Abstract
Background
Renal organic ion transporters and uromodulin (UMOD) play the important roles in renal urate excretion and function. Hyperuricemia is considered as a risk factor for the development of renal dysfunction. The flavonoid quercetin in diets exerts the hypouricemic and nephroprotective effects.
Purposes
To evaluate the effects of quercetin on renal organic ion transporters and UMOD in hyperuricemic mice.
Methods
Kun-Ming mice were divided into normal and hyperuricemic groups receiving water, 25, 50 and 100 mg/kg quercetin, 5 mg/kg allopurinol, respectively. Hyperuricemic mice were orally gavaged with 250 mg/kg oxonate daily for 1 week. Quercetin and allopurinol were orally gavaged on the day when oxonate or water was given 1 h later. After 1 week, serum uric acid, creatinine and blood urea nitrogen concentrations, excretion of urate and creatinine, and fractional excretion of uric acid were measured. The mRNA and protein levels of renal urate transporter 1 (mURAT1), glucose transporter 9 (mGLUT9), organic anion transporter 1 (mOAT1) and organic cation/carnitine transporters (mOCT1, mOCT2, mOCTN1 and mOCTN2) in mice were analyzed. Simultaneously, UMOD levels in serum, urine and kidney, as well as renal UMOD mRNA expression were detected.
Results
Quercetin significantly restored oxonate-induced abnormalities of these biochemical indexes compared with normal vehicle group. Furthermore, it remarkably prevented expression changes of renal organic ion transporters and UMOD, and UMOD level alteration in hyperuricemic mice.
Conclusions
These results suggest that quercetin has the uricosuric and nephroprotective actions mediated by regulating the expression levels of renal organic ion transporters and UMOD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperuricemia is associated with the development of renal dysfunction, gout, hypertension, hyperlipidemia, diabetes and obesity [1, 2]. The impairment of reabsorption and secretion of urate in the kidney is an important cause of hyperuricemia. Renal organic anion transporters (OATs) of the SLC22 gene family are suggested to regulate renal urate excretion. OAT1 (SLC22A6) and OAT3 (SLC22A8) at the basolateral membranes of renal proximal tubules are responsible for primary urate transport [3, 4]. The reabsorptive urate transporter 1 (URAT1, SLC22A12), as well as the SLC2 gene family SLC2A9 encoding glucose transporter 9 (GLUT9) at apical membranes of renal proximal tubules in human mainly control urate homeostasis [5–7]. Mutations in SLC2A9 gene develop low serum uric acid concentration and high fractional excretion of uric acid (FEUA) [5]. On the other hand, uptake and excretion of kidney organic cations are mediated by renal organic cation and carnitine transporters (OCTs and OCTNs) of the SLC22 gene family [8–10]. Expression of renal rOCT1 (SLC22A1) and rOCT2 (SLC22A2) is down-regulated in streptozotocin-induced diabetic rats with kidney dysfunction [8]. OCTN1 (SLC22A4) is a susceptibly factor in the etiopathology of autoimmune disorders [9]. Defect mutations in hOCTN2 (SLC22A5) cause the impaired reabsorption and secretion of organic cations [10]. OAT1 and OAT3 mediate uptake of anion toxic from the blood into renal proximal tubules to induce nephrotoxicity [11]. These observations indicate that abnormalities in the expression levels of these renal organic ion transporters may be involved in the pathogenesis of hyperuricemia and renal dysfunction. Uromodulin (UMOD), the most abundant protein in normal urine, is exclusively expressed in the thick ascending limb of Henle’s loop. Mutations in UMOD gene lead to familial juvenile hyperuricemic nephropathy [12, 13]. Mouse missense mutation of UMOD also develops kidney dysfunction [14]. Therefore, UMOD may be a useful marker of renal dysfunction in hyperuricemia with the abnormality of renal organic ion transporters.
Quercetin (3,3′,4′,5,7-pentahydroxyflavone), a kind of flavonoids, is abundant in fruits, vegetables and herbal food with health beneficial properties for humans, such as anti-inflammation, anti-oxidation, anti-carcinogen, anti-hypertension and anti-apoptosis [15, 16]. Our previous studies confirmed that quercetin reduced serum uric acid levels in hyperuricemic mice induced by uricase inhibitor potassium oxonate [17, 18], and increased FEUA with the enhancement of renal urate excretion in excess fructose consumption-induced hyperuricemic rats [19]. In addition, quercetin decreases serum creatinine and blood urea nitrogen (BUN) concentrations in ischemia/reperfusion-induced renal injury [20], cadmium-induced nephrotoxicity [21] and fructose-induced renal dysfunction [19] in rats. It is noted that 96% of quercetin is metabolized in rat kidneys [22]. A recent study shows that kidney contains significantly higher concentrations than plasma after long-term dietary intake of quercetin [23]. Therefore, the kidney may be considered as a primary target of quercetin’s uricosuric and renal protective effects. Furthermore, quercetin is found to significantly inhibit cellular uptake of (3H)-p-aminohippurate mediated by hOAT1 in MDCK cells [24] and of (14C)-tetraethylammonium mediated by pOCT2 in LLC-PK1 cells [25, 26]. In our previous study, quercetin was found to attenuate fructose-induced dysregulation of renal rSLC2A9v2, renal-specific transporter, rOAT1, rOCT1 and rOCT2 in rats [19]. These observations have implications for the involvement of renal organic ion transporters in the uricosuric and renal protective actions of quercetin in hyperuricemia with kidney dysfunction. It is well known that renal urate transport system in mice is similar to that in humans [3, 27]. The research of renal organic ion transporters from mice will provide insights into renal urate transport system in humans. Therefore, the purpose of the present study was to determine whether quercetin regulates the expressing levels of renal organic ion transporters, which contributes to its protection against hyperuricemia and renal dysfunction in oxonate-induced hyperuricemic mice. In order to improve our understanding of the role of UMOD in hyperuricemia with renal dysfunction and find new therapeutic target, UMOD level and expression were also examined in hyperuricemic mice treated with quercetin.
Materials and methods
Material
Quercetin, uric acid, allopurinol and potassium oxonate were purchased from Sigma (St. Louis, MO, USA). Trizol reagent was purchased from Invitrogen. Reverse transcriptase moloney murine leukemia virus (M-MLV) used for cDNA synthesis was from Promega. Taq DNA polymerase and polymerase chain reaction buffer mixture were from Genescript Company limited. R. P. China. Assay kits of creatinine and BUN were purchased from Jiancheng Biotech (Nanjing, P. R. China). ELISA assay kit of UMOD was purchased from R&D (Minneapolis, USA). mURAT1 (001046-R), mGLUT9 (001051-R), mOAT1 (001019-R), mOAT3 (001020-R), mOCT1 (001017-R), mOCT2 (001018-R) antibodies were obtained from Cellchip Biotech (Beijing, P. R. China). mOCTN1 (OCTN11-A) and mOCTN2 (OCTN21-A) antibodies were purchased from Alpha Diagnostic International Inc. (San Antonio, USA). Mouse mGAPDH monoclonal antibody (KC-5G5) was from Kangcheng Biotech (Shanghai, P. R. China). Rabbit mNa+–K+ ATPase antibody (#3010S) was from Cell Signaling Technology, Inc. (Boston, MA, USA). Goat anti-rabbit-IgG-HRP (SB-200) was obtained from Jingmei Biotech (Shanghai, P. R. China).
Animals and drug administration
The Animal Experiment Committee of Nanjing University approved the protocol of animal study. Male Kum-Ming strain of mice (20 ± 2 g) was purchased from the animal centre of Qing-Longshan (Nanjing, Jiangsu Province, P. R. China). Hyperuricemia was developed by uricase inhibitor potassium oxonate, as described previously [28]. Mice were divided into ten groups: hyperuricemic groups receiving water (vehicle), 25, 50 and 100 mg/kg quercetin, 5 mg/kg allopurinol, and normal groups receiving water (vehicle), 25, 50 and 100 mg/kg quercetin, 5 mg/kg allopurinol, respectively. Oxonate, quercetin and allopurinol at various concentrations were dissolved or suspended in distilled water. Standard diets (60% vegetable starch, 11% fat and 29% protein), but not water, were withdrawn from the animals 1 h prior to the administration. Briefly, mice were administered in a volume of 15 mL/kg by gavage once daily with oxonate (250 mg/kg) or water (vehicle) at 8:00 a.m. for seven consecutive days. Quercetin or allopurinol was orally gavaged at 9: 00 a.m. on the day when oxonate was given. Dosages of quercetin were determined based on the conversions from clinical adult dosages [29–32] and our previous and preliminary studies. According to the reports in subjects, daily supplementation dosages of quercetin in subjects are 1,000 mg [29], 730 mg [30] and 150 mg [15, 31], respectively. Equivalently, for mice, these dosages are 130, 94.9 and 19.5 mg/day calculated by the formula that converts dosage of human into that of mouse according to the respective body surface areas according to the Chinese Medicine Pharmacology Research Technology [32]. Moreover, pretreatment of 30 mg/kg quercetin markedly restores ischemia/reperfusion-induced renal dysfunction and morphological alterations in rodents [20]. Quercetin at 50 mg/kg improves gentamicin-induced renal dysfunction and degenerative changes in glomeruli and tubules of rats [33]. Our previous studies showed that quercetin at 50 and 100 mg/kg could lower serum urate levels in acute oxonate-treated mice and chronic fructose-fed rats with renal dysfunction [17, 19]. In addition, our preliminary experiment demonstrated that three doses of quercetin at 25, 50 and 100 mg/kg were suitable for the present study.
Blood, urine and tissue sample collection
From 24 h before final administration on the seventh day, urine sample for each mouse in 24-h was collected in a metabolic cage and urine volume was recorded. Urine samples were centrifuged at 2,000×g for 10 min to remove the particulate contaminants. Whole blood samples were collected 1 h after final administration on the seventh day by tail vein bleeding, and then centrifuged at 10,000×g for 5 min to obtain the serum. Serum and urine samples were stored at −20 °C until biochemical assays. Simultaneously, mice were anesthesized by intraperitoneal injection of xylacin hydrochloride (10 mg/kg) and ketamine (100 mg/kg). Kidney cortex tissues were rapidly and carefully removed on ice-plate, and stored at −70 °C for assays.
Determination of uric acid, creatinine and BUN levels
Serum uric acid levels (Sur) and urinary uric acid levels (Uur) were determined by the phosphotungstic acid method [17]. Excretion of urine uric acid in 24-h was calculated using the formula: Volume of urine in 24-h × Uur. Serum and urine creatinine levels (Scr, Ucr) were determined spectrophotometrically using trinitrophenol colorimetry kit. Excretion of urine creatinine in 24-h was calculated using the formula: Volume of urine in 24-h × Ucr. FEUA was calculated using the formula: FEUA = (Uur × Scr)/(Sur × Ucr) × 100, expressed as percentage [34]. BUN levels were determined using urease ultraviolet kit.
Determination of UMOD
The whole kidney was homogenized in 100 volume/wet weight of sodium chloride and centrifuged at 5,000×g for 10 min at 4 °C. The supernatant was for kidney UMOD level assay. Serum, urine and kidney UMOD levels were determined using ELISA kit.
RNA isolation and semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
RNA analyses of mOAT1, mOAT3, mGLUT9, mURAT1, mOCT1, mOCT2, mOCTN1 and mOCTN2 in kidney cortex were performed by a semiquantitative RT-PCR as described previously [28]. PCR amplification was carried out using gene-specific PCR primers. cDNA was amplified using the Promega Access RT-PCR System with the following program: one cycle at 95 °C, 2 min; followed by 30–40 cycle each including denaturation at 94 °C 30 s, 35 s at appropriate anneal temperature, extension 45 s at 72 °C and one cycle at 72 °C, 10 min. The sequences of gene-specific PCR primers, numbers of cycles and anneal temperature were showed in Table 1. Relative quantitation for PCR products was calculated after normalization to the amount of mGAPDH mRNA levels.
Preparation of kidney tissue extraction and Western blot analysis
The protein extractions of renal cortical brush-border membrane vesicles for mURAT1, mOCTN1, mOCTN2 and mNa+–K+ ATPase analysis, and of renal cortex for mOAT1, mOAT3, mGLUT9, mOCT1, mOCT2 and mGAPDH analysis were prepared as previously described [28]. Western blot analysis was performed as previously described [28]. The contents of target proteins were analyzed densitometrically using the sensiansys software (Peiqing tech. company Ltd. Shanghai, P. R. China) and normalized by the respective blotting from mGAPDH or mNa+–K+ ATPase, respectively.
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM). Effects of quercetin or allopurinol on biochemical indicators in normal and hyperuricemic mice were analyzed by a 2-way analysis of variance (ANOVA) to determine the level of significance. The results of PCR and Western Blotting experiments were analyzed by LSD post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Serum uric acid levels
In order to monitor the efficacy of quercetin treatment, uric acid levels in serum were determined in hyperuricemic mice (Table 2). As expected [28], oral administration of 250 mg/kg oxonate for 1 week significantly induced elevation of serum uric acid levels (P < 0.001) compared to normal vehicle group. Quercetin at 25, 50 and 100 mg/kg effectively decreased serum levels of uric acid (P < 0.05, P < 0.01, P < 0.001) in hyperuricemic mice compared to hyperuricemia vehicle group, with dose-dependent manner (quercetin: P < 0.01, 100 mg/kg compared to 25 mg/kg; P < 0.001, 100 mg/kg compared to 50 mg/kg). Allopurinol at 5 mg/kg had similar effect (P < 0.001). These data confirmed that quercetin reversed oxonate-induced serum uric acid elevation in mice. 2-way ANOVA revealed significant effects of oxonate, quercetin and oxonate × quercetin in quercetin-treated mice, while 2-way ANOVA also revealed significant effects of oxonate factor, allopurinol factor and oxonate × allopurinol interaction in allopurinol-treated mice (Table 2). In addition, quercetin did not affect serum uric acid levels in normal mice, however, allopurinol remarkably decreased the levels (P < 0.001) (Table 2).
Urate excretion and renal function
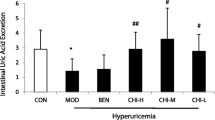
As shown in Table 2, there were no differences in 24-h volume of urine among all groups. Hyperuricemic mice exhibited 24-h under-excretion of uric acid (P < 0.01) and creatinine (P < 0.01) compared to normal vehicle animals (Table 2). FEUA, related renal uric acid handling parameter, was also remarkably decreased in this model (P < 0.01) (Table 2). Oxonate induced significant elevation in serum levels of creatinine (P < 0.001) and BUN (P < 0.01) in mice (Table 2). These data further confirmed that oxonate-treated mice developed renal urate excretion reduction and function impairment. Quercetin at 25, 50 and 100 mg/kg significantly increased excretion of uric acid (P < 0.05, P < 0.05, P < 0.001) and creatinine (P < 0.01, P < 0.01, P < 0.001) in hyperuricemic mice compared to hyperuricemia vehicle group (Table 2), without significant dose-dependent manner.
Furthermore, administration of quercetin to hyperuricemic mice was effective in reversing oxonate-induced elevation of serum levels of creatinine (P < 0.01, P < 0.001, P < 0.001) and BUN (P < 0.01, P < 0.01, P < 0.05), thereby elevated FEUA (P < 0.01, P < 0.01, P < 0.001) (Table 2). Effects of quercetin on serum creatinine levels (P < 0.05, 100 mg/kg; P = 0.072, 50 mg/kg compared to 25 mg/kg) and FEUA (P < 0.05, 100 mg/kg compared to 25 mg/kg) exhibited dose-dependent manners. 2-way ANOVA revealed significant effects of quercetin factor and oxonate × quercetin interaction on excretion of uric acid and creatinine, serum creatinine and BUN levels, as well as of oxonate factor and quercetin factor on FEUA in quercetin-treated mice (Table 2). Allopurinol at 5 mg/kg attenuated oxonate-induced alterations of uric acid (P < 0.05) and creatinine (P < 0.001) excretion, serum creatinine (P < 0.01) and BUN (P < 0.001) levels, and FEUA (P < 0.001) in mice (Table 2). 2-way ANOVA revealed significant effects of oxonate factor, allopurinol factor and oxonate × allopurinol interaction on creatinine excretion, FEUA and BUN levels, and of oxonate factor and oxonate × allopurinol on uric acid excretion, while 2-way ANOVA showed significant effects of allopurinol factor and oxonate × allopurinol interaction on serum creatinine levels (Table 2).
In addition, quercetin and allopurinol did not produce any alternations in normal mice.
Expressions of renal organic anion transporters
To determine whether the enhancement of renal urate excretion was due to regulate renal organic anion transporters, the expression levels of renal mOAT1, mOAT3, mURAT1 and mGLUT9 were examined, respectively. The decreased renal mOAT1 mRNA levels (P < 0.01) and increased renal mURAT1 (P < 0.001) and mGLUT9 (P < 0.001) mRNA levels were observed in hyperuricemic mice compared to normal vehicle group (Fig. 1a). Quercetin at 50 and 100 mg/kg effectively up-regulated mOAT1 (P < 0.001, P < 0.01) and down-regulated mGLUT9 (P < 0.001, P < 0.001) at mRNA levels in hyperuricemic mice compared to hyperuricemia vehicle group (Fig. 1a). Quercetin also decreased renal mURAT1 mRNA levels of hyperuricemic mice at the tested doses (P < 0.05, P < 0.001, P < 0.001) (Fig. 1a). Allopurinol at 5 mg/kg completely reduced mURAT1 (P < 0.001) and increased mOAT1 (P < 0.05) mRNA levels but failed to significantly affect mGLUT9 mRNA levels in this model (Fig. 1a). Furthermore, renal mOAT1 protein levels (P < 0.001) was decreased in hyperuricemic mice, which were attenuated by quercetin significantly (P < 0.01, P < 0.001, P < 0.001) (Fig. 1b). The protein levels of renal mURAT1 (P < 0.001) and mGLUT9 (P < 0.01) were significantly increased in hyperuricemic mice compared to normal vehicle animals (Fig. 1b). Administration of 50 and 100 mg/kg quercetin to hyperuricemic mice resulted in recovery of mURAT1 (P < 0.05, P < 0.01) and mGLUT9 (P < 0.01, P < 0.001) at protein levels compared to hyperuricemia vehicle group (Fig. 1b). Allopurinol at 5 mg/kg could reverse oxonate-induced alterations of renal mURAT1 (P < 0.05) and mOAT1 (P < 0.001) protein levels, but fail to significantly affect renal mGLUT9 protein levels (Fig. 1b). In addition, there was no remarkable difference in renal mOAT3 expression between the tested groups (data not shown). Quercetin and allopurinol did not produce expression changes of these renal organic anion transporters in normal groups.
Regulation of quercetin (Que) and allopurinol (AP) on renal organic anion transporters in normal and oxonate (OX)-induced hyperuricemic mice. a mRNA levels of renal mOAT1, mGLUT9 and mURAT1 were determined by semiquantitative reverse transcriptase-polymerase chain reaction analysis. b Protein levels of renal mOAT1, mGLUT9 and mURAT1 were determined by Western blotting analysis. Values represented the mean ± SEM (n = 4). ++ P < 0.01, +++ P < 0.001 versus normal vehicle group (Non-OX). *P < 0.05, **P < 0.01, ***P < 0.001 versus hyperuricemia vehicle group (OX)
Expressions of renal organic cation and carnitine transporters
To explore the protective mechanisms of quercetin against renal dysfunction in oxonate-induced hyperuricemic mice, the expression changes for renal organic cation/carnitine transporters mOCT1, mOCT2, mOCTN1 and mOCTN2 were also examined. In comparison to normal vehicle mice, oxonate significantly caused down-regulation of renal mOCT1, mOCT2, mOCTN1 and mOCTN2 at mRNA levels (P < 0.001, P < 0.001, P < 0.001, P < 0.001) in mice (Fig. 2a). Quercetin at 25, 50 and 100 mg/kg effectively up-regulated renal mOCT1 (P < 0.05, P < 0.05, P < 0.001) and mOCT2 (P < 0.001, P < 0.001, P < 0.001) mRNA levels compared to hyperuricemia vehicle group (Fig. 2a). 50 and 100 mg/kg quercetin could significantly up-regulate mOCTN1 (P < 0.05, P < 0.01) and mOCTN2 (P < 0.01, P < 0.001) mRNA levels in hyperuricemic mice (Fig. 2a). Allopurinol at 5 mg/kg remarkably elevated renal mOCT1 (P < 0.001), mOCT2 (P < 0.001), mOCTN1 (P < 0.01) and mOCTN2 (P < 0.01) mRNA levels in hyperuricemic mice (Fig. 2a). The reduced protein levels of renal mOCT1, mOCT2, mOCTN1 and mOCTN2 (P < 0.001, P < 0.001, P < 0.001, P < 0.001) were observed in hyperuricemic mice, which were attenuated by 50 and 100 mg/kg quercetin (mOCT1: P < 0.01, P < 0.001; mOCT2: P < 0.01, P < 0.01; mOCTN1: P < 0.01, P < 0.001; mOCTN2: P < 0.01, P < 0.001) compared to hyperuricemia vehicle group (Fig. 2b). Additionally, 25 mg/kg quercetin could up-regulate renal mOCT1, mOCTN1 and mOCTN2 protein levels (P < 0.05, P < 0.05, P < 0.05). Allopurinol at 5 mg/kg also significantly increased renal mOCT1, mOCT2, mOCTN1 and mOCTN2 protein levels (P < 0.001, P < 0.01, P < 0.001, P < 0.001) in this model (Fig. 2b). In addition, there was no significant alteration in renal organic cation/carnitine transporters of normal mice treated with quercetin and allopurinol.
Regulation of quercetin (Que) and allopurinol (AP) on renal organic anion transporters in normal and oxonate (OX)-induced hyperuricemic mice. a mRNA levels of renal mOCT1, mOCT2, mOCTN1 and mOCTN2 were determined by semiquantitative reverse transcriptase-polymerase chain reaction analysis. b Protein levels of renal mOCT1, mOCT2, mOCTN1 and mOCTN2 were determined by Western blotting analysis. Values represented the mean ± SEM (n = 4). ++ P < 0.01, +++ P < 0.001 versus normal vehicle group (Non-OX). *P < 0.05, **P < 0.01, ***P < 0.001 versus hyperuricemia vehicle group (OX)
UMOD level and mRNA expression
In order to confirm the role of UMOD in oxonate-induced hyperuricemia and renal dysfunction in mice, UMOD concentrations in serum, urine and kidney, as well as renal UMOD mRNA expression were determined. Hyperuricemic mice showed that UMOD concentrations were significantly reduced in urine (P < 0.01) (Table 3), but increased in serum (P < 0.01) and kidney (P < 0.001) (Table 3) compared to normal vehicle animals. Quercetin at 25, 50 and 100 mg/kg effectively reversed oxonate-induced alternations of UMOD concentrations in serum (P < 0.001, P < 0.001, P < 0.01) and kidney (P < 0.05, P < 0.01, P < 0.01) in mice compared to hyperuricemia vehicle group (Table 3). Quercetin at two high doses lowered urinary UMOD levels (P < 0.001, P < 0.01) (Table 3) in hyperuricemic mice. Moreover, 2-way ANOVA displayed significant effects of oxonate and quercetin on urinary UMOD concentrations, quercetin and oxonate × quercetin interaction on serum UMOD concentrations, and oxonate, quercetin and their interaction on renal UMOD concentrations. Allopurinol at 5 mg/kg succeeded in decreasing UMOD concentrations in urine (P < 0.001), serum (P < 0.01) and kidney (P < 0.05) in hyperuricemic mice, exhibiting oxonate × allopurinol interaction. Meanwhile, up-regulation of renal UMOD mRNA levels was observed in this model (P < 0.01) (Fig. 3). Administration of 50 and 100 mg/kg quercetin to hyperuricemic mice resulted in recovery of renal UMOD mRNA expression (P < 0.05, P < 0.01) (Fig. 3). Allopurinol at 5 mg/kg also reduced renal UMOD mRNA levels in hyperuricemic animals (P < 0.05). Quercetin and allopurinol had no significant effects on UMOD level and expression of normal animals.
Regulation of quercetin (Que) and allopurinol (AP) on renal mUMOD mRNA levels in normal and oxonate (OX)-induced hyperuricemic mice. mUMOD mRNA levels were determined by semiquantitative reverse transcriptase-polymerase chain reaction analysis. Values represented the mean ± SEM (n = 4). ++ P < 0.01 versus normal vehicle group (Non-OX). *P < 0.05, **P < 0.01 versus hyperuricemia vehicle group (OX)
Discussion
The present study confirmed that quercetin promoted renal excretion of uric acid with the uricosuric effect and kidney function improvement. It was noted that quercetin showed hypouricemic effect only in hyperuricemic mice, but not in normal mice. However, allopurinol reduced serum urate levels in normal group. Several clinical trials have shown that quercetin is safe and efficient [35–37]. These observations suggest that quercetin may bring fewer side effects than allopurinol in the amelioration of hyperuricemia and renal dysfunction.
Organic anion transporters at the proximal tubules of renal cortex can control kidney urate secretion and reabsorption. Consistent with our previous study that quercetin increased renal rOAT1 expression in fructose-induced hyperuricemic rats [19], the present study found that quercetin up-regulated mOAT1 expression in hyperuricemic mice, indicating that quercetin may mediate urate secretion in hyperuricemia. Quercetin reduces cellular uptake of (3H)-p-aminohippurate mediated by hOAT1 in MDCK cells [24]. Recent study show that renal OAT1 mediates the basolateral uptake of quercetin and isoflavonoids conjugates in the kidney. Systemic availability of these flavonoids is limited when interacting with OATs [38]. Thus, these observations may provide the mechanism of drug-food containing flavonoids interaction via inhibition of renal uptake mediated by OAT1. URAT1 and GLUT9 mediate renal urate reabsorption in renal proximal tubules [3, 27]. Down-regulation of renal mURAT1 and mGLUT9 was found in quercetin-treated hyperuricemic mice, suggesting that quercetin may inhibit renal urate reabsorption in hyperuricemia. Noticeably, allopurinol had no effect on mGLUT9 expression in hyperuricemic mice. These data indicate that the action of quercetin on renal urate reabsorption appears to be more subtle than that of allopurinol in hyperuricemic mice. Interestingly, quercetin-mediated regulation of these renal organic anion transporters was correlated to its enhancement of urate excretion and reduction of serum urate levels in hyperuricemic mice. Thus, mOAT1, mURAT1 and mGLUT9 may be targeted by quercetin for the amelioration of hyperuricemia in mice.
Organic ion transporters at renal proximal tubules play a predominant role in modifying kidney transports of cations and anions. In this study, administration of quercetin to hyperuricemic mice largely up-regulated the expression levels of renal mOCT1, mOCT2, mOCTN1 and mOCTN2, which were partly consistent with our previous report that quercetin increased renal rOCT1 and rOCT2 in fructose-fed rats with hyperuricemia and kidney dysfunction [19]. Regulation of these renal organic ion transporters by quercetin was in parallel with its renal function improvement in hyperuricemic mice. Of note, flavonoids including quercetin, as common components of daily nutrition, bear the ability to interfere with secretory intestinal transport processes mediated by OCTs [25]. Accordingly, the regulative effect of quercetin on OCTs may attenuate the accumulation of endogenous and exogenous noxious substances in the kidney. Clearly, these possibilities remain to be experimentally validated.
UMOD alteration at renal distal convoluted tubules is suggested to be associated with hyperuricemia and kidney dysfunction [12, 13]. More importantly, we first found that abnormality of UMOD level and expression was related to renal dysfunction in oxonate-induced hyperuricemic mice with kidney dysfunction. These results were consistent with the observations in patients with UMOD-associated kidney disease [39]. On the other hand, UMOD affect sodium reabsorption in kidney thick ascending limb, where is known to interact with Na+–K+–2Cl− cotransporter associated with circulating levels of uric acid [40]. UMOD defect exhibits an up-regulation of proximal mechanisms for sodium reabsorption, partly leading to increase of uric acid reabsorption, and subsequently to hyperuricemia [41]. Oxonic acid-induced hyperuricemia produces the increased K+ and decreased Na+ excretion in Sham and 5/6 nephrectomy rats [42]. Thus, UMOD may be considered as a usefully predictive factor for hyperuricemia with renal dysfunction. In this study, restoration of quercetin on UMOD alteration was parallel with its regulation on renal organic ion transporters in hyperuricemic mice. These findings further provide the evidence supporting that renal protective role of quercetin is partly mediated by regulating UMOD expression in distal convoluted tubule, which may cause the attenuation of renal organic ion transport abnormality in patients with hyperuricemia and renal dysfunction.
Quercetin at high doses has no side effects in normal individuals. Based on the ratio of surface area (human/mouse) and the doses of 25, 50 and 100 mg/kg tested in mice of the present study, the corresponding doses in human are 192.3, 384.6 and 769.2 mg/day. Treating mice with up to 3,000 mg/kg quercetin exhibit no toxicity [43]. Twenty-two patients administrated with 500 mg of quercetin twice a day for 4 weeks experience none negative side effects [37]. Therefore, oral administration of quercetin as nutritional dietary supplement may safely and effectively protect against hyperuricemia and renal dysfunction. On the other hand, quercetin-containing human food includes onions, apples, propolis, berries, tea, red wine and other dietary supplements. For examples, 220 g onions can provide 114 mg quercetin [44], and quercetin concentrations in propolis reaches 10–30 mg/g [45]. Apples and apple products possess a wide range of biological activities which may contribute to health beneficial effects against cardiovascular disease, asthma and pulmonary dysfunction, diabetes, obesity and cancer [46]. Onion peel extract containing high quercetin ameliorates hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats [47]. Daily supplementation with 730 mg quercetin for 28 days reduces systolic, diastolic and mean arterial pressure in subjects with stage 1 hypertension [30], as well as with 150 mg provides protection against cardiovascular disease in overweight subjects [31] and reduces niacin-induced flush in humans [32]. Recently, it is reported that male volunteers take 500 mg of quercetin twice daily over 4 weeks show immunoregulatory effects [29]. Thus, it is possible that quercetin may exert some of these activities at the tested dosages attained by consuming quercetin-containing human food.
More evidence has demonstrated several molecular targets of quercetin in treating diseases. Raf and MEK protein kinases, which are firmly associated with pathogesis of cancer, are suggested to be direct molecular targets for the chemopreventive effect of quercetin in mouse skin epidermal cells [48]. Quercetin exhibits anti-inflammation mediated by the inhibition of TNF-α via modulation of NFκβ1 and Iκβ in human peripheral blood mononuclear cells [49]. Chronic dietary intake of quercetin can mainly recover PPAR-α expression to avoid fat accumulation and metabolic syndrome in the liver of western diet-fed mice [50]. Chronic 1% quercetin diet lead to up-regulation of pulmonic 3-Hydroxy-3-methylglutaryl-CoA synthase 2, Enoyl CoA hydratase 1, aconitase 1, propionyl CoA carboxylase, lipoprotein lipase and acetyl-CoA acyltransferase 2, affecting fatty acid catabolism [51]. Quercetin treatment also protects cadmium nephrotoxicity by preventing overexpression of inducible nitric oxide synthase and cyclooxygenase-2 and increasing metallothionein expressions in rats [52] and attenuates ischemic renal injury by regulating renal expressions of normal T-cell expressed and secreted, monocyte chemoattractant protein-1 and allograft inflammatory factor in rats [53]. To our knowledge, the effects of quercetin on the expression levels of renal organic ion transporters have never been investigated. The present study provides new molecular targets of quercetin for the treatment of hyperuricemia and renal dysfunction.
In conclusion, renal mOAT1, mURAT1, mGLUT9, mOCT1, mOCT2, mOCTN1, mOCTN2 and UMOD are both involved in quercetin-mediated uricosuric and renal protective actions in hyperuricemic mice with kidney dysfunction. Quercetin is a consumed flavonoid with beneficial effects on human health. It is confirmed that quercetin concentrations nearly double in subjects fed with high-vegetable, -berry and -other fruit diets [54], so it is possible that quercetin may exert the beneficial effects on hyperuricemia and renal dysfunction by consuming quercetin-containing human food. The present study provides the evidence that quercetin may be a potential therapeutic candidate in the amelioration of hyperuricemia and renal dysfunction. Of note, other quercetin derivatives modulate a variety of biological activities [55–58]. Our previous study showed that rutin, as a glycoside of quercetin, can restore renal dysfunction and regulate the expression levels of renal organic ion transporters in fructose-fed rats [19]. Thus, quercetin as a model for the discovery of quercetin-like molecules which can regulate renal organic ion transporters and UMOD to prevent hyperuricemia and renal dysfunction is further investigated.
Abbreviations
- OAT:
-
Organic anion transporter
- URAT1:
-
Urate transporter 1
- GLUT9:
-
Glucose transporter 9
- OCT:
-
Organic cation transporter
- OCTN:
-
Organic cation/carnitine transporter
- FEUA:
-
Fractional excretion of uric acid
- Sur:
-
Serum uric acid concentration
- Scr:
-
Serum creatinine concentration
- Uur:
-
Urine uric acid concentration
- Ucr:
-
urine creatinine concentration
- UMOD:
-
Uromodulin
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- BBMV:
-
Brush border membrane vesicles
- BUN:
-
Blood urea nitrogen
References
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821
Richette P, Bardin T (2010) Gout. Lancet 375:318–328
Hosoyamada M, Ichida K, Enomoto A et al (2004) Function and localization of urate transporter 1 in mouse kidney. J Am Soc Nephrol 15:261–268
Nigam SK, Bush KT, Bhatnagar V (2007) Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues. Nat Clin Pract Nephrol 3:443–448
Vitart V, Rudan I, Hayward C et al (2008) SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40:437–442
Preitner F, Bonny O, Laverrière A et al (2009) Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci USA 106:15501–15506
Enomoto A, Kimura H, Chairoungdua A et al (2002) Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417:447–452
Grover B, Buckley D, Buckley AR et al (2004) Reduced expression of organic cation transporters rOCT1 and rOCT2 in experimental diabetes. J Pharmacol Exp Ther 308:949–956
Tokuhiro S, Yamada R, Chang X et al (2003) An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 35:341–348
Glube N, Closs E, Langguth P (2007) OCTN2-mediated carnitine uptake in a newly discovered human proximal tubule cell line (Caki-1). Mol Pharm 4:160–168
Rizwan AN, Burckhardt G (2007) Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res 24:450–470
Dahan K, Devuyst O, Smaers M et al (2003) A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol 14:2883–2893
Hart TC, Gorry MC, Hart PS et al (2002) Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 39:882–892
Kemter E, Rathkolb B, Rozman J et al (2009) Novel missense mutation of uromodulin in mice causes renal dysfunction with alterations in urea handling, energy, and bone metabolism. Am J Physiol Renal Physiol 297:1391–1398
Egert S, Bosy-Westphal A, Seiberl J et al (2009) Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr 102:1065–1074
Perez-Vizcaino F, Duarte J, Jimenez R et al (2009) Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep 61:67–75
Zhu JX, Wang Y, Kong LD et al (2004) Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J Ethnopharmacol 93:133–140
Mo SF, Zhou F, Lv YZ et al (2007) Hypouricemic action of selected flavonoids in mice: structure-activity relationships. Biol Pharm Bull 30:1551–1556
Hu QH, Wang C, Li JM et al (2009) Allopurinol, rutin, and quercetin attenuate hyperuricemia and renal dysfunction in rats induced by fructose intake: renal organic ion transporter involvement. Am J Physiol Renal Physiol 297:1080–1091
Singh D, Chander V, Chopra K (2004) The effect of quercetin, a bioflavonoid on ischemia/reperfusion induced renal injury in rats. Arch Med Res 35:484–494
Renugadevi J, Prabu SM (2010) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol 62:471–481
Graf BA, Ameho C, Dolnikowski GG et al (2006) Rat gastrointestinal tissues metabolize quercetin. J Nutr 136:39–44
Bieger J, Cermak R, Blank R et al (2008) Tissue distribution of quercetin in pigs after long-term dietary supplementation. J Nutr 138:1417–1420
Hong SS, Seo K, Lim SC et al (2007) Interaction characteristics of flavonoids with human organic anion transporter 1 (hOAT1) and 3 (hOAT3). Pharmacol Res 56:468–473
Ofer M, Wolffram S, Koggel A et al (2005) Modulation of drug transport by selected flavonoids: involvement of P-gp and OCT? Eur J Pharm Sci 25:263–271
Taur JS, Rodriguez-Proteau R (2008) Effects of dietary flavonoids on the transport of cimetidine via P-glycoprotein and cationic transporters in Caco-2 and LLC-PK1 cell models. Xenobiotica 38:1536–1550
Bibert S, Hess SK, Firsov D et al (2009) Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Renal Physiol 297:612–619
Hu QH, Jiao RQ, Wang X et al (2010) Simiao pill ameliorates urate underexcretion and renal dysfunction in hyperuricemic mice. J Ethnopharmacol 128:685–692
Nickel T, Hanssen H, Sisic Z et al (2011) Immunoregulatory effects of the flavonol quercetin in vitro and in vivo. Eur J Nutr 50:163–172
Edwards RL, Lyon T, Litwin SE et al (2007) Quercetin reduces blood pressure in hypertensive subjects. J Nutr 137:2405–2411
Kalogeromitros D, Makris M, Chliva C et al (2008) A quercetin containing supplement reduces niacin-induced flush in humans. Int J Immunopathol Pharmacol 21:509–514
Chen Q (1994) Chinese medicine pharmacology research technology. The people’s medical publishing house, Beijing
Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA (2009) Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull 32:61–67
Perez-Ruiz F, Calabozo M, Erauskin GG et al (2002) Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 47:610–613
Okamoto T (2005) Safety of quercetin for clinical application (review). Int J Mol Med 16:275–278
Harwood M, Danielewska-Nikiel B, Borzelleca JF et al (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 45:2179–2205
Katske F, Shoskes DA, Sender M et al (2001) Treatment of interstitial cystitis with a quercetin supplement. Tech Urol 7:44–46
Wong CC, Botting NP, Orfila C et al (2011) Flavonoid conjugates interact with organic anion transporters (OATs) and attenuate cytotoxicity of adefovir mediated by organic anion transporter 1 (OAT1/SLC22A6). Biochem Pharmacol 81:942–949
Bleyer AJ, Hart TC, Willingham MC et al (2005) Clinico-pathologic findings in medullary cystic kidney disease type 2. Pediatr Nephrol 20:824–827
Cappuccio FP, Strazzullo P, Farinaro E et al (1993) Uric acid metabolism and tubular sodium handling. Results from a population-based study. JAMA 270:354–359
Cappuccio FP, Iacone R, Strazzullo P (1991) Serum uric acid and proximal sodium excretion: an independent association in man (the Olivetti Study). J Hypertens Suppl 9:280–281
Eräranta A, Kurra V, Tahvanainen AM et al (2008) Oxonic acid-induced hyperuricemia elevates plasma aldosterone in experimental renal insufficiency. J Hypertens 26:1661–1668
Ruiz MJ, Fernández M, Picó Y et al (2009) Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J Agric Food Chem 57:3180–3186
Janssen K, Mensink RP, Cox FJ et al (1998) Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: results from an in vitro and a dietary supplement study. Am J Clin Nutr 67:255–262
Wang XP, Lin L, Bai JQ (2009) Detection of quercetin concentrations in propolis from different manufacturing locations by HPLC. Acta Yunnan Collage Tradit Chin 3:30–32 (in Chinese)
Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutr J 3:5
Jung JY, Lim Y, Moon MS et al (2011) Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr Metab (Lond) 8:18
Lee KW, Kang NJ, Heo YS et al (2008) Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res 68:946–955
Nair MP, Mahajan S, Reynolds JL et al (2006) The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol 13:319–328
Kobori M, Masumoto S, Akimoto Y et al (2011) Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol Nutr Food Res 55:530–540
de Boer VC, van Schothorst EM, Dihal AA et al (2006) Chronic quercetin exposure affects fatty acid catabolism in rat lung. Cell Mol Life Sci 63:2847–2858
Morales AI, Vicente-Sánchez C, Jerkic M et al (2006) Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol Appl Pharmacol 210:128–135
Shoskes DA (1998) Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation 66:147–152
Erlund I, Freese R, Marniemi J et al (2006) Bioavailability of quercetin from berries and the diet. Nutr Cancer 54:13–17
Femia AP, Caderni G, Ianni M et al (2003) Effect of diets fortified with tomatoes or onions with variable quercetin-glycoside content on azoxymethane-induced aberrant crypt foci in the colon of rats. Eur J Nutr 42:346–352
Ahmad NS, Farman M, Najmi MH et al (2008) Pharmacological basis for use of Pistacia integerrima leaves in hyperuricemia and gout. J Ethnopharmacol 117:478–482
Mulholland PJ, Ferry DR, Anderson D et al (2001) Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann Oncol 12:245–248
Panda S, Kar A (2007) Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-o-glucoside. Biofactors 31:201–210
Acknowledgments
The research was supported by grants from NSFC (No. 81025025), JSNSF (BK2010365) and PCSIRT (IRT1020) to Ling-Dong Kong (L. D. Kong). Kong Ling-Dong contributed to the experimental design. Qing-Hua Hu performed the experiments described. Xian Zhang and Xing Wang participated in the part of the experiments. Qing-Hua Hu and Xian Zhang undertook the statistical calculations, graphed the data for visual inspection and analysis. Rui-Qing Jiao and Qing-Hua Hu wrote the first draft of the manuscript. Kong Ling-Dong was involved in writing the final manuscript which was reviewed and approved by all authors.
Conflicts of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, QH., Zhang, X., Wang, X. et al. Quercetin regulates organic ion transporter and uromodulin expression and improves renal function in hyperuricemic mice. Eur J Nutr 51, 593–606 (2012). https://doi.org/10.1007/s00394-011-0243-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0243-y