Abstract
Background
DNA methylation is an important epigenetic process for transcriptional control of human genome including those genes involved in cancer initiation and progression. Clinical studies have suggested that biological explanation to the protective effect of some nutrients could be linked with the DNA methylation. Folate is a primary methyl donor nutrient; it has been shown to play a key role in DNA methylation, repair and synthesis, by acting as co-factors and/or substrates in this metabolic pathway. Likewise, activity of a key enzyme, the methylenetetrahydrofolate reductase (MTHFR) has also been shown to influence DNA methylation. Overall, these findings support the notion that dietary intake as well as genetic factors play a role in one-carbon metabolism.
Aim of the study
This study is to evaluate the dietary intake of nutrients involved in one-carbon metabolism and the genotype of MTHFR 677 C > T with respect to GC risk.
Methods
We carried out in January 2004 a population-based case–control study in the metropolitan area of Mexico City. A total of 248 histological confirmed GC patients were recruited from nine tertiary hospitals, along with 478 age and sex-matched controls. Nutrient intake was estimated from food frequency questionnaire; the MTHFR 677C > T genotype was determined by PCR-RFLP analysis.
Results
A significant reduction in diffuse GC risk was observed for MTHFR 677 TT genotype among individuals with high consumption of folate (OR = 0.23; 95% CI 0.06–0.84), choline (OR = 0.55; 95% CI 0.33–0.9) and Vitamin B6 (OR = 0.59; 95% CI 0.36–0.96) compared to MTHFR 677 CC + CT carriers. Among subjects with low consumption of methionine, a reduced risk of diffuse GC was also detected (OR = 0.40; 95% CI 0.16–0.97). In contrast, carriers of the MTHFR 677 TT genotype with a low consumption of folate had a significant increased risk of intestinal GC (OR = 1.88 95% CI 1.02–3.47). A folate–MTHFR 677 C > T interaction in the borderline of significance (P = 0.055) was detected.
Conclusions
It is probable that GC prevention requires dietary recommendations according to the individual genotype; nevertheless, the available information to this respect is still very limited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence and mortality of gastric cancer (GC) have decreased for the past 70 years; the reduction was mainly restricted to intestinal GC [8]. GC is now the fourth most frequent and the second most deadly cancer at a global level [8]. In 2000, about 75% of diagnosed subjects die due to this neoplasia [8]. In Mexico, incidence and mortality of GC have not decreased; it is the second cause of death by malignant neoplasias [23, 29] and, as opposed to other countries, diffused GC is more prevalent than the intestinal type [28].
Gastric cancer is a multifactorial disease; its marked geographical variation and the migratory relation in its incidence suggest that environmental factors and lifestyle are major contributors to its aetiology [8]. Infection by Helicobacter pylori (H. pylori) is considered a necessary cause [10, 27]; however, the infection by itself is not the only determinant, since only 3% of H. pylori seropositive individuals eventually develop the tumour [32]. Consumption of salty foods and low consumption of certain types of vegetables (non-starchy vegetables and alliums) and fruits increase GC risk [38].
Folate, mainly from vegetables, some fruits and fortified cereals [13], together with other nutrients, such as methionine, choline and vitamins B6 and B12 are key components in one-carbon metabolism by acting as methyl donors or cofactors [6, 17]. Deficiency of these nutrients reduces the concentration of S-adenosylmethionine (SAM) and increases levels of S-adenosylhomocysteine (SAH); that in turn decreases the degree of methylation [9] and impairs DNA repair [24]. The protective association of dietary folate consumption has been shown in diverse types of cancer [19, 20, 31]; however, inconsistent evidence is available in relation to GC. A recent cohort study show no association between blood folate levels and GC risk among Europeans [36], while others reported inverse associations of dietary folate and GC risk [19, 38]. Also, there is limited, inconsistent evidence, about the association between dietary methionine intake and risk of GC [37]. No information is available regarding choline and GC. Sparse and contrasting results relate Vitamin B12 with GC risk, and suggestive negative associations are reported regarding Vitamin B6 and GC risk [37].
DNA methylation has been shown to be correlated with the activity of a key one-carbon metabolizing enzyme, Methylenetetrahydrofolate reductase (MTHFR) [9]; the carriers of the variant genotype (MTHFR 677TT) had twofold increase in GC risk compared to those with the MTHFR 677CC genotype [2, 25, 41].
Herein, we conducted a population-based case–control study that evaluates the dietary intake of nutrients involved in one-carbon metabolism (folate, choline, methionine and vitamins B6 and B12) and the genotype of MTHFR 677 C > T with respect to GC risk in a Mexican population.
Materials and methods
We carried out a population-based case–control study in the metropolitan area of Mexico City in January 2004. The cases were later followed to evaluate factors associated with survival; results of the follow-up study have been published elsewhere [11]. This study was approved by the IRB of the National Institute of Public Health (INSP) and written informed consent was obtained from all participants.
Cases
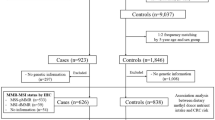
Cases were individuals, newly diagnosed and histological confirmed, with adenocarcinoma of the stomach, older than 20 years of age, with at least 3 years of residency in Mexico City and/or surrounding areas, with no history of cancer. Gastric tumours were classified by a single expert pathologist, according to the TNM system [21] as well as Lauren’s histological type (diffuse vs. intestinal). Patients were recruited from oncology and/or gastroenterology units of nine tertiary care hospitals in the metropolitan area of Mexico City (Hospital de Oncologia, Hospital de Especialidades la Raza, Hospital de Especialidades del Centro Médico Siglo XXI, Instituto Nacional de Cancerología, Hospital General de México, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Hospital Juárez, Hospital Adolfo López Mateos, and Hospital 20 de Noviembre). A total of 263 eligible individuals were identified and 257 agreed to participate (rate of participation 97.7%). The total number of identified cases corresponds to almost 60% of all GC cases in the area reported to the National Registry of cancer during the recruitment period of this study.
Controls
For each index case, up to two healthy individuals were recruited of the same age (±5 years) and sex, which was at least 20 years old, without a history of any type of cancer and with permanent residency in Mexico City and surrounding area. Eligible controls were identified by means of the master sampling framework, built by the INSP and used to select dwellings for the national health surveys. Based on household census, a probabilistic list of almost 1400 blocks, with different number of houses, was available for this study. When there was more than one eligible control in the same house, he/she was chosen randomly using Simple Random Sampling; when no one was eligible, the interviewer went to the next house to the right. Finally, 478 of 507 eligible controls agreed to participate (response rate of 94.3%).
Interviews
Eligible subjects were interviewed about their sociodemographic characteristics, clinical history, lifestyles and dietary patterns. The dietary information went back 3 years before the appearance of symptoms, for the cases, and 3 years before the interview, for the controls. Although the interviewers knew the condition of being a case or a control, they were blind to the hypothesis of the study. Cases were interviewed at the hospital and controls in their homes.
Nutrients involved in one-carbon metabolism intake
Intake of folate, choline, methionine and vitamins B6 and B12 was estimated through a semi-quantitative food frequency questionnaire (FFQ) which was previously validated [12, 14]. It included 127 foods with pre-determined portions, which were divided into the following groups: milk products, fruits, meats, vegetables, legumes, cereals, drinks, oils, local dishes, sodas and candies. It also included 10 categories of consumption frequency, from “never” up to “six times a day.” The complete of FFQ took an average of 25 min.
The frequency of consumption of fruits and vegetables was adjusted according to their availability in the market. In addition, information was obtained on the cooking method used with 74 foods, in order to have a more precise calculation of folate intake [34]. Consumption frequencies of individual foods were converted to grams per day.
Intake of calories and nutrients was estimated by means of the computerized Food Intake Analysis System (FIAS 3.0), whose nutritional values were adapted to Mexican foods and the methodology has been previously published [22]. Briefly, this methodology consisted of a comparison between the nutrient values of the foods in this programme and those corresponding to the ones reported in the Tables of Food Composition, designed by the “Salvador Zubirán” National Institute of Nutrition [26]. When a nutritional value differed by more than 10% when comparing both sources of information, we took the one from the second source.
For the purpose of this study, the nutrient tables were updated with choline values reported by the U.S. Department of Agriculture [15] and for methionine those contained in the food composition tables of the “Salvador Zubirán” National Institute of Nutrition [26].
Nine subjects were excluded from the subsequent analysis, who reported a daily caloric intake >4500 kcal, as a control measure of information quality, so the final sample size was 248 cases and 478 controls.
Blood samples
A 15 ml of blood was obtained from each patient, before they received treatment, and refrigerated for up to 2 h until their separation in plasma, buffy coat and red cells. Plasma and buffy coat samples were stored at −70 °C until further analysis.
Determination of H. pylori CagA serology status
The presence of antibodies (IgG) against H. pylori CagA+ antigen was determined by an ELISA method based on the presence of serum IgG antibodies against orv220, a 65,000 Dalton recombinant cagA-encoded protein purified from Escherichia coli [1], according to the methodology and control quality described previously [1].
MTHFR 677 C > T genotyping
Genomic DNA from cases was extracted from buffy coat, using QIAamp DNA Blood Mini Kit (Qiagen, Inc., Valencia CA) following the manufacturer’s protocol. MTHFR 677C > T genotypes were determined by PCR-RFLP, according to Chen et al. [4].
Statistical analysis
Selected general characteristics between cases and controls (age, sex, education, total energy intake, etc.) were compared by χ 2 and t-test accordingly.
The observed distribution of the MTHFR 677C > T genotypes in the total population was compared with the expected one using the Hardy–Weinberg equilibrium test and the medians of dietary consumption of methyl donors adjusted by residual energy, age and sex, were compared between cases and controls using the Mann–Whitney test.
Unconditional logistic regression models were used to assess the association between dietary intake and genetic polymorphism in relation to risk of GC. The dietary intake was categorized into tertiles based on the distribution in the control group. Odds ratios were adjusted by age, sex, education and energy intake. The association between MTHFR 677 C > T genotype and GC was estimated according to following genetic models: codominant model (CC vs. CT vs. TT), recessive model (CC + CT vs. TT), dominant model (CC vs. CT + TT) and allele carriers (allele C vs. allele T). Due to small sample size, only the adjusted ORs of the recessive model by histological type are presented according to the dietary recommended allowances (RDA) for folate, choline and vitamins B12 and vitamin B6 intake (low folate < 400 μg/d vs. high folate ≥ 400 μg/g; low choline < 425 mg/d vs. high choline ≥ 425 mg/d; low vitamin B12 < 2.4 μg vs. high vitamin B12 ≥ 2.4 μg; low vitamin B6 < 1 mg/d vs. high vitamin B6 ≥ 1 mg/d), except for methionine where the median intake among controls was used as a cut off point. (low < 1653 mg/d vs. high ≥ 1653 mg/d) [3, 16] since no RDA is not available. The final models included as confounding variables, age, sex, energy and education that were selected a priori, as well as H. pylori CagA+, vegetables, beans, salt use, chilli consumption because they resulted significantly associated with the risk of CG. All the analyses were performed using the statistical software Stata 9:0 (Stata, College Station, TX, USA).
Results
The age (mean of 58 years) and sex (54% males) distributions were similar between cases and controls. The cases had significant higher years of education and total energy intake compared to controls (data not included in the tables). The consumption of vegetables and beans showed a significant inverse association with GC, in contrast with the intake of salt and chilli pepper as well as H. pylori CagA+ status that significantly increased the risk of GC. A marginal association between alcohol consumption and GC risk was also detected (Table 1).
Folate consumption was significantly higher among controls compared to cases. A non-significant increase in median intakes of choline and vitamin B12 was also observed among controls. In the meantime, the mean intake of methione was non-significantly higher in the cases; vitamin B6 consumption was the same in both groups (Table 2).
The observed distribution of the MTHFR 677 C > T genotype was in agreement with Hardy–Weinberg equilibrium. There was no apparent association between the MTHFR 677 C > T genotype and overall GC risk. Only after stratifying by the histological type, we observed a significant association between the MTHFR 677 C > T genotype and intestinal GC risk (OR = 2.67 CI 95%1.16–6.16).
A significant reduction in diffuse GC risk was observed for MTHFR 677 TT genotype among individuals with high consumption of folate (OR = 0.23; 95% CI 0.06–0.84), choline (OR = 0.55; 95% CI 0.33–0.9) and vitamin B6 (OR = 0.59; 95% CI 0.36–0.96) compared to MTHFR 677 CC + CT carriers. Among subjects with low consumption of methionine, a reduced risk of diffuse GC was also detected (OR = 0.40; 95% CI 0.16–0.97). In contrast, carriers of the MTHFR 677 TT genotype with a low consumption of folate had a significant increased risk of intestinal GC (OR = 1.88 95% CI 1.02–3.47) (Table 3).
Among diffuse GC, a folate–MTHFR 677 C > T interaction in the borderline of significance (P = 0.055) was detected: carriers of the MTHFR 677 TT genotype with low consumption of folate had an increased risk of GC, in contrast, high consumption of folate in this group showed a protective significant association of GC (OR = 0.18, CI 95% 0.05–0.65) (Table 4).
Discussion
Results of this study provide evidence that one-carbon metabolism may play an important role in GC aetiology; association between dietary methyl intake and GC risk may be modified by the key genes in the one-carbon metabolic pathway.
Only one previous study had simultaneously evaluated the MTHFR 677 genotypes and plasma B vitamins showing no significant gene–nutrient associations among Europeans’ unfortunately authors did not show results stratifying by histological type of GC [36] to compare our results. Results from our study need to be replicated in other populations.
Similar to this study, several studies had reported an increased risk of GC among MTHFR 677 TT carriers [2]. To our knowledge, this is the first report on an inverse association between choline on diffuse GC. Only two recent epidemiologic studies, with contrasting results, reported the association of choline intake and the risk of colorectal adenoma [5] and breast cancer, respectively [39]. The scarce information available in this regard might be partially explained by the absence of food composition data [15, 40]. A protective relation of vitamin B6 for GC has been found in three previous studies [37], which are on the line of the results among diffuse MTHFR TT carriers of this study.
Also it is important to mention that the protective association of folate, choline and vitamin B6 emerged among those individuals with adequate dietary consumptions. A current debate regarding the potential pro-carcinogenic effect of high folate intake has argued for the importance of timing and dosage of folate supplementation for cancer prevention [18, 35].
Diet has been traditionally related to the risk of intestinal GC, where a carcinogenic pathway with clear precancerous lesions (chronic to atrophic gastritis, intestinal metaplasia and dysplasia) has been described [7]. In contrast, risk factors for diffuse GC have not been fully described. Our results suggest that one-carbon metabolism may play an important role for diffuse GC; larger studies including gene-nutrient evaluations are needed to elucidate diffuse gastric pathogenesis.
In the interpretation of our results, there are various methodological considerations. We believe there is a low probability of a differential report between cases and controls on consumption of methyl-related nutrients since the general population as well as the interviewers were blinded to the study hypothesis. Also, folate intake is only an estimate of the real intake, since, it is important to remember that the FFQ, as other questionnaires, has a sensitivity and specificity that are <100%, besides, no information of folate supplementation was available for this study, although national figures show a low prevalence of this supplementation among adults over 20 years of age (18.2%) [30], and thus our results are conservative results of the real association. In addition, no assessment of MTHFR 1298 polymorphism was performed due to the low percentage of the variant allele among Mexicans [33]. Finally, the statistical power for diet–gene interaction is limited because of the moderate sample size.
It is probable that GC prevention requires dietary recommendations according to the individual genotype; nevertheless, the available information to this respect is still very limited.
References
Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A (1995) Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 55:2111–2115
Boccia S, Hung R, Ricciardi G, Gianfagna F, Ebert MP, Fang JY, Gao CM, Gotze T, Graziano F, Lacasana-Navarro M, Lin D, Lopez-Carrillo L, Qiao YL, Shen H, Stolzenberg-Solomon R, Takezaki T, Weng YR, Zhang FF, Van Duijn CM, Boffetta P, Taioli E (2008) Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol 167:505–516
Bourges H, Casanueva E, Rosado J (2005) Recomendaciones de ingestión de nutrimentos para la población mexicana. Tomo 1. Editorial Médica Panamericana, México, pp 163–175
Chen J, Giovannucci E, Kelsey K, Rimm EB, Stampfer MJ, Colditz GA, Spiegelman D, Willett WC, Hunter DJ (1996) A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res 56:4862–4864
Cho E, Willett WC, Colditz GA, Fuchs CS, Wu K, Chan AT, Zeisel SH, Giovannucci EL (2007) Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl Cancer Inst 99:1224–1231
Choi SW, Mason JB (2000) Folate and carcinogenesis: an integrated scheme. J Nutr 130:129–132
Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M (1975) A model for gastric cancer epidemiology. Lancet 2:58–60
Crew KD, Neugut AI (2006) Epidemiology of gastric cancer. World J Gastroenterol 12:354–362
Davis CD, Uthus EO (2004) DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood) 229:988–995
Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F (1991) Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 302:1302–1305
Galvan-Portillo M, Oñate-Ocaña LF, Perez-Perez GI, Chen J, Herrera-Goepfert R, Chihu-Amparan L, Flores-Luna L, Mohar-Betancourt A, Lopez-Carrillo L (2009) Dietary folate and vitamin B12 intake before diagnosis decreases gastric cancer mortality risk among susceptible MTHFR 677 TT carriers. Nutrient (in press)
Galvan-Portillo MV, Wolff MS, Torres-Sanchez LE, Lopez-Cervantes M, Lopez-Carrillo L (2007) Assessing phytochemical intake in a group of Mexican women. Salud Publica Mex 49:126–131
Gutierrez-Valenzuela V, Casanueva E (2005) Folatos. In: Bourges B, Casanueva E, Rosado JL (eds) Recomendaciones de ingestión de nutrimentos para la población mexicana. Tomo 1. Editorial Médica Panamericana, México, pp 163–175
Hernandez-Avila M, Romieu I, Parra S, Hernandez-Avila J, Madrigal H, Willett W (1998) Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex 40:133–140
Howe JC, Williams J, Holden JM, Zeisel SH, Mar M (2004) USDA database for the choline content of common foods—March 2004. U.S. Department of Agriculture. Agricultural Research Service. and Beltsville Human Nutrition Research Center. Nutrient Data Laboratory. Beltsville, Maryland, USDA
Institute of Medicine National Academy of Sciences USA (1998) Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotine and choline. National Academic Press, Washington, DC
Kim YI (1999) Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem 10:66–88
Kim YI (2004) Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 80:1123–1128
Larsson SC, Giovannucci E, Wolk A (2006) Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology 131:1271–1283
Larsson SC, Giovannucci E, Wolk A (2007) Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 99:64–76
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 64:31–49
Lopez-Carrillo L, Lopez-Cervantes M, Ward MH, Bravo-Alvarado J, Ramirez-Espitia A (1999) Nutrient intake and gastric cancer in Mexico. Int J Cancer 83:601–605
Lopez-Carrillo L, Vega-Ramos B, Costa-Dias R, Rascon-Pacheco RA (1997) Histological types of gastric cancer in Mexico. Int J Epidemiol 26:1166–1171
Mason JB, Choi SW (2000) Folate and carcinogenesis: developing a unifying hypothesis. Adv Enzyme Regul 40:127–141
Miao X, Xing D, Tan W, Qi J, Lu W, Lin D (2002) Susceptibility to gastric cardia adenocarcinoma and genetic polymorphisms in methylenetetrahydrofolate reductase in an at-risk Chinese population. Cancer Epidemiol Biomarkers Prev 11:1454–1458
Muñoz M, Ledesma JA (2002) Loa alimentos y sus nutrientes. Tablas del valor nutritivo de alimentos, México, pp 174–177
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325:1127–1131
Rizo-Ríos P, Sierra-Colindres MI, Vázquez-Piñon G, Cano-Guadiana M, Mohar A (2007) Registro Hospitalario de cáncer:compendio de cáncer 2000–2004. Cancerología 2:203–287
Registro Histopatológico de Neoplasias Malignas (2002) Compendio de Cáncer/2002. Secretaría de Salud, México
Rivera Dommarco J, Shamah Levy T, Villalpando Hernández S, González de Cossío T, Hernández Prado B, Sepúlveda J (2001) Encuesta Nacional de Nutrición 1999. Estado nutricio de niños y mujeres en México, México
Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ (2005) Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer 113:825–828
Torres J, Lopez L, Lazcano E, Camorlinga M, Flores L, Munoz O (2005) Trends in Helicobacter pylori infection and gastric cancer in Mexico. Cancer Epidemiol Biomarkers Prev 14:1874–1877
Torres-Sanchez L, Chen J, Diaz-Sanchez Y, Palomeque C, Bottiglieri T, Lopez-Cervantes M, Lopez-Carrillo L (2006) Dietary and genetic determinants of homocysteine levels among Mexican women of reproductive age. Eur J Clin Nutr 60:691–697
U.S. Department of Agriculture, Agricultural Research Service (2003) USDA table of nutrient retention factors, Release 5. Beltsville, Maryland, USDA
Ulrich CM, Potter JD (2007) Folate and cancer—timing is everything. JAMA 297:2408–2409
Vollset SE, Igland J, Jenab M, Fredriksen A, Meyer K, Eussen S, Gjessing HK, Ueland PM, Pera G, Sala N, Agudo A, Capella G, Del Giudice G, Palli D, Boeing H, Weikert C, Bueno-de-Mesquita HB, Carneiro F, Pala V, Vineis P, Tumino R, Panico S, Berglund G, Manjer J, Stenling R, Hallmans G, Martinez C, Dorronsoro M, Barricarte A, Navarro C, Quiros JR, Allen N, Key TJ, Bingham S, Linseisen J, Kaaks R, Overvad K, Tjonneland A, Buchner FL, Peeters PH, Numans ME, Clavel-Chapelon F, Boutron-Ruault MC, Trichopoulou A, Lund E, Slimani N, Ferrari P, Riboli E, Gonzalez CA (2007) The association of gastric cancer risk with plasma folate, cobalamin, and methylenetetrahydrofolate reductase polymorphisms in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 16:2416–2424
WCRF and AICR (2007) WCRF/AICR expert report food, nutrition, physical activity, and the prevention of cancer: a global perspective systematic literature review—Support Resource. AICR, Washington, DC
World Cancer Research Fund/American Institute for Cancer Research (2007) 7.5 Stomach. In: AICR (ed) Food, nutrition, physical activity and the prevention of cancer: a global perspective. American Institute for Cancer Research, Washington, DC, pp 265–270
Xu X, Gammon MD, Zeisel SH, Lee YL, Wetmur JG, Teitelbaum SL, Bradshaw PT, Neugut AI, Santella RM, Chen J (2008) Choline metabolism and risk of breast cancer in a population-based study. FASEB J 22(6):2045–2052
Ziegler RG, Lim U (2007) One-carbon metabolism, colorectal carcinogenesis, chemoprevention—with caution. J Natl Cancer Inst 99:1214–1215
Zintzaras E (2006) Association of methylenetetrahydrofolate reductase (MTHFR) polymorphisms with genetic susceptibility to gastric cancer: a meta-analysis. J Hum Genet 51:618–624
Acknowledgment
The authors thanks Ms. Verónica López for the logistic field coordination as well as Dr. Guillermo I Perez-Perez and Dr. Lilia Chihu for their laboratory support. This grant was supported by CONACYT (Salud-2002-001-7107).
Conflict of interest statement
None declared
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galván-Portillo, M.V., Cantoral, A., Oñate-Ocaña, L.F. et al. Gastric cancer in relation to the intake of nutrients involved in one-carbon metabolism among MTHFR 677 TT carriers. Eur J Nutr 48, 269–276 (2009). https://doi.org/10.1007/s00394-009-0010-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0010-5