Abstract
Background
Nutritional support is an established element of therapy for various indications. However, its impact on the mucosal barrier function is not well understood.
Aim of the study
We investigated the influence of EN and PN on intestinal epithelial cells and peripheral blood (PBMC) and lamina propria mononuclear cells (LPMC), all of which are involved in the mucosal defense against bacterial translocation and systemic inflammation.
Methods
Integrity of epithelial cells was measured as transepithelial electrical resistance (TER) of confluent Caco-2 monolayers in the presence of 1% EN, PN and a parenteral amino acid mixture (AM). To determine wound healing capacities, an established migration model with IEC-6 cells was used. Furthermore, we investigated apoptosis, cell activation, proliferation and cytokine secretion of Caco-2, HT29 and of stimulated PBMC and LPMC cultured with or without 1 and 5% EN, AM or PN.
Results
We demonstrated that EN, AM and PN promoted the integrity of the epithelial monolayer and reconstituted epithelial cell continuity TGF-β-dependently and -independently. Interestingly, only PN induced apoptosis and decreased the mitochondrial membrane potential. The activation status of PBMC was significantly reduced by EN and AM. Specifically, EN leads to an increased apoptosis rate, inhibited cell cycle progression and increased pro-inflammatory cytokine secretion. Both EN and PN reduced the activation status and the release of pro- and anti-inflammatory cytokines.
Conclusions
Our study provides evidence that by promoting wound healing and regulating T cell function, EN, AM, and PN potently interact with the intestinal barrier and immune system, thus justifying its use in diseases accompanied by impaired mucosal barrier function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutritional support is an established element in the therapy of various indications. In general, total parenteral nutrition (PN) is required in the treatment of critically ill patients who are unable to ingest foods via the enteral route. Aside from the nutritional benefits for malnourished patients, several studies have indicated increased infectious complications associated with mucosal atrophy and increased intestinal permeability [15, 33]. Although the underlying mechanisms remain elusive, there is increasing evidence that PN leads to alterations in cytokine production and decreased IgA levels in the mucin layer of the mucosa, provoking bacterial overgrowth and translocation [18]. As a result, enteral nutrition (EN) is currently the preferred method of supplying nutritional support. The successful application of EN, however, requires adequate gut function [13]. Thus, the purpose of the gut mucosa is not only to emphasize the physical intestinal barrier, its primary function is to prevent luminal microorganisms and toxins from entering the systemic circulation [33].
The conjunction of adjacent epithelial cells is controlled by tight junctions, which regulate intestinal permeability and membrane leakiness. Besides the tightness of adjacent epithelial cells structural changes of the epithelium are also responsible for impaired barrier function, mainly induced by the inhibition of epithelial cell cycle progression or by the induction of apoptosis [16]. Once the epithelial surface is damaged, the reconstitution of the intestinal barrier is accomplished by increased epithelial cell migration into the wound to cover the injured area. Insufficient capacity to repair epithelial damage and ongoing leakiness results in the recognition of luminal antigens by lymphocytes of the immune system. These translocated microorganisms and falsely recognized luminal antigens perpetuate cellular immune responses resulting in uncontrolled intestinal and systemic inflammation [2, 14]. Therefore, next to achieving target requirements by nutritional support, the question of whether EN and PN have the capacity to induce structural and cellular changes of the intestinal and peripheral immune system needs to be addressed.
Materials and methods
Reagents and antibodies
Anti-CD3 monoclonal antibody (mAb) (clone OKT3; kindly provided by Janssen-Cilag, Neuss, Germany) and CD2 mAb (T112 and T113; kindly provided by Dr. Ellis Reinherz, Boston, MA) were used for T cell activation. For flow cytometry, FITC-labeled anti-cyclin B1 and Annexin-V as well as the PE-labeled CD25 were purchased from BD Pharmingen (Heidelberg, Germany). PI was purchased from Calbiochem (Schwalbach, Germany) and rhodamine123 from Sigma-Aldrich (Taufkirchen, Germany). FITC mouse IgG1 and PE mouse IgG1κ from BD Pharmingen were used as isotype controls. Recombinant human TGF-β-1 and pan-specific immunoneutralizing TGF-β antibody were obtained from R&D Systems (Wiesbaden, Germany). The cytometric bead array (CBA) kit was purchased from BD Pharmingen.
Nutrition
Enteral nutrition (EN, Fresubin high fiber), amino acid mixture (AM, Aminoven 10%) and total PN (Structokabiven) with defined ingredients (Table 1) were purchased from Fresenius-Kabi (Bad Homburg, Germany).
Cell lines and cell culture
Caco-2 cells, HT29 cells and the rat intestinal cell line IEC-6 were purchased from ATCC, Rockville, MD. Cells were maintained at 37°C with 5% CO2 in DMEM with 10% FCS (both from Invitrogen, Karlsruhe, Germany), 1% penicillin/streptomycin (Biochrom, Berlin, Germany), 2% l-glutamine (PAA Laboratories, Cölbe, Germany) and 1% sodium pyruvate (Invitrogen, Karlsruhe, Germany). For flow cytometric analysis 1 × 105 cells were seeded on a 12-well cell culture plate (BD Pharmingen). Cells were incubated for 48 h with or without 1 or 5% (vol/vol) EN, AM or PN.
Isolation and culture of peripheral blood and lamina propria mononuclear cells
Peripheral blood (PBMC) and lamina propria (LPMC) mononuclear cells were isolated, cultured and stimulated as previously described from healthy volunteers or from surgical specimens obtained from patients admitted for bowel resection for malignant and nonmalignant conditions of the large intestine, including colon cancer and benign polyps, respectively [27, 29, 30]. PBMC and LPMC were cultured in RPMI 1640 containing 10% fetal calf serum, 1.5% HEPES buffer, 2.5% penicillin–streptomycin (all from Biowhittaker, Walkersville, MD, USA). 3 × 105 PBMC were activated via their T cell receptor by cross-linked plate-bound anti-CD3 mAb (OKT, 10 µg/ml) for 1.5 h on a 96-well cell culture plate (BD Pharmingen). For flow cytometric analysis the cells were incubated with or without the nutritional additives for 48 h. LPMC are hyporesponsive towards CD3 stimulation. However the alternative pathways mediated by CD2 or CD28 is largely preserved [20, 34]. Therfore 3 × 105 LPMC were stimulated by anti-CD2 mAB (T112 and T113; 1 µg/ml, respectively) and incubated for 48 h with or without 1 or 5% (vol/vol) EN, AM or PN on a 96-well culture plate (BD Pharmingen). Approval of the protocol was granted by the local ethics committee of the Charité (Berlin, Germany).
Flow cytometric analyses
Flow cytometric analysis was used to determine cell activation, cell cycling, apoptosis and cytokine secretion, essentially as described previously [31]. Cells were incubated with the respective FITC-or PE-conjugated Ab at predetermined saturating concentrations or with isotype-matched non-specific mouse IgG mAb (BD Pharmingen) as an isotype control and analyzed by a flow cytometer (FACSCalibur; Beckman Coulter, Fullerton, CA) using CellQuest software (BD Pharmingen). Rhodamine123 staining to assess the mitochondrial membrane potential was previously described [29]. The binding of annexin-V was used to determine apoptosis and staining was performed as described earlier [27, 29, 30].
Wound healing model
Confluent monolayers of IEC-6 cells were incubated in 60 mm plastic dishes (BD Pharmingen) for 12 h in serum-deprived medium (0.1% FCS). Subsequently cells were scraped with a razor blade to produce wounds of ∼20 mm width followed by washing with PBS to remove residual cell debris. Wounded monolayers were then cultured for 24 h in DMEM medium in the presence or absence of EN, AM or PN as described earlier [32]. Migration of IEC-6 cells was assessed by counting the number of cells across the wound border in a blinded fashion.
Measurement of transepithelial electrical resistance (TER)
To measure the TER Caco-2 cells were seeded at confluent density on a 12 well culture plate in Transwell inserts with a pore size of 0.4 µm (Corning Costar Corporation, Cambridge, MA). The integrity of the monolayer was evaluated with a Millicell-ERS Voltohmmeter (Millipore, Schwalbach, Germany). To a monolayer with a stable (non-rising) TER of more than 170 Ω cm2 1% of EN, AM or PN was added to the insert. Measurements were taken three times daily to analyze changes in Caco-2 monolayer permeability.
Statistical analysis
Data are expressed as mean ± the standard deviation of the mean. Statistical analysis for significant differences was performed using analysis of variance, the Student t test for parametric samples (GraphPad Prism, San Diego, CA, USA).
Results
Effect of enteral and PN on transepithelial electrical resistance (TER)
Intestinal permeability is increased during intestinal inflammation, thus permitting bacterial translocation [15, 16, 22]. In our study we first evaluated the effect of EN and PN on the TER, a measure of monolayer integrity and tight-junction permeability. We cultured Caco-2 cells for 21 days until they reached confluency and the TER remained stable. When adding EN, AM, or PN to confluent Caco-2 cells at time-point 0, compared to the control group, the TER increased significantly in all three groups within 24 h (Fig. 1).
Effect of enteral and PN on intestinal epithelial cell restitution and proliferation
Intestinal epithelial cell restitution was assessed using a previously well-characterized wounding and migration model with confluent IEC-6 cells [7]. As shown in Table 2, EN, AM and interestingly also PN, significantly enhanced epithelial cell migration over the wound edge. Several cytokines and chemokines enhance epithelial cell migration TGF-β-1-dependently [7]. Thus, we cultured IEC-6 cells in the presence and absence of neutralizing anti-TGF-β antibodies. Interestingly, blocking TGF-β had a distinct effect on nutrition-mediated cell migration. Although the TGF-β blockade nearly completely abrogated EN- and PN-mediated enhanced cell migration, it was less capable of inhibiting AM-induced enhanced migration (Table 2). As a control cells were also cultured in the presence of TGF-β, resulting in an insignificant increase in migration of all groups compared to migration without TGF-β (Table 2).
During the process of wound healing and closure process cell expansion is essential to replenish the reduced cell pool [26]. Consequently, we determined the effect of EN and PN on cell cycling, determined by DNA staining. Excluding dead cells by proper gating, cell cycle progression of Caco-2 and HT29 cells through the S- and G2/M-phase was not significantly impaired by either EN, AM or PN (Fig. 2).
Distinct effect of enteral and PN on intestinal epithelial cell apoptosis
Using the externalization of phosphatidylserine as a marker of cell apoptosis and positive DNA staining as an indicator of membrane leakage, we tested the effect of EN and PN nutrition on epithelial cell death. Although the addition of 5% EN reduced the number of dead cells significantly, direct contact of PN to the cells significantly induced cell necrosis, as indicated by the increase in PI positive cells (Fig. 3). The addition of AM to HT29 cells reduced the number of apoptotic and necrotic cells, but failed statistical significance (Fig. 3). The induction of Caco-2 and HT29 cell death by PN were confirmed by trypan blue staining (data not shown). Additionally, we determined the de-energizing of the Caco-2 mitochondrial membrane potential by rhodamine123 fluorescence [23], confirming the preservation of cell integrity by EN and AM, but severe cell damage in the case of PN (Fig. 4).
Regulation of enteral and PN on PBMC and LPMC activation
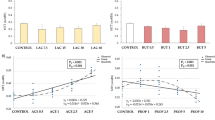
Within the lamina propria, LPMC play a major role and the increased activation and disabled apoptosis of Lamina propria T lymphocytes (LPT) is critically involved in the pathogenesis of IBD [11, 27, 28, 30]. Given the different delivery routes, PN comes into direct contact with peripheral blood T cells after infusion, whereas EN first comes into contact with mucosal T cells in the presence of a leaky intestinal epithelial cell barrier. Thus, we isolated PBMC and LPMC, stimulated the cells with anti-CD3 or -CD2 mAb, respectively, and investigated the effect of EN, AM, and PN on critical parameters of T cell functions. Robust stimulation of PBMC and LPMC is shown in the supplemental material (Fig. 1). When PBMC were stimulated for 48 h in the presence of EN, AM, and PN, CD25 expression was dose-dependently reduced by 5% of EN, AM, and PN. The latter, however, failed statistical significance (Fig. 5a). We then isolated and activated LPMC and cultured the cells for 48 h in the presence or absence of EN, AM or PN. As depicted in Fig. 5b, all compounds tested (EN, AM, and PN) significantly inhibited CD25 expression dose dependently (Fig. 5b).
The activation status of stimulated PBMC is reduced by EN and AM, of stimulated LPMC by EN, AM and PN. CD3 stimulated PBMC (a) and CD2 stimulated LPMC (b) were cultured for 48 h with or without EN, AM or PN for 48 h. Mean ± SEM of 6 individual experiments. *P < 0.05 and **P < 0.01 for change versus 0%
Effect of enteral and PN on PBMC and LPMC cell cycling
Activation of PBMC and LPMC initiates cell cycle entry and progression. Thus, we next sought to determine the influence of EN, AM and PN on the cell cycle progression of PBMC and LPMC using DNA staining. When PBMC were activated by anti-CD3 mAb, EN, AM and PN inhibited T cell cycle progression through the S- and G2/M-phase dose dependently (P < 0.05 vs. 0%, Fig. 6a). Independently tested, cyclin-B1 staining confirmed the results from the DNA content measurement (data not shown). Interestingly, when LPMC were activated and cultured for 48 h in the presence of the investigational agents, only EN reduced the cell cycling of anti-CD2 stimulated LPMC (P < 0.05 vs. 0%, Fig. 6b). Again, cyclin-B1 staining confirmed the results from the DNA content measurement (data not shown).
EN, AM and PN distinctively modulate cell cycle progression of PBMC and LPMC. In PBMC all nutritional additives reduced cell cycle progression whereas in LPMC only EN inhibited cell cycling. CD3 stimulated PBMC and CD2 stimlated LPMC were cultured with or without 1 and 5% EN, AM or PN for 48 h. Mean ± SEM of five individual experiments. *P < 0.05 for change versus 0%
Modulation of cytokine secretion in stimulated PBMC and LPMC by EN and PN
T cell cycling is linked to cytokine secretion, and the release of cytokines is crucial for the differentiation of T cells [21]. As depicted in Fig. 7a, EN significantly reduced IL-12 and IL-10 secretion in PBMC, while increasing TNF-α, IL-6 and IL-1β release. AM did not modulate PBMC cytokine secretion patterns. In contrast to EN, PN only reduced IL-10 secretion (Fig. 7a). In LPMC, EN reduced TNF-α and IL-10 secretion (Fig. 7b). In contrast to PBMC, AM reduced TNF-α release while PN reduced TNF-α, IL-10 and IL-6 levels (Fig. 7b).
EN, AM and PN distinctively modulate cytokine secretion of stimulated PBMC and LPMC. CD3 stimulated PBMC (a) and CD2 stimulated LPMC (b) were cultured with or without 1% EN, AM or PN for 48 h and supernatants were collected to determine cytokine secretion by CBA analysis. Mean ± SEM of three individual experiments. *P < 0.05 for change versus 0%
Effect of enteral and PN on PBMC and LPMC apoptosis
Comparable to the experimental setting described above, we also investigated the effect of EN, AM, and PN on PBMC and LPMC apoptosis. When PBMC were stimulated and cultured for 48 h in the presence and absence of 1 and 5% EN, AM or PN, annexin-V staining showed a dose-dependent and significant increase of apoptotic cells through the addition of EN, but not AM or PN (Fig. 8a). As expected [11, 27], activation of LPMC increased the number of apoptotic cells. Although AM and PN had no influence on the rate of dead mucosal T cells, comparable to PBMC, EN significantly increased LPMC apoptosis (Fig. 8b).
Effect of enteral and PN on PBMC function through an intact epithelial barrier
Above we provided evidence that EN, AM, and PN potently modulate T cell function. Finally, we aimed to investigate whether this feature can be still observed when the cells are separated from EN by an intact epithelial barrier. To address this question we cultured Caco-2 cells to confluency on a cell insert and cultured activated T cells in the basal compartment. On adding nutrition to the upper compartment for 48 h, EN, AM and PN lost their capacity to modulate T cell activation (CD25) (9a), cell cycling (cells in the S- and G2/M-phase) (Fig. 9b), or apoptosis (annexin-V) (Fig. 9c).
EN, AM and PN do not modulate activation, cell cycling and apoptosis in activated PBMC through an intact epithelial monolayer. Confluent Caco-2 cells were cultured with or without 1% EN, AM or PN and cocultured with CD3 stimulated PBMC in the basal compartment for 48 h. Mean ± SEM of three individual experiments. *P < 0.05 for change versus 0%
Discussion
In critical ill patients, bacterial translocation of viable resident bacteria or endotoxins from the gastrointestinal lumen into normally sterile tissues like the mesenteric lymph nodes or other internal organs is a common and feared clinical problem [3, 6]. In addition, the lack of enteral feeding in those patients results in further a loss of the mucosal immune function [5]. Normally, one of the major tasks of the epithelial cell layer is to provide an effective barrier against the luminal content [2, 17]. This feature is achieved by tight epithelial cell–cell contact which seals the monolayer and prevents bacterial translocation. By measuring the transepithelial resistance, a sensitive tool for determining the integrity of the epithelial cell barrier, we showed that not only EN, but also PN promoted the sealing of the epithelial monolayer. Given the different administration routes, PN normally does not come into contact with gastrointestinal epithelial cells and it is, to date, a matter of speculation as to whether the compounds of EN might diffuse from the gastrointestinal vessels to the basal side of the epithelial cell line. However, our study shows that the ingredients of PN are, in principle capable of promoting the epithelial resealing. In line with this finding, all three tested nutrition formulas, EN, AM and PN, significantly enhanced epithelial cell migration over the wound edge, a process which is essential for restoring the continuity of the epithelial surface following mucosal injuries. We and other investigators have previously shown that a number of cytokines enhance epithelial cell restitution in vitro through a TGF-β-dependent pathway [1, 7, 32]. Indeed, a neutralizing pan-specific TGF-β antibody was able to prevent EN- and PN-mediated enhancement of IEC-6 migration, indicating that EN and PN stimulate IEC-6 cell migration by a TGF-β-dependent mechanism. Interestingly, and verifying the more complex nutritional regime of EN and PN, regulation of cell migration by AM was not significantly altered by blocking TGF-β.
Next to migration into the wounded area, cell proliferation enhances closure of an injured area [4, 26]. Interestingly, in contrast to their profound effect on epithelial cell migration, neither EN, AM nor PN promoted Caco-2 and HT29 cell proliferation. This distinct effect shows not only that migration and proliferation are distinctively regulated in epithelial cells, but also that EN and PN have comparable but seemingly specific effects on different aspects of the wound healing process. The epithelial layer serves as an interface between the organism and the contents of the gastrointestinal tract, underlining the importance of regulating cellular viability despite an onslaught of luminal pathogens [9]. Thus, a tight balance between cellular proliferation and apoptosis is necessary to maintain this critical barrier. Our study showed that by decreasing epithelial cell apoptosis and necrosis, EN enhanced epithelial cell viability. In contrast, PN was found to induce epithelial cell death when it was directly added to HT29 cells under our experimental conditions where PN is in direct contact with epithelial cells. Several groups observed a correlation between a lower calorie content and a higher rate of apoptotic epithelial cells which is responsible for villus destruction in vivo [8, 33]. In our study, EN and PN had almost the same calorie content. Thus, based on our data, we can not confirm that the induction of epithelial cell death is related to the caloric delivery. However, since apoptosis was not induced by AM, it may be assumed that the fat compound of PN is responsible for this effect. Furthermore the beneficial effect of EN might be related to the fiber supplements which other investigators declared as effective in reducing PN-induced bacterial translocation [35].
Inflammatory bowel diseases are not only characterized by a disrupted epithelial cell barrier and disabled wound healing capacities but also by chronic tissue damage mediated by a disregulated mucosal T cell function [10]. In addition, in IBD the function of peripheral blood T cells is also disturbed [10], cells which are immediately contacted by PN after infusion. Interestingly, but in line with the different cell cycling of peripheral and mucosal T cells [20, 27, 34], EN, AM and PN had a different effect on PBMC and LPMC activation. In PBMC, the addition of EN and AM reduced the activation status of antigen stimulated T cells. In contrast, PN did not restrict the ability of peripheral T cells to become activated, thereby triggering a sufficient adaptive immune response. Interestingly, all nutritional additives tested significantly inhibited the activation of anti-CD2 stimulated LPMC significantly. As shown in our study, an intact epithelial barrier protects the underlining T cells from the inhibitory effect of EN. Given the disruption of the intestinal barrier, capillary leakage and increased activation of mucosal T cells in IBD [25, 27, 28], although the bioavailability of nutrients on immune cells is unclear, it might be speculated that during the active inflammatory process, EN and PN might come into direct contact with mucosal T cells, having an anti-inflammatory effect.
Cell activation induces entry of cells into the cell cycle, leading finally to cell division and expansion. Therefore next to an inhibited activation status of intestinal and peripheral lymphocytes a decreased cell cycle progression can also ameliorate inflammatory processes [31]. The number of cycling PBMC in S- and G2/M-phase was reduced by all three compounds tested emphasizing the anti-inflammatory effect of EN and AM on T cell activation and proliferation. In LPMC, only EN decreased the number of cycling cells, again underlining the possibility of a direct influence in case of an increased epithelial permeability. Immunological homeostasis is maintained by a tight balance between anti- and proinflammatory cytokines. In PBMC, EN induced the secretion of proinflammatory cytokines and abolished the release of the anti-inflammatory IL-10. However, Crohn’s disease is characterized by an uncontrolled Th1 response triggered by the release of IL-12 [19], while the inhibition of IL-12 release by EN indicates that EN influences the cytokine balance in both ways. In the gastrointestinal mucosa, TNF-α is a key mediator of inflammatory processes and in IBD, mucosal TNF-α levels are elevated [24]. Interestingly, EN, AM, and PN reduced TNF-α secretion of activated mucosal T cells, highlighting the potent immuno-regulatory capacities of the nutritional additives. In immune cells, cytokine secretion is linked to cell cycling [21]. Thus, remaining in a specific cell cycle phase results in less cell activation and proliferation but might increase the secretion of cytokines produced in this specific cell cycle phase.
Unrestricted cell activation and proliferation leads to inflammation, autoimmunity or cancer. In CD, apoptosis is disabled and unrestrained T cell activation promotes mucosal inflammation [12]. As shown in our study, EN is capable of inducing peripheral and mucosal T cell apoptosis. However, as demonstrated by our insert experiments, an intact epithelial barrier prevents this effect, providing evidence that a disrupted epithelial barrier is a necessary prerequisite for inducing T cell death by EN.
Our results systematically and sequentially investigated the effect of enteral and PN on critical elements of the gastrointestinal mucosal function. We were able to demonstrate that both EN and PN reconstitute epithelial cells and increase the epithelial barrier function, but distinctively modulate epithelial cell death. With regard to their effect on peripheral and mucosal T cells, EN and PN differentially inhibit T cell activation and reduce the release of pro-inflammatory cytokines. Given the frequent use of EN and PN in diseases accompanied by impaired mucosal function, the evidence that EN and PN both beneficially modulate epithelial and T cell functions might contribute to a better understanding of the impact of artificial nutrition in diseases in which the epithelial barrier is disrupted and mucosal T cells are at an increased status of activation such as in IBD.
References
Basson MD, Modlin IM, Flynn SD, Jena BP, Madri JA (1992) Independent modulation of enterocyte migration and proliferation by growth factors, matrix proteins, and pharmacologic agents in an in vitro model of mucosal healing. Surgery 112:299–307
Baumgart DC, Dignass AU (2002) Intestinal barrier function. Curr Opin Clin Nutr Metab Care 5:685–694
Berg RD, Garlington AW (1979) Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in the gnotobiotic mouse model. Infect Immun 23:403–411
Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J (2007) Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87:545–564
Debaveye Y, Van den Berghe G (2006) Risks and benefits of nutritional support during critical illness. Annu Rev Nutr 26:513–538
Deitch EA (2001) Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care 7:92–98
Dignass AU, Podolsky DK (1993) Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology 105:1323–1332
Dray X, Marteau P (2005) The use of enteral nutrition in the management of Crohn’s disease in adults. J Parenter Enteral Nutr 29:S166–S172
Edelblum KL, Yan F, Yamaoka T, Polk DB (2006) Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis 12:413–424
Fiocchi C (1998) Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182–205
Ina K, Itoh J, Fukushima K, Kusugami K, Yamaguchi T, Kyokane K, Imada A, Binion DG, Musso A, West GA, Dobrea GM, McCormick TS, Lapetina EG, Levine AD, Ottaway CA, Fiocchi C (1999) Resistance of Crohn’s disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. J Immunol 163:1081–1090
Itoh J, de La MC, Strong SA, Levine AD, Fiocchi C (2001) Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn’s disease. Gut 49:35–41
Jeejeebhoy KN (2007) Enteral nutrition versus parenteral nutrition the risks and benefits. Nat Clin Pract Gastroenterol Hepatol 4:260–265
Jiang XH, Li N, Li JS (2003) Intestinal permeability in patients after surgical trauma and effect of enteral nutrition versus parenteral nutrition. World J Gastroenterol 9:1878–1880
MacFie J (2000) Enteral versus parenteral nutrition: the significance of bacterial translocation and gut-barrier function. Nutrition 16:606–611
Mankertz J, Schulzke JD (2007) Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol 23:379–383
Mccole DF, Barrett KE (2007) Varied role of the gut epithelium in mucosal homeostasis. Curr Opin Gastroenterol 23:647–654
Minard G, Kudsk KA (1998) Nutritional support and infection: does the route matter? World J Surg 22:213–219
Peluso I, Pallone F, Monteleone G (2006) Interleukin-12 and Th1 immune response in Crohn’s disease: pathogenetic relevance and therapeutic implication. World J Gastroenterol 12:5606–5610
Qiao L, Schurmann G, Betzler M, Meuer SC (1991) Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology 101:1529–1536
Richter A, Lohning M, Radbruch A (1999) Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J Exp Med 190:1439–1450
Sanders DSA (2005) Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn’s disease. J Clin Pathol 58:568–572
Scaduto RC Jr, Grotyohann LW (1999) Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76:469–477
Schreiber S, Nikolaus S, Hampe J, Hamling J, Koop I, Groessner B, Lochs H, Raedler A (1999) Tumour necrosis factor a and interleukin 1 beta in relapse of Crohn’s disease. Lancet 353:459–461
Steeber DA, Tedder TF (2000) Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res 22:299–317
Sturm A, Dignass AU (2008) Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 14:348–353
Sturm A, Itoh J, Jacobberger JW, Fiocchi C (2002) p53 negatively regulates intestinal immunity by delaying mucosal T cell cycling. J Clin Invest 109:1481–1492
Sturm A, Leite AZ, Danese S, Krivacic KA, West GA, Mohr S, Jacobberger JW, Fiocchi C (2004) Divergent cell cycle kinetics underlie the distinct functional capacity of mucosal T cells in Crohn’s disease and ulcerative colitis. Gut 53:1624–1631
Sturm A, Lensch M, Andre S, Kaltner H, Wiedenmann B, Rosewicz S, Dignass AU, Gabius HJ (2004) Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol 173:3825–3837
Sturm A, Mohr S, Fiocchi C (2002) Critical role of caspases in the regulation of apoptosis and proliferation of mucosal T cells. Gastroenterology 122:1334–1345
Sturm A, Rilling K, Baumgart DC, Gargas K, Abou-Ghazale T, Raupach B, Eckert J, Schumann RR, Enders C, Sonnenborn U, Wiedenmann B, Dignass AU (2005) Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. Infect Immun 73:1452–1465
Sturm A, Sudermann T, Schulte KM, Goebell H, Dignass AU (1999) Modulation of intestinal epithelial wound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology 117:368–377
Sun X, Spencer AU, Yang H, Haxhija EQ, Teitelbaum DH (2006) Impact of caloric intake on parenteral nutrition-associated intestinal morphology and mucosal barrier function. JPEN J Parenter Enteral Nutr 30:474–479
Targan SR, Deem RL, Liu M, Wang S, Nel A (1995) Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J Immunol 154:664–675
Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH (2005) Lack of enteral nutrition—effects on the intestinal immune system. J Surg Res 123:8–16
Acknowledgments
We thank Anne Carney for critical reading of the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (STU247/3-2), the Forschungsförderung of the Charité, Universitätsmedizin Berlin and an unrestricted grant from Travacare GmbH, Hallbergmoos, Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guzy, C., Schirbel, A., Paclik, D. et al. Enteral and parenteral nutrition distinctively modulate intestinal permeability and T cell function in vitro. Eur J Nutr 48, 12–21 (2009). https://doi.org/10.1007/s00394-008-0754-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-008-0754-3