Summary
Background
Vitamin B12 deficiency in infancy may cause failure to thrive, severe neurological disorders and megaloblastic pancytopenia. It is well known that infants born with deficient vitamin B12 storage have increased the risk of vitamin B12 deficiency. Vitamin B12 deficiency is more prevalent in infancy in Sanliurfa province (at the southeast region of Turkey).
Aim of the study
The aim of this study was to determine the frequencies of vitamin B12, folic acid and iron deficiencies in pregnants and their babies at birth and to what extend the mothers’ deficiency becomes effective on babies’ deficiencies.
Methods
The study groups were constituted by 180 pregnant women and their single and term babies. Venous blood samples of pregnants were obtained 1–3 h before delivery and babies’ cord bloods were collected at birth. Vitamin B12 and folic acid levels were measured with electro chemiluminiscence method; serum iron and iron binding capacities were measured by colorimetric method and complete blood counts were performed by automatic blood counter.
Results
Mean vitamin B12 levels in maternal and cord blood serum were 130 ± 61.7 pg/ml and 207 ± 141 pg/ml; mean folic acid levels were 8.91 ± 6.46 ng/ml and 17.8 ± 11.8 ng/ml; mean serum iron levels were 56.9 ± 37.5 µg/dl and 147 ± 43.2 µg/dl; and mean transferrin saturations were 11.8 ± 8% and 65.6 ± 24%, respectively. There were vitamin B12 deficiency (<160 pg/ml) in 72% of the mothers and 41% of the babies, and severe deficiency (<120 pg/ml) in 48% of the mothers and 23% of the babies. Folic acid deficiency was found in 12% of the mothers, but was not found in the babies. There were iron deficiency in 62% of the mothers and 1% of the babies. There were statistically significant correlation between maternal and cord blood serum vitamin B12 levels (r = 0.395, P < 0.001) and folic acid levels (r = 0.227, P = 0.017), while there were no correlation between maternal and cord blood iron levels and transferrin saturations.
Conclusion
The study results showed that vitamin B12 deficiency is prevalent in pregnants in this region and that 41% of infants have born with deficient vitamin B12 storages. Therefore, prophylactic use of vitamin B12 by pregnant women in Sanliurfa and other poor communities could have considerable benefits to prevent vitamin B12 deficiency and its complications in infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin B12 deficiency is a rare condition in infants who are born with enough vitamin B12 storage. During pregnancy, vitamin B12 is actively transported to the fetus by the placenta. But severe deficiency may occur in infants if the mother is vitamin B12 deficient during pregnancy, and lactation [1–6]. It is reported that vitamin B12 deficiency in mother’s diet at gestational period causes severe retardation of myelination of baby’s nervous system and brain atrophy [7]. In infancy, vitamin B12 deficiency may cause failure to thrive, irritability, anorexia, delay and regression of neurological development, hypotonia, coma and convulsions, and severe megaloblastic pancytopenia because of delayed DNA synthesis and myelination defects [4–9].
Anemia, failure to thrive and neurological disorders due to vitamin B12 deficiency in infancy are common in the Sanliurfa Province (at the southeast region of Turkey), and some of these children are brought to the hospital in severe coma [10]. Most of these children’s mothers also have vitamin B12 deficiency. We think that one of the possible important causes of the high frequency of vitamin B12 deficiency in infants is inadequate transfer of this vitamin from mothers both in pregnancy and in breast-feeding to the baby. So, we aimed to investigate vitamin B12 status of pregnants and their infants, and correlation of vitamin B12 in maternal and cord blood serum at birth. We also investigated other hematic substances, i.e., folic acid and iron, and hematologic values since nutritional deficiencies, especially iron deficiency, are high in pregnancy.
Materials and methods
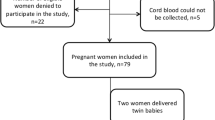
The study groups were constituted by 188 healthy parturients who gave birth at the Health Minister Sanliurfa Maternity Hospital, and their babies, but 8 pairs were excluded from the study due to insufficient sampling of cord blood or broken tubes. All parturients had normal gestations lasting 38–42 weeks and gave birth to normal babies weighing more than 2500 g by uncomplicated vaginal delivery. The data associated with mothers’ ages, pregnancy numbers, and medications in pregnancy were obtained from mothers and their hospital records before delivery. All pregnants gave informed consent for themselves and for their babies. The study was performed according to national and international ethical guidelines under the supervision of a medical ethic specialist.
Venous blood samples of pregnants were taken 1–3 h before delivery and babies’ cord blood samples were collected at birth into two tubes one with and one without EDTA. Blood specimens in the tubes without EDTA were left to stand at room temperature for approximately 3 h and then centrifuged. The sera were collected and immediately frozen at −20 °C until tested. Vitamin B12 and folic acid levels were measured with electrochemiluminiscence method (Elecsys 2010), serum iron and iron binding capacities, were measured by colorimetric method (Boehringer-Meinhaim). Complete blood counts were performed by automatic blood counter (Celldyn 3500) in the same day. Vitamin B12 and folic acid measurements and complete blood counts were performed in all subjects; serum iron, iron binding capacities and transferrin saturations were measured in 100 of them.
Vitamin B12 levels lower than 160 pg/ml were accepted as deficiency and lower than 120 pg/ml were considered as severe deficiency for both pregnants and infants [11]. Serum folic acid levels lower than 4 ng/ml were accepted as deficiency and lower than 2 ng/ml were accepted as severe deficiency for both pregnants and infants [5, 12]. If serum iron level was lower than 60 µg/dl and transferrin saturation was lower than 16%, it was considered as iron deficiency for both pregnants and infants [11]. Hb levels lower than 12 g/dl in pregnants and lower than 13.5 g/dl in infants were considered as anemia. When MCV was lower than 80 fl in mothers and lower than 98 fl in infants, it was accepted as microcytosis [11].
Statistical analyses were performed by using SPSS computer program (version 11.5). The correlations between mothers’ and babies’ values were evaluated with Spearman correlation coefficient. Unpaired Student t test was used for comparison of mean serum concentrations of vitamin B12, folic acid, iron, iron binding capacity, and transferrin saturations of pregnants and their babies. In addition, pregnants and their babies were grouped according to pregnants’ ages, pregnancy numbers, vitamin B12 status (adequate: ≥160 pg/ml, mildly deficient: 120–160 pg/ml, and severely deficient: <120 pg/ml), and iron or multivitamin receival; and each subgroup results were also compared with other subgroups results with unpaired Student t test and one-way ANOVA test (in multiple comparisons) with Bonferroni test for post-hoc multiple comparisons. Chi Square (χ2) test was used in comparison of deficiency rates of subgroups according to pregnants’ vitamin B12 status.
Results
Mean age of pregnants was 27 + 5.79 years. Twenty-eight (16%) of pregnants were younger than 20 years of age, 54 (30%) were between 21–25 years of age, 59 (33%) were between 26 and 30 years of age, and 39 (22%) were in 31 years of age or older. Fifty-four (30%) of these mothers had their first pregnancy, 56 (31%) had their second or third pregnancy, 42 (23%) had their fourth or fifth pregnancy, and 28 (16%) had six or more pregnancies. Mean pregnancy number was 3.22 + 2.16.
Data of maternal and cord blood serum levels of vitamin B12, folic acid and transferrin saturation were presented in Table 1. The differences between maternal and cord blood levels were significant (P < 0.001) for all parameters.
As shown in Table 2, the frequency of vitamin B12 deficiency were significantly high in both mothers and babies, while only 1% of the babies was iron-deficient and none of the babies was folic acid-deficient. Seventeen (77%) of 22 pregnants who had folic acid deficiency and 36 (58%) of 62 pregnants who had iron deficiency also had vitamin B12 deficiency.
Eighty-four (48%) of the pregnants had anemia (24 (13%) had Hb levels lower than 10.5 g/dl) (Table 2). Anemia was microcytic in 45 (54%) of these 84 women and normocytic in others. Only five (3%) infants had anemia and two (1%) of these infants had microcytosis.
We found a statistically significant correlation between maternal and cord blood serum vitamin B12 levels (r = 0.395, P < 0.001) and folic acid levels (r = 0.227, P = 0.017), while there were no correlation between maternal and cord blood serum iron levels, iron binding capacities and transferrin saturations (Table 1).
There were no statistically important correlations between both pregnants’ ages and pregnancy numbers and serum levels of vitamin B12, folic acid, iron, and iron saturation of mothers and infants (P > 0.05 for all comparison). When the infants were grouped according to pregnants’ ages and pregnancy numbers, there were no significant differences between mean serum vitamin B12 and folic acid concentrations and transferrin saturations (P > 0.05 for all comparisons).
When the infants were grouped according to mothers’ vitamin B12 status, mean vitamin B12 levels of the infants whose mothers were in mild and severe vitamin B12 deficient groups were significantly lower than mean level of the infants whose mothers were in vitamin B12 adequate group (F = 6.541, P = 0.002) (Table 3 and Fig. 1). There were no important differences between mean folic acid levels and transferrin saturations of infants according to pregnants’ vitamin B12 status (P > 0.05 for each comparison).
The frequency of severe vitamin B12 deficiency status in infants whose mothers had severe vitamin B12 deficiency was significantly higher than the infants whose mothers had mild deficiency and had adequate vitamin B12 (Table 4).
According to data obtained from pregnant, 127 (71%) of them had received neither iron nor multivitamin preparation at pregnancy, while 11 (6%) had used iron and 34 (19%) had used multivitamin (5 (3%) had used each of iron and multivitamin) at least one package. Eight (4%) of the pregnants did not answer this question. There were no important differences in mean serum levels of vitamin B12, folic acid and transferrin saturations between pregnants who had ever received iron or multivitamin preparations and who had not, and also between their babies (P > 0.05 for all comparison). These results suggested that iron or multivitamin receival by pregnants were insufficient in this region.
Discussion
This study demonstrated that the frequency of vitamin B12 deficiency was significantly high in pregnants of this region, and an important proportion of infants have born with deficient vitamin B12 storages. This results showed that vitamin B12 deficiency in pregnants is an important cause of biochemical vitamin B12 deficiency in newborns in our region, although vegetarian nutrition is uncommon.
There is little consensus on appropriate cut-off point for determining normal and abnormal vitamin B12 and folic acid levels. However, the levels which are frequently used for vitamin B12 are 160 and 200 pg/ml [11–14]. Our laboratory used <200 pg/ml as the cut-off point for vitamin B12 during our investigation. But, since serum vitamin B12 concentrations physiologically fall during pregnancy [15–17], we accepted 160 pg/ml for vitamin B12 as cut-off point.
There is also no complete consensus about cut-off level of serum folic acid. In Frery et al. study [13], folic acid was considered deficient when its plasma level was less than 2.5 ng/ml. Perkins has given 2 ng/ml for normal lower limits of folic acid [11]. Whitehead et al. [5] have given 4–20 ng/ml as normal range and less than 3 ng/ml as deficiency. According to another textbook, if the serum folate concentration is more than 4 ng/ml, folate deficiency is effectively ruled out [12]. Our laboratory’s lower limit of normal range for folic acid was also 4 ng/ml. Therefore, we used 4 ng/ml for cut-off point for folic acid in this study.
One of the important causes of high frequency of vitamin B12 deficiency in this region is inadequate consumption of animal products due to poverty. High frequency of iron deficiency, which was found in pregnants, supported this suggestion. Another important cause is the local tradition of some people at this region that, meat consumption of woman during pregnancy or lactation might be harmful to her fetus or infant. One of the other causes may be intestinal bacterial overgrowth [5], due to poor hygienic conditions of the region.
One of the nutritional characteristics of the people in this region is meat products are frequently consumed with some vegetal foods such as dry or fresh pepper and eggplant. Some foods that are usually consumed with meat may also inhibit vitamin B12 and iron absorption, and the subject must be investigated from this perspective. Some intestinal parasitic infections may result in vitamin B12 malabsorption [6], and intestinal parasitic infections, which are more common in this region, may be one of the causes of this deficiency [18, 19]. Therefore, further studies are required to determine the exact causes of vitamin B12 deficiency in pregnancy.
We found that cord blood vitamin B12 levels were significantly correlated with mothers’ vitamin B12 levels in contrast to the cases of iron levels. Frery et al. [13] and Guerr-Shinohar et al. [20] also reported that cord blood vitamin B12 levels were highly correlated with mothers’ vitamin B12 levels. Monagle et al. [3] reported that maternal vitamin B12 deficiency is the most frequently seen cause of infantile megaloblastosis, and 50% of these mothers were asymptomatic. It was shown that pregnants’ ages or pregnancy numbers have no important effect on the infants’ vitamin B12 levels, but pregnants’ vitamin B12 status were significantly effective on the infants’ vitamin B12 levels. As seen in Tables 3 and 4, especially the infants, whose mothers have severe vitamin B12 deficiency, are under the risk of severe vitamin B12 deficiency; but an important proportion of infants, whose mothers have mild deficiency may also be under the risk for severe deficiency. All these results have suggested that if mothers have inadequate vitamin B12 storage during pregnancy, the babies probably born with deficient vitamin B12 storage [1–5].
Specker et al. [21] reported that milk vitamin B12 was correlated with maternal vitamin B12 concentration and infant urinary methylmalonic acid (UMMA) concentration (an important biochemical indication of vitamin B12 deficiency) were inversely related to milk vitamin B12 concentration. Therefore, the deficiency may become more evident especially in breast-fed infants because of the low milk concentration of vitamin B12 [21, 22]. In contrary, although iron deficiency was more prevalent in pregnant women in our study, it was rare in infants, and this situation supports the assertion that even if mothers had iron deficiency, the fetus could get iron that was needed.
Interestingly, we found that 6% of the infants whose mothers had adequate vitamin B12 levels, had vitamin B12 levels lower than 120 pg/ml, and 10% of this infants had vitamin B12 levels between 120 and 160 pg/ml. We think that these pregnants might have received vitamin B12 sources in the final days of their pregnancy which was sufficient to correct their own serum vitamin B12 levels, but was not enough to correct their infants serum levels.
In some poor communities, quite high rate of vitamin B12 and folic acid deficiencies in pregnants have been reported [23, 24]. Ackurt et al. [25] reported that the frequencies of vitamin B12 and folate deficiencies in pregnant were 48.8% and 59.7% in early stages of pregnancies, 80.9% and 76.4% in late stages of pregnancy, and 60% and 73.3% in postnatal stages, respectively, in Istanbul and Izmit, two developed cities in the north-west region of Turkey. In Ackurt et al.’s study, the frequency of vitamin B12 deficiency in women in late stages of pregnancy was quite similar with the frequency of deficiency that was found in our study. These similar results show that vitamin B12 deficiency in pregnancy is not a restricted problem of south-east region, but also an important problem in the other regions of Turkey.
Generally, folic acid deficiency during pregnancy is more common than vitamin B12 deficiency [26]. But, the frequency of folic acid deficiency in pregnants was lower than the frequency of vitamin B12 deficiency, and none of the infants had folic acid deficiency at birth in this study. One of the possible causes of this result may be that the sources of folic acid [5] are more common and cheaper in our region.
This study also showed that the use of iron and multivitamin by pregnants is insufficient in this region. When it is considered that most of the physicians prescribe iron and multivitamins to pregnants, the results suggested that the insufficient follow-ups of the pregnants by the physicians, and multivitamin and iron medication is also insufficient in pregnants who reported that they receive iron or multivitamin.
It is important to diagnose vitamin B12 deficiency in pregnants since it can result in low vitamin B12 stores in infants at birth [1, 3, 5, 13, 20]. Children who are born with low vitamin B12 stores, if not diagnosed and treated, may show developmental and severe neurological problems within the first years of life. Treatment may resolve these complications, but permanent neurologic damage and long-term intellectual impairment may occur [2–9]. Therefore, prevention of this deficiency is an important measure for healthy development of infants and children. Ajayi et al. [27] reported that receiving the capsules that contain ferrous fumarate, folic acid, vitamin B12, vitamin C, magnesium sulfate and zinc (Chemiron) by normal pregnants instead of conventional ferrous glucanate and folic acid, had better hematological effect. There is no known teratogenic effect of vitamin B12 if received by pregnants. In contrast, it was shown that it could reduce the teratogenic effects that induced by valproic acid and dexamethasone, and could reduce the frequency of malformation in animal experiments [28, 29]. Therefore, vitamin B12 administration to pregnants will be beneficial to prevent infantile vitamin B12 deficiency.
In conclusion, this study showed that, an important proportion of infants in Sanliurfa had vitamin B12 deficiency at birth due to maternal vitamin B12 deficiency. Due to the potential serious results of vitamin B12 deficiency for infants, prenatal screening studies may be performed to prevent the deficiency of vitamin B12 in infants. We suggest that prophylactic use of vitamin B12 by pregnant women, similar to iron and folic acid prophylaxis, in addition to nutrition education programs and food enrichment, will greatly benefit to prevent vitamin B12 deficiency and its complications in the first years of life, in Sanliurfa and other less advantaged communities.
Vitamin B12 can be given to pregnants in routine physician control; or it can be given by nurses or midwives in village clinics to pregnants who are not in regular physician follow-ups, at least one in each trimester of gestation as 1 mg ampoules, that is quite cheap. Vitamin B12 can also be given to infants at first week of life similar to vitamin K injection in poor communities. The addition of vitamin B12 in multivitamin preparations for infants will also have beneficial effects to prevent vitamin B12 deficiency in infancy.
References
Davis JR, Goldenring J, Lubin BH (1981) Nutritional vitamin B-12 deficiency in infants. Am J Dis Child 135:566–567
McPhee AJ, Davidson GP, Leahy M, Beare T (1988) Vitamin B12 deficiency in a breast-fed infant. Arch Dis Child 63:921–923
Monagle PT, Tauro GP (1997) Infantile megaloblastosis secondary to maternal vitamin B12 deficiency. Clin Lab Hematol 19:23–25
Grattan-Smith PJ, Wilcken B, Procopis PG, Wise GA (1997) The neurological syndrome of infantile cobalamin deficiency: developmental regression and involuntary movements. Mov Disord 12:39–46
Whitehead VM, Rosenblatt DS, Cooper BA (2003) Megaloblastic anemia. In: Nathan DG, Orkin SH, Ginsburg D, Look AT (eds) Nathan and Oski’s hematology of infancy and childhood, 6th edn. W.B. Saunders Comp., Philadelphia, pp 419–455
Ramussen SA, Fernboff PM, Scanlon KS (2001) Vitamin B12 deficiency in children and adolescents. J Pediatr 138:10–17
Lovblad K, Ramelli G, Remonda L et al (1997) Retardation of myelination due to dietary vitamin B12 deficiency: cranial MRI findings. Pediatr Radiol 27:155–158
Hall CA (1990) Function of vitamin B12 in the central nervous system as revealed by congenital defects. Am J Hematol 34:121–127
Graham SM, Arvela OM, Wise GA (1992) Long-term neurologic consequences of nutritional vitamin B12 deficiency in infants. J Pediatr 121:710–714
Koc A, Soran M, Tatli MM, Sevinc E, Vural H (1999) Vitamin B12 deficiency in the etiology of childhood coma. 1st National Congress of Pediatric Hematology, Antalya, Turkey
Perkins SL (1999) Normal blood and bone marrow values in humans. In: Lee GR, Foerster J, Lukens J, Paraskevas F, Greer JP, Rodgers GM (eds) Wintrobe’s clinical hematology, 10th edn. Williams & Wilkins, Baltimore, pp 2798–2748 (Table A.3 and Table A.17)
Antony AC (2000) Megaloblastic anemias. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LH, McGlave P (eds) Hematology: basic principles and practice, 3rd edn. Churchill Livingstone, New York, pp 446–485
Frery N, Huel G, Leroy M et al (1992) Vitamin B12 among parturients and their newborns and its relationship with birthweight. Eur J Obstet Gynecol Reprod Biol 45:155–163
Lanzkowsky P (2000) Manual of pediatric hematology and oncology. Academic Press, San Diego, pp 51–72
Metz J, McGrath K, Bennett M, Hyland K, Bottiglieri T (1995) Biochemical indices of vitamin B12 nutrition in pregnant patients with subnormal serum vitamin B12 levels. Am J Hematol 48:251–255
Allen LH (1994) Vitamin B12 metabolism and status during pregnancy, lactation and infancy. Adv Exp Med Biol 352:173–186
Pardo J, Gindes L, Orvieto R (2004) Cobalamin (vitamin B12) metabolism during pregnancy. Int J Gynaecol Obstet 84:77–78
Ulukanligil M, Seyrek A (2003) Anthropometric status, anaemia and intestinal helminthic infections in shantytown and apartment schoolchildren in the Sanliurfa province of Turkey. Eur J Clin Nutr 58:1056–1061
Koc A, Kocyigit A, Ulukanligil M, Demir N (2005) The frequency of vitamin B12 and folic acid deficiency in 9–12 years of age children in Sanliurfa Region and their relation with intestinal helminthes. Cocuk Sagligi ve Hastaliklari Dergisi 48:308–315 (Abstracts in English)
Guerr-Shinohar EM, Paiva AA, Rondo PH, Yamasaki K, Terzi CA, D’Almedia V (2002) Relationship between total homocysteine and folate levels in pregnant women and their newborn babies according to maternal serum levels of vitamin B12. BJOG 109:784–791
Specker BL, Black A, Allen L, Morrow F (1990) Vitamin B-12: low milk concentrations are related to low serum concentrations in vegetarian women and to methylmalonic aciduria in their infants. Am J Clin Nutr 52:1073–1076
Casterlina JE, Allen LH, Ruel MT (1997) Vitamin B-12 deficiency is very prevalent in lactating Guatemalan women and their infants at three months postpartum. J Nutr 127:1966–1972
Msolla MJ, Kinoba JL (1997) Prevalence of anaemia in pregnant women during the last trimester. Int J Food Sci Nutr 48:265–270
House JD, March SB, Ratnam S et al (2000) Folate and vitamin B12 status of women in Newfounland at their first prenatal visit. CMAJ 162:1557–1559
Ackurt F, Wetherilt H, Loker M, Hacibekiroglu M (1995) Biochemical assessment of nutritional status in pre-and post-natal Turkish women and outcome of pregnancy. Eur J Clin Nutr 49:613–622
Lee Gr (1999) The anemias associated with renal disease, liver disease, endocrine disease, and pregnancy. In: Lee GR, Foerster J, Lukens J, Paraskevas F, Greer JP, Rodgers GM (eds) Wintrobe’s clinical hematology, 10th edn. Williams & Wilkins, Baltimore, pp 1497–1517
Ajayi GO, Fadiran EO (1998) The effect of 61 days of combined iron (Chemiron) and single iron therapy on haemoglobin, packed cell volume, platelets and reticulocytes during pregnancy. Preliminary report. Clin Exp Obstet Gynecol 25:107–111
Elmazar MM, Thiel R, Nau H (1992) Effect of supplementation with folic acid, vitamin B6, and vitamin B12 on valproic acid-induced teratogenesis in mice. Fundam Appl Toxicol 18:389–394
Natsume N, Narukawa T, Kawai T (1986) Teratogenesis of dexamethasone and preventive effect of vitamin B12. Int J Oral Maxillofac Surg 15:752–755
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koc, A., Kocyigit, A., Soran, M. et al. High frequency of maternal vitamin B12 deficiency as an important cause of infantile vitamin B12 deficiency in Sanliurfa province of Turkey. Eur J Nutr 45, 291–297 (2006). https://doi.org/10.1007/s00394-006-0598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-006-0598-7