL-17A, KC, and TNF-α in the colon were analyzed. IL-17A secreted mainly by Th 17 cells is an important cytokine involved in the progression of colitis [14]. IL-17A promotes the production of CXC chemokines and inflammatory cytokines, thereby modulating the recruitment of neutrophils [37]. KC is a murine homolog of the human CXC chemokine. TNF-α is primarily produced by mononuclear phagocytes. CXC chemokines and TNF-α are important cytokines associated with colonic inflammation that mediate neutrophil recruitment [38]. In this study, we also measured plasma haptoglobin. Haptoglobin is an acute-phase protein. It is a marker of systemic inflammation and can be used to monitor disease activity in murine experimental colitis [16]. Higher plasma haptoglobin concentrations and KC and TNF-α mRNA expressions in colon tissues of the S-DSS group indicated the occurrence of systemic inflammation, and certain amounts of macrophages and neutrophils were attracted to sites of inflammation. The findings of this study showed that colon IL-17A, KC, and TNF-α gene expressions were lower in the 2 Gln-intervention groups, suggesting fewer leukocytes were recruited to the colon when Gln was administered. Our recent report also showed that compared to the S-DSS group, blood IL-17A, IL-17F, and IFN-γ percentages were lower in the Gln-injected group [39].
HSP expression is vital to cellular and tissue protection after stress or injury [40]. The Hsp70 family is the most conserved and best investigated class of all HSPs. Of these, the two best studied members are the constitutive or cognate Hsp70 (Hsc70) and a stress-inducible form of cytosolic Hsp70 (Hsp72). The cytoprotective effects of Hsp72 are related to its chaperone property and the ability to inhibit apoptosis via both caspase-dependent and -independent pathways [41, 42]. Thus, higher Hsp72 expression may indicate that the severity of tissue damage can be attenuated. A previous study demonstrated that Gln protected gut mucosa from injury by enhancing Hsp72 expression [43]. We observed that pretreatment with Gln had a more-profound influence on alleviating the inflammatory reaction induced by colitis, since the colon length and Hsp72 and mucin gene expressions were higher, whereas NF-κB p65 were lower than those seen with post-colitis Gln administration.
A layer of mucus lining the gastrointestinal tract acts as a lubricant and protective physical barrier between the mucosal surface and luminal contents. This layer is formed by mucins. Muc2 is one of the predominant mucins detected in the colorectum. Muc2 is normally specific to goblet cells [44]. Trefoils are a group of small peptides that have important roles in epithelial protection and mucosal healing [45]. There are 3 trefoil peptides that are referred to as trefoil factor family (TFF) peptides. Tff3 is found throughout the small and large intestines. The findings of this study showed that Muc2 and Tff3 mRNA expressions in the G-DSS group were higher than those of the S-DSS group, whereas these alterations were not found in the DSS group administered Gln after colitis. DSS results in epithelial damage and an inflammatory response in the colon that last for several days. Pretreatment with Gln may have provided sufficient fuel and protection for enterocytes, while Gln administered in the recovery phase was not potent enough to improve healing of the mucosa.
In summary, this study showed that although Gln administered after the colitis had a few effects on attenuating inflammatory response that were not observed with the DSS reference group, pretreatment with Gln had more-pronounced beneficial effects in reducing inflammatory markers and enhancing recovery of the colon mucosa in acute DSS-induced colitis.
References
Baumgart DC, Sandborn WJ (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657
Brown SJ, Mayer L (2007) The immune response in inflammatory bowel disease. Am J Gastroenterol 102:2058–2069
Kelsall BL (2008) Innate and adaptive mechanisms to control pathological intestinal inflammation. J Pathol 214:242–259
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Zum Buschenfelde KHM (1995) Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 102:448–455
Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I (1996) Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 38:365–375
Palsson-McDermott EM, O’Neill LA (2004) Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153–162
Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T et al (2002) Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 122:1987–2000
Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Mraz M et al (2008) Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol 151:34–41
Ohkawara T, Takeda H, Miyashita K, Nishiwaki M, Nakayama T, Taniguchi M et al (2006) Regulation of Toll-like receptor 4 expression in mouse colon by macrophage migration inhibitory factor. Histochem Cell Biol 125:575–582
Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H et al (2003) Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol 170:3977–3985
Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG et al (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385–391
Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T (1998) Involvement of CD4 + T cells in the development of dextran sulfate sodium-induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol 31:477–481
Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW (2000) Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 62:240–248
Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R et al (2008) Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun 377:12–16
Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J et al (2006) Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol 146:330–338
Melgar S, Karlsson A, Michaelsson E (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol 288:G1328–G1338
Wang WW, Qiao SY, Li DF (2009) Amino acids and gut function. Amino Acids 37:105–110
Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB (2001) Glutamine reduces cytokine release, organ damage, and mortality in a rat model of endotoxemia. Shock 16:398–402
White JS, Hoper M, Parks RW, Clements WD, Diamond T (2005) Glutamine improves intestinal barrier function in experimental biliary obstruction. Eur Surg Res 37:342–347
Hering NA, Schulzke JD (2009) Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis 27:450–454
Rao RK, Samak G (2012) Role of glutamine in protection of intestinal epithelial tight junctions. J Epithelial Biol Pharmacol 5:47–54
Belmonte L, Coëffier M, Le Pessot F, Miralles-Barrachina O, Hiron M, Leplingard A et al (2007) Effects of glutamine supplementation on gut barrier, glutathione content and acute phase response in malnourished rats during inflammatory shock. World J Gastroenterol 13:2833–2840
Mok E, Constantin B, Favreau F, Neveux N, Magaud C, Delwail A et al (2008) Hankard R. l-Glutamine administration reduces oxidized glutathione and MAP kinase signaling in dystrophic muscle of mdx mice. Pediatr Res 63:268–273
Iba Y, Sugimoto Y, Kamei C, Masukawa T (2003) Possible role of mucosal mast cells in the recovery process of colitis induced by dextran sulfate sodium in rats. Int Immunopharmacol 3:485–491
Hulsewé KW, van der Hulst RW, van Acker BA, von Meyenfeldt MF, Soeters PB (2004) Inflammation rather than nutritional depletion determines glutamine concentrations and intestinal permeability. Clin Nutr 23:1209–1216
Shiomi Y, Nishiumi S, Ooi M, Hatano N, Shinohara M, Yoshie T et al (2011) GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm Bowel Dis 17:2261–2274
Israeli E, Berenshtein E, Wengrower D, Aptekar L, Kohen R, Zajicek G et al (2004) Prophylactic administration of topical glutamine enhances the capability of the rat colon to resist inflammatory damage. Dig Dis Sci 49:1705–1712
Vicario M, Amat C, Rivero M, Moreto M, Pelegri C (2007) Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J Nutr 137:1931–1937
Xue H, Sufit AJ, Wischmeyer PE (2011) Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr 35:188–197
Wang F, Zhao HY, Zhang ST, Gong YZ, Zhang HF, Zhang C (2011) Effect of enteral nutrition on dextran sulfate sodium-induced colitis in rats. J Dig Dis 12:453–458
Kretzmann NA, Fillmann H, Mauriz JL, Marroni CA, Marroni N, Gonzalez-Gallego J et al (2008) Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm Bowel Dis 14:1504–1513
Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS et al (2006) Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131:862–877
Ungaro R, Fukata M, Hsu D, Hernandez Y, Breglio K, Chen A et al (2009) A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol 296:G1167–G1179
Simeonidis S, Stauber D, Chen G, Hendrickson WA, Thanos D (1999) Mechanisms by which IκB proteins control NF-κB activity. Proc Natl Acad Sci USA 96:49–54
Magne N, Toillon RA, Bottero V, Didelot C, Houtte PV, Gerard JP et al (2006) NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett 231:158–168
Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE (2000) The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4 + lymphocytes. J Immunol 165:1743–1754
Kolls JK, Linden A (2004) Interleukin-17 family members and inflammation. Immunity 21:467–476
Strieter RM, Kunkel SL, Keane MP, Standiford TJ (1999) Chemokines in lung injury: Thomas A. Neff lecture. Chest 116:103S–110S
Chu CC, Hou YC, Pai MH, Chao CJ, Yeh SL (2011) Pretreatment with alanyl-glutamine suppresses T-helper-cell-associated cytokine expression and reduces inflammatory responses in mice with acute DSS-induced colitis. J Nutr Biochem [Epub ahead of print]
Garrido C, Schmitt E, Cande C, Vahsen N, Parcellier A, Kroemer G (2003) HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2:579–584
Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C (2008) Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med 12:743–761
Sreedhar AS, Csermely P (2004) Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther 101:227–257
Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB (1997) Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol 272:G879–G884
Shirazi T, Longman RJ, Corfield AP, Probert CS (2000) Mucins and inflammatory bowel disease. Postgrad Med J 76:473–478
Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK (1995) Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology 109:516–523
Acknowledgments
This study was supported by a research grant (NSC100-2320-B-038-007-MY3) from the National Science Council, Taipei, Taiwan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, YC., Chu, CC., Ko, TL. et al. Effects of alanyl-glutamine dipeptide on the expression of colon-inflammatory mediators during the recovery phase of colitis induced by dextran sulfate sodium. Eur J Nutr 52, 1089–1098 (2013). https://doi.org/10.1007/s00394-012-0416-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0416-3