Abstract
Background
Intake of fish and long-chain n-3 fatty acids has been of wide interest due to their beneficial effects on cardiovascular risk factors and lower coronary heart disease (CHD) risk.
Aim of the study
The aim of this pilot study was to examine the effects of fatty fish and lean (white) fish on fatty acid composition of serum lipids and cardiovascular risk factors in subjects with CHD using multiple drugs for this condition.
Methods
The study was an 8-week controlled, parallel intervention. Inclusion criteria were myocardial infarction or unstable ischemic attack, age under 70 years, use of betablockers and presence of sinus rhythm. The subjects were randomized to one of the following groups: 4 meals/week fatty fish (n = 11), 4 meals/week lean fish (n = 12) and control diet including lean meat (n = 10).
Results
The mean (±SD) of reported fish meals per week was 4.3 ± 0.4, 4.7 ± 1.1 and 0.6 ± 0.4 in the groups, respectively. The proportions of eicosapentaenoic and docosahexaenoic acids in serum lipids increased in the fatty fish group only (P < 0.05). Systolic and diastolic blood pressure levels decreased in the lean fish group (0 vs. 8 week: 3.5 ± 3.2 and 4.6 ± 3.6%, respectively, P < 0.05). Serum total triglyceride concentration did not significantly change. HDL cholesterol concentration change differed among groups but without significant post hoc differences. Apolipoprotein A-1 concentration decreased in the control group (0 vs. 8 week, P < 0.05). Coagulation factors, 25-hydroxy vitamin D, and heart rate variability (24 h Holter) did not change among the groups.
Conclusions
Our results suggest that intake of lean fish at least four times per week could reduce blood pressure levels in CHD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with coronary heart disease (CHD) are recommended to eat at least two portions of fish weekly, preferably fatty fish [11, 20]. Fish, especially fatty fish, is the major dietary source of the long-chain n-3 fatty acids, eicosapentaenoic and docosahexaenoic acids (EPA, DHA). The intake of EPA plus DHA should be 1 g/d in subjects with CHD. This recommendation is based on the beneficial effects of fish and fish oils on CHD mortality and non-fatal events [43], as well as on cardiovascular risk factors like serum triglyceride concentration, blood pressure, arrhythmias and inflammation [7]. The hypotriglyceridemic effect has been well documented, while the effects on blood pressure, arrhythmias and inflammation have been less consistent.
Observational data suggest that the type of fish and its preparation method affect its association with the risk of CHD death [31]. However, studies on the effects of different types of fish in secondary prevention of CHD are limited. Majority of the previous intervention trials have focused on the effects of fish oils rather than inclusion of fish in the diet. Moreover, the effects of lean fish, which provides lower amounts of EPA and DHA as compared to fatty fish, have been less investigated [28]. However, lean fish provides other nutrients like protein or vitamin D that can be associated with cardiovascular risk factors [1, 14]. Total fish intake is an important source of vitamin D providing almost half of it in the Finnish diet [23]. The effects of fish protein could be related to peptides inhibiting angiotensin converting enzyme [41]. One possibility is that fish in the diet substitutes foods increasing risk of CHD (e.g. fatty meat products). Our aim was to study the effects of at least four portions of fatty or lean fish weekly on fatty acid profile of serum lipids, blood pressure, serum lipids, coagulation and heart rate variability in men and women with established CHD. Our hypothesis is that the fatty fish and lean fish diets will have different effects than the control diet and furthermore the effects of lean fish will differ from that of fatty fish due to different nutrient composition.
Methods
Subjects
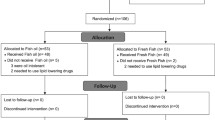
Subjects who had been in the Kuopio University Hospital due to myocardial infarction (MI) or unstable ischemic attack during the previous 3–36 months were identified from the discharge lists, and to those without major exclusion criteria an invitation letter to participate in the study was sent. Altogether, 59 subjects called the study nurse, out of which 44 were screened for eligibility (Fig. 1). The inclusion criteria were as follows: use of betablockers, age under 70 years, normal sinus rhythm verified by electrocardiogram, fasting serum triglyceride ≤3.5 mmol/l, fasting serum cholesterol ≤8 mmol/l, body mass index (BMI) 18.5–30 kg/m2 and fasting plasma glucose ≤7.0 mmol/l. Furthermore, subjects using other antiarrhythmic medications than betablockers or psychotropic drugs, or subjects with diabetes, atrial fibrillation, abnormal liver, thyroid or kidney function, excessive use of alcohol or inflammatory bowel disease were excluded. Recent (<3 months) use of fish oil supplements or high habitual use of fish (≥3 meals/week) was not allowed. Of the screened subjects, 35 subjects were eligible for the study and were randomized to one of the intervention groups. The subjects that were screened but not randomized did not differ from the randomized subjects (not randomized subjects: 3 women, 6 men, mean (±SD) age 62 ± 8 years, BMI 26.5 ± 4.7 kg/m2, systolic blood pressure 125 ± 13 mmHg and diastolic blood pressure 81 ± 5 mmHg). Two of the subjects dropped out from the study due to medical reasons.
The study subjects gave written consent for participation in the study. The study plan was approved by the Research Ethics Committee, Hospital District of Northern Savo.
Intervention
The duration of the intervention was 8 weeks with measurements performed at baseline, 4 and 8 weeks. All the subjects were instructed by a clinical nutritionist to follow a diet recommended for CHD patients [33] with group specific instructions about use of fish, fish products, meat and meat products. During the fish diets the amount of fish was instructed to be 100–150 g/meal consumed at least four times per week. The following fish species were advised to be used during the FATTY FISH diet: salmon, rainbow trout, Baltic herring, whitefish, vendace and tuna, and during the LEAN FISH diet: pike, pike-perch, perch, saithe and cod. Subjects in the CONTROL group were instructed to consume meals made with lean meat (beef or pork) or chicken without skin and less than 1 fish meal per week. In food preparation, subjects were advised to avoid sources of saturated fat like butter and cream. Use of fish oil supplements was not allowed in any of the groups. Subjects continued to use the medications prescribed by their physicians.
Patients’ habitual dietary intake was estimated by a 4-day food record at the baseline. Dietary compliance was monitored by using 7-day food record performed at weeks 3 and 7. The portion sizes were estimated using household measures. Nutrient intake was calculated using the Micro-Nutrica® dietary analysis program (version 2.5, Finnish Social Insurance Institute, Turku, Finland). The mean (±SD) ratio of reported energy intake to estimated energy expenditure was 0.62 (±0.13) indicating lower energy intake than the predicted one based on Schofield equations and cut off factor (1.46) [17]. Furthermore, the subjects kept a diary where they recorded any fish meals eaten during the whole study period.
Measurements
Anthropometric measurements included height and weight. BMI was calculated. Blood samples were drawn after a 12-h overnight fast from an antecubital vein at screening, baseline, 4 and 8 weeks. Blood pressure was measured twice after the subject had rested for at least 5 min with a 1 min interval between them in the morning (8.00–10.00 a.m.). The mean of the two values was used in the analyses. The measurements were taken by the same well-trained nurse from the right arm in the sitting position with a calibrated mercury sphygmomanometer. Subjects filled in a questionnaire about physical activity, use of medications and dietary supplements at the baseline and the end of the study.
Serum total, LDL and HDL cholesterol and triglyceride were analyzed using commercial kits (981813, 981656, 981655 and 981786, respectively, Thermo Electron corporation, Vantaa, Finland) and Thermo Fisher Konelab 20XTi Analyzer (Thermo Electron Corporation, Vantaa, Finland). Serum apolipoprotein A1 and B and plasma glucose were analyzed with the same analyzer using kits 981662, 981663 and 981779, respectively. Serum insulin was analyzed with chemiluminescent immunoassay (ACS 180 Plus Automated Chemiluminescence System, Bayer Diagnostics, Tarrytown, NY, USA). Hemoglobin A1c levels were analyzed with cation exchange chromatography (TOSOH HLC-723G7, Tessenderlo, Belgium). Plasma fibrinogen was analyzed using Fibri-prest Automate 2 and Thrombolyzer compact XR Analyzer (Behnk Electronic, Norderstedt, Germany). D-dimer was analyzed with Biopool International Autodimer 1431, Trinity Biotech, Co Wicklow, Ireland and Konelab60i/Konelab30 Clinical Analyzer, Thermo Electron Corporation, Vantaa, Finland). Analysis of plasminogen activator inhibitor-1 (PAI-1) was performed with Stachrom PAI kit and STA Compact Thrombolyzer Analyzer (Diagnostica Stago, Inc, Parsippany, NJ, USA). Serum 25-hydroxy vitamin D (25(OH)D) was analyzed using enzymeimmonoassay (Immunodiagnostic Systems Ldt, Tyne&Wear, UK).
For the fatty acid analyses, lipids were extracted from the serum sample with chloroform-methanol (2:1) [45]. Lipid fractions were separated with an aminopropyl column. Fatty acids in lipid fractions were transmethylated with 14% borontrifluoride in methanol at 100°C for 1 h. Finally, fatty acid methyl esters were analyzed with a gas-chromatograph (Hewlett-Packard 5890 series II, Hewlett-Packard Company, Waldbronn, Germany) equipped with FFAP-column (length 25 m, inner diameter 2 mm and film thickness 0.3 µm, Agilent) and helium as the carrier gas. Fatty acids are presented as molar percentage of total fatty acids. Heptadecanoate acid (17:0) was used as an internal standard.
Twenty-four hour ambulatory ECG-recordings were performed using Medilog® FD4 recorders and Oxford Medilog Exel2 analyses (Oxford Instruments Ltd, Abingdon, UK). Semiautomated visually assisted analysis was performed by trained nurses. Heart rate variability was evaluated by using time domain parameters: standard deviation of normal to normal RR-interval (SDNN), square root of the mean of the sum of squares of differences between adjacent intervals (r-MSSD) and percentage of differences between adjacent RR intervals that are greater than 50 ms (pNN50%) [39]. SDNN is a global index of heart rate variability and reflects depolarization of sinus node as well as the long-term components and circadian rhythms for variability. r-MSSD and pNN50% are parameters based on interval differences and correspond to short-term changes in heart rate variability.
Statistical methods
Statistical analyses were performed with SPSS 14.0.1 (SPSS, Chicago, IL). The results are presented as means ± SD. Normal distribution of the variables was tested with Shapiro-Wilks test and for those variables not complying with the assumption, logarithmic or other arithmetic transformations were used in statistical testing. Characteristics at screening among the groups were tested with ANOVA or χ 2 test, when appropriate. The effect of dietary interventions on measured outcomes was tested using general linear models (GLM) for repeated measurements. The post hoc comparisons were tested using independent samples t test (between-group) and paired t tests (within-group) with Bonferroni correction. Two-tailed P value of <0.05 was considered as statistically significant.
Results
The mean (±SD) age of the subjects was 61.0 ± 5.8 years (Table 1). BMI did not differ among the groups. Serum total cholesterol concentration tended to be higher in the CONTROL group (P = 0.09) and the number of subjects having total cholesterol >5 mmol/l was higher in the CONTROL group (P = 0.007). Accordingly, the LDL cholesterol level tended to be higher in the CONTROL group (P = 0.07), however, the prevalence of high LDL cholesterol did not significantly differ among the groups. There were more subjects with low HDL cholesterol in the LEAN FISH groups (P = 0.06). The serum triglyceride concentrations or blood pressure levels did not significantly differ among the groups at screening. The number of subjects with MI was higher in the LEAN FISH group than in the other groups, but the difference was not statistically significant. All the subjects were using betablockers and statins, and continued their medications throughout the study, with the exception of one subject in the CONTROL group who stopped using a statin. The use of medications did not differ among the groups (Table 1).
The mean reported number of fish meals was 4.3 ± 0.4, 4.7 ± 1.1 and 0.6 ± 0.4 per week in the FATTY FISH, LEAN FISH and CONTROL groups, respectively, during the study. Based on the food records, there were changes in the energy intake among the groups (P = 0.05), however, the within-group changes were not significant (Table 2). There was a significant increase in the intake of polyunsaturated fat and EPA + DHA in the FATTY FISH group as compared to baseline (P = 0.05 and P = 0.003, respectively). The variance in alcohol consumption was high in the CONTROL group due to one subject with higher alcohol consumption. The intake of vitamin D tended to be higher in the FISH groups than in the CONTROL group (P = 0.06).
The analysis of serum lipid fatty acids showed that the proportion of γ-linolenic acid in cholesteryl esters decreased in the FATTY FISH group (Table 3). The proportions of EPA and DHA in serum lipids were higher in the FATTY FISH group than in the other groups at 8 weeks (Table 3 and Fig. 2). In the LEAN FISH and CONTROL groups, there were no significant changes in the proportions of fatty acids in cholesteryl esters (Table 3).
The mean (±SD) proportions of eicosapentaenoic (EPA) and docosahexaenoic acids (DHA) in serum cholesteryl esters (CE), triglycerides (TG) and phospholipids (PL) in FATTY FISH (n = 11), LEAN FISH (n = 12) and CONTROL (n = 10) groups at 0 and 8 weeks (black bars denote 0 week and white bars 8 weeks). Letters denote differences at P < 0.05, a 0 vs. 8 weeks, b fatty fish vs. lean fish and control at 8 weeks, c lean fish vs. control at 8 weeks
Interestingly, systolic and diastolic blood pressure levels at 8 weeks as compared to 0 week decreased in the LEAN FISH group (3.5 ± 3.2 and 4.6 ± 3.6%, respectively, P < 0.05), but not in the FATTY FISH group (Table 4). The different response among the groups remained significant after adjustment for changes in BMI, self-reported frequency of fitness training, dietary sodium or alcohol intake during the study or history of myocardial infarction. The change in dietary sodium intake was, however, associated with systolic blood pressure (P = 0.05) and diastolic blood pressure (P = 0.09) in the repeated measures GLM. The mean (95% confidence interval) changes in systolic blood pressure (mmHg) between the time points 0 vs. 4 weeks and 4 vs. 8 weeks were 3.5 (−0.6; 7.7) and 1.5 (−2.5; 5.5) in FATTY FISH, −2.4 (−5.7; 0.8) and −1.8 (−5.4; 1.3) in LEAN FISH and −3.9 (−8.8; 1.0) and 3.3 (−2.0; 6.6) in the CONTROL group, respectively. Concentrations of glucose, insulin, hemoglobin A1c, 25(OH)D, fibrinogen and PAI-1 did not change significantly during the study. The change in the concentration of D-dimer among the groups was significant, however, in post hoc tests there were no significant within or between group differences.
There were no significant differences in the concentrations of total and LDL cholesterol and triglycerides among the groups (Table 5). The change in HDL cholesterol concentration during the study was different among the groups, however, in post hoc comparisons the increase in HDL cholesterol in the FATTY FISH group and the decrease in the CONTROL group were not significant. The concentration of apolipoprotein A-1 decreased in the CONTROL group (0 vs. 8 weeks, P < 0.05).
There were no significant changes in the heart rate or measures of heart rate variability derived from the 24 h Holter recording (Table 6).
Discussion
The results of this pilot intervention showed that consumption of fatty fish at least 4 meals per week increased EPA and DHA proportions in serum lipids at 8-weeks and that the feasibility of the diet regimen was good. The results also showed that lean fish consumption reduced blood pressure levels in CHD patients using multiple medications. However, fish intake did not significantly affect serum triglyceride, serum glucose and insulin, 25(OH) D, coagulation factors and heart rate variability.
Dietary adherence was monitored by dietary records and analysis of fatty acids in serum lipid fractions. The subjects were compliant to the fish eating regimen on the basis of self-report. In addition, fatty acids in serum lipids confirmed that. Previously, it has been shown that serum lipid long-chain n-3 fatty acids are a valid marker of their dietary intake [3, 19, 42].
Blood pressure levels decreased in the LEAN FISH, but not in the FATTY FISH group. The change remained significant after adjustment for dietary sodium intake. According to the dietary records the sodium intake increased by 0.3 g/d in the FATTY FISH group, but decreased by 0.2 g/d in the LEAN FISH group. In some previous studies, reductions in blood pressure levels have been reported after fish intake [4, 21], although negative results have also been published [28]. The beneficial effect of fish on blood pressure has often been ascribed to the increased intake of long-chain n-3 fatty acids. However, the hypotensive effect of n-3 fatty acids seems to be limited to hypertensive subjects and the reductions occur at relatively high doses, which may explain that in the FATTY FISH group there was no effect on blood pressure [4, 15]. Secondly, fish protein has been suggested to affect blood pressure levels in animals [1]. In humans, limited data from epidemiological studies have suggested an inverse association or no association between fish intake and blood pressure [27, 35, 38], while data about the inverse association between vegetable protein and blood pressure has been more consistent [2]. Bioactive peptides possessing hypotensive properties by inhibition of angiotensin converting enzyme have been identified in several fish species [40]; however, their bioavailability is less investigated [41]. Thirdly, it has been reported that vitamin D status could be associated with risk of hypertension [14]. Vitamin D intake increased during both fish diets as could be expected, however, no significant increases were observed in the concentrations of serum 25(OH)D nor was change in 25(OH)D associated with change in blood pressure.
Our results on HDL cholesterol are compatible with reports showing increased HDL cholesterol concentration after high intake of salmon in healthy adults and in CHD patients [21, 37]. In contrast, two to three weekly portions of oily fish did not affect serum HDL cholesterol concentrations [13, 28]. No significant changes in serum triglyceride concentration were observed in our study. In previous studies, fish as a source of EPA and DHA [21, 28, 29, 37] or supplementation of EPA and DHA [16, 44] has lowered triglyceride concentrations, even in statin-treated subjects [44]. The intake of EPA + DHA was 1.07 g/d in the FATTY FISH group, which may have been too low, since 2–4 g/d is the recommended amount for hypotriglyceridemic purposes [18, 20]. Even more plausible explanations for the lack of significant effect on serum triglyceride are that all the subjects were using lipid lowering drugs and the sample size of this pilot study was small.
The effect of long-chain n-3 fatty acids on coagulation and fibrinolytic factors has not been consistent [32]. We did not observe any significant effect of fish intake either on fibrinogen or PAI-1 levels. Similarly, fibrinogen concentration did not change in subjects with MI after 6 months fish oil treatment in the GISSI-Prevenzione trial [16]. In healthy adults, consumption of oily or lean fish has not affected fibrinogen or PAI-1 levels [13, 21, 28].
Marked reduction in the risk of sudden cardiac death has been reported after long-chain n-3 fatty acid supplementation [24] suggesting an antiarrhythmic effect of these fatty acids. In fact, the antiarrhythmic effect of long-chain n-3 fatty acids has been shown in animal studies [25] and in many observational studies [22]. However, results from clinical trials have remained conflicting. Consistent with our results, use of supplement providing EPA and DHA in a similar range than the intake in our study (810 mg/d) did not affect overall heart rate variability in men with MI [34]. Reduced heart rate variability is a predictor of arrhythmic events and mortality [39]. However, greater dose of EPA and DHA has increased heart rate variability in patients with MI [9] and post-MI heart failure [36]. Positive associations between DHA in cell membranes and heart rate variability have been reported [8, 10]. Inconsistent results are suggested to be due to different arrhythmogenic mechanisms in different populations [12]. To the best of our knowledge there are no interventions on fish consumption with regard to heart rate variability.
We did not observe any significant effect of fish diets on heart rate. It could be that the baseline heart rate was too low and the duration of the study too short to observe such an effect, as suggested by a review reporting that heart rate reduction after fish oil supplementation is limited to subjects with high baseline heart rate (≥69 bpm) and interventions lasting ≥12 weeks [30].
Two major limitations could have prevented us to observe significant effects. First, the sample size was small. Frequent prevalence of exclusion criteria (diseases, medications, older age) limited the number of eligible subjects. Second, the use of multiple medications affecting cardiovascular risk factors could have impeded the possible effects of fish. However, the selected study population is important because daily intake of 1 g long-chain n-3 fatty acids from fatty fish is recommended in secondary prevention of CHD [11, 20] and the data from studies testing use of fish in this population are limited [5, 6, 37]. Even though, we cannot exclude the possibility that a longer treatment period could have been needed, a recent study showed that maximum levels of EPA and DHA in myocardial phospholipids are reached already in 30 days [26], which would imply that cellular effects could have been achieved in the current study.
The results suggest that four meals of lean fish per week reduce systolic and diastolic blood pressure levels in CHD patients. We found this blood pressure lowering effect very interesting, and it needs to be confirmed by further studies.
References
Ait-Yahia D, Madani S, Savelli JL, Prost J, Bouchenak M, Belleville J (2003) Dietary fish protein lowers blood pressure and alters tissue polyunsaturated fatty acid composition in spontaneously hypertensive rats. Nutrition 19:342–346
Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM (2006) Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47:296–308
Arterburn LM, Hall EB, Oken H (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83:1467S-1476S
Bao DQ, Mori TA, Burke V, Puddey IB, Beilin LJ (1998) Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension 32:710–717
Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC (2003) Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr 57:193–200
Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM (1989) Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 2:757–761
Calder PC (2004) n-3 fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci 107:1–11
Christensen JH, Christensen MS, Dyerberg J, Schmidt EB (1999) Heart rate variability and fatty acid content of blood cell membranes: a dose–response study with n-3 fatty acids. Am J Clin Nutr 70:331–337
Christensen JH, Gustenhoff P, Korup E, Aaroe J, Toft E, Moller J, Rasmussen K, Dyerberg J, Schmidt EB (1996) Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomised controlled trial. Br Med J 312:677–678
Christensen JH, Korup E, Aaroe J, Toft E, Moller J, Rasmussen K, Dyerberg J, Schmidt EB (1997) Fish consumption, n-3 fatty acids in cell membranes, and heart rate variability in survivors of myocardial infarction with left ventricular dysfunction. Am J Cardiol 79:1670–1673
Cooper A, Skinner J, Nherera L, Feder G, Ritchie G, Kathona M, Turnbull N, Shaw G, MacDermott K, Minhas P, Packham C, Squires H, Thomson D, Timmis A, Walsh J, Williams H, White A (2007) Clinical guidelines and evidence review for post myocardial infarction: secondary prevention in primary and secondary care for patients following myocardial infarction. National Collaborating Centre for Primary Care and Royal College for General Practitioners, London
Den Ruijter HM, Berecki G, Opthof T, Verkerk AO, Zock PL, Coronel R (2007) Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res 73:316–325
Elvevoll EO, Barstad H, Breimo ES, Brox J, Eilertsen KE, Lund T, Olsen JO, Osterud B (2006) Enhanced incorporation of n-3 fatty acids from fish compared with fish oils. Lipids 41:1109–1114
Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC (2007) Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49:1063–1069
Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ (2002) Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 20:1493–1499
GISSI-Prevenzione Investigators (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354:447–455
Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM (1991) Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 45:569–581
Harris WS (1997) n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 65(suppl):1645S–1654S
Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M (1997) Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38:2012–2022
Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757
Lara JJ, Economou M, Wallace AM, Rumley A, Lowe G, Slater C, Caslake M, Sattar N, Lean ME (2007) Benefits of salmon eating on traditional and novel vascular risk factors in young, non-obese healthy subjects. Atherosclerosis 193:213–221
London B, Albert C, Anderson ME, Giles WR, Van Wagoner DR, Balk E, Billman GE, Chung M, Lands W, Leaf A, McAnulty J, Martens JR, Costello RB, Lathrop DA (2007) Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office of Dietary Supplements Omega-3 fatty acids and their role in cardiac Arrhythmogenesis workshop. Circulation 116:e320–e335
Männistö S, Ovaskainen M, Valsta L (2003) The national Findiet 2002 study. National Public Health Institute, Helsinki
Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RS, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F, on behalf of the GISSI-Prevenzioni Investigators (2002) Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardido (GISSI)-Prevenzione. Circulation 105:1897–1903
Matthan NR, Jordan H, Chung M, Lichtenstein AH, Lathrop DA, Lau J (2005) A systematic review and meta-analysis of the impact of omega-3 fatty acids on selected arrhythmia outcomes in animal models. Metabolism 54:1557–1565
Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG (2007) Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr 85:1222–1228
Miura K, Greenland P, Stamler J, Liu K, Daviglus ML, Nakagawa H (2004) Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men: the Chicago Western Electric Study. Am J Epidemiol 159:572–580
Moore CS, Bryant SP, Mishra GD, Krebs JD, Browning LM, Miller GJ, Jebb SA (2006) Oily fish reduces plasma triacylglycerols: a primary prevention study in overweight men and women. Nutrition 22:1012–1024
Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ (1999) Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr 70:817–825
Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB (2005) Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation 112:1945–1952
Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS (2003) Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation 107:1337–1372
Mutanen M, Freese R (2001) Fats, lipids and blood coagulation. Curr Opin Lipidol 12:25–29
National Cholesterol Education Program (NCEP) Expert Panel (2002) Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421
O’Keefe JH Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS (2006) Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol 97:1127–1130
Panagiotakos DB, Zeimbekis A, Boutziouka V, Economou M, Kourlaba G, Toutouzas P, Polychronopoulos E (2007) Long-term fish intake is associated with better lipid profile, arterial blood pressure, and blood glucose levels in elderly people from Mediterranean islands (MEDIS epidemiological study). Med Sci Monit 13:CR307–CR312
Radaelli A, Cazzaniga M, Viola A, Balestri G, Janetti MB, Signorini MG, Castiglioni P, Azzellino A, Mancia G, Ferrari AU (2006) Enhanced baroreceptor control of the cardiovascular system by polyunsaturated fatty acids in heart failure patients. J Am Coll Cardiol 48:1600–1606
Seierstad SL, Seljeflot I, Johansen O, Hansen R, Haugen M, Rosenlund G, Froyland L, Arnesen H (2005) Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur J Clin Invest 35:52–59
Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, Jacobs DR Jr (2005) Associations of plant food, dairy product, and meat intakes with 15-year incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr 82:1169–1177
Task Force of the European Society of Cardiology, the North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17:354–381
Vercruysse L, VanCamp J, Smagghe G (2005) ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: a review. J Agric Food Chem 53:8106–8115
Vermeirssen V, Van Camp J, Verstraete W (2004) Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br J Nutr 92:357–366
Vidgren HM, Ågren JJ, Schwab U, Rissanen T, Hänninen O, Uusitupa MI (1997) Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 32:697–705
Wang C, Harris WS, Chung M, Lichtenstein AH, Balk E, Kubelnick B, Jordan HS, Lau J (2006) n-3 fatty acids from fish or fish oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary and secondary prevention studies: a systematic review. Am J Clin Nutr 84:5–17
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369:1090–1098
Ågren JJ, Julkunen A, Penttilä I (1992) Rapid separation of serum lipids for fatty acid analysis by a single aminopropyl column. J Lipid Res 33:1871–1876
Acknowledgments
The study was supported by The Finnish Cultural Foundation, North-Savo Regional Fund of The Finnish Cultural Foundation, Yrjö Jahnsson Foundation, Juho Vainio Foundation and the Kuopio University Hospital (grant number 510RA07). Vanessa D.F. de Mello was supported by grants from Conselho Nacional de Desenvolvimento de Ciência e Tecnologia (CNPq), Brazil. We thank laboratory technologist Tuomas Onnukka for skillful work.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Erkkilä, A.T., Schwab, U.S., de Mello, V.D.F. et al. Effects of fatty and lean fish intake on blood pressure in subjects with coronary heart disease using multiple medications. Eur J Nutr 47, 319–328 (2008). https://doi.org/10.1007/s00394-008-0728-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-008-0728-5