Abstract
Objectives
Behcet’s disease (BD) is a systemic vasculitis characterized by cardiovascular complications. Early diagnosis of these complications can reduce morbidity and mortality. Carotid artery intima–media thickness (cIMT) and the logarithmic value of triglyceride to high density lipoprotein ratio (atherogenic index of plasma, AIP) are good markers of atherosclerosis. The purpose of this study was to investigate whether AIP is a predictive marker of subclinical atherosclerosis in BD patients.
Patients and methods
A total of 84 BD patients (60 male, 24 female) and 84 healthy control individuals (58 male, 26 female) were included in this study. cIMT measurements were made, and AIP values were calculated.
Results
cIMT (p < 0.001) and AIP (p < 0.001) values of the BD patients were higher than those of the control group. A strong independent relationship was found between the AIP value and cIMT (β = 0.232, p = 0.018). In the subgroup analysis, the cIMT and AIP values of male BD patients were higher than those of female BD patients.
Conclusion
Increased AIP and cIMT values can be a good marker for subclinical atherosclerosis in BD patients, especially in male BD patients.
Zusammenfassung
Zielsetzung
Bei Morbus Behçet („Behcet’s disease“, BD) handelt es sich um eine systemische Vaskulitis, die durch kardiovaskuläre Komplikationen charakterisiert ist. Eine frühzeitige Diagnose dieser Komplikationen kann die Morbidität und Mortalität reduzieren. Die Intima-Media-Dicke der A. carotis (cIMT) und der Logarithmus aus dem Quotienten aus Triglyceriden und Lipoproteinen mit hoher Dichte (atherogener Plasma-Index, AIP) sind geeignete Marker für eine Atherosklerose. Ziel dieser Studie war es, zu untersuchen, ob der AIP ein prädiktiver Marker für eine subklinische Atheroskerlose bei BD-Patienten ist.
Patienten und Methoden
Insgesamt 84 BD-Patienten (60 männlich, 24 weiblich) und 84 gesunde Kontrollpersonen (58 männlich, 26 weiblich) wurden in diese Studie eingeschlossen. Es wurden Messungen der cIMT vorgenommen und die AIP-Werte berechnet.
Ergebnisse
Die cIMT- (p < 0,001) und AIP-Werte (p < 0,001) der BD-Patienten waren höher als die der Kontrollgruppe. Es wurde eine starke unabhängige Beziehung zwischen dem AIP- und dem cIMT-Wert festgestellt (β = 0,232, p = 0,018). In der Subgruppenanalyse waren die cIMT- und AIP-Werte der männlichen BD-Patienten höher als die der weiblichen.
Schlussfolgerung
Erhöhte AIP- und cIMT-Werte können einen geeigneten Marker für die subklinische Atherosklerose bei BD-Patienten, insbesondere bei männlichen Patienten, darstellen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Behçet’s disease (BD) is a disease with systemic involvement, the most important of which is vascular damage. Cardiovascular manifestations such as atherosclerotic heart disease and myocardial infarction in BD patients are the most important causes of mortality [1]. The vascular involvement ratio in BD varies between 1 and 40 %, according to studies [2]. Atherosclerosis and complications of atherosclerosis are the leading causes of morbidity and mortality around the world [3]. The cellular biology of atherosclerotic plaque development and rupture is actually an inflammatory condition [4]. With verification of this inflammatory basis, a correlation between systemic inflammatory markers and clinical events due to plaque rupture was shown in many studies in the rheumatologic patient population [5]. BD especially advances with increased cardiovascular risk in the younger population by common vascular involvement [1, 2]. The understanding of cellular and molecular interactions that determine the formation and progression of atherosclerosis and the definition of endothelial dysfunction as the initial lesion, especially in BD patients, are very important in this population [6]. Generally, early atherosclerotic lesions progress asymptomatically. In BD, cardiovascular involvement can be seen even in young patients, according to the duration of the disease [7]. Therefore, the determination of proper diagnosis, treatment, and prevention tools in this population who are cardiologically asymptomatic continues to draw attention as a major challenge. In many studies about BD, carotid artery intima–media thickness (cIMT) measurement has been shown to be a strong marker of subclinical atherosclerosis risk [8, 9].
In many populations, the index of atherogenic plasma lipoprotein profiles, such as low density lipoprotein (LDL)/high density lipoprotein (HDL) or total cholesterol (TC)/HDL, were shown to be important markers for predicting coronary artery disease [10–12]. Elevated triglycerides (TG) and a low HDL level are strong markers of cardiovascular diseases [13, 14]. Recently, it was determined that the atherogenic index of plasma (AIP) value, which is acquired by the logarithmic transformation of the number found by dividing plasma TG value to HDL value, can be a good marker for the risk of atherosclerosis and cardiovascular disease [15].
In this study, we aimed to investigate what AIP values were or were not high in patients with BD and to investigate the correlation of AIP with cIMT, which is a good marker of subclinical atherosclerosis.
Methods
Patient population
This cross-sectional case–control study was conducted with outpatients who visited the rheumatology, internal medicine, and cardiology clinics of our university hospital between June 2015 and January 2016. A total of 60 male and 24 female BD patients were included in this study, and this group was compared with a healthy control group of 84 patients that included 58 males and 26 females of comparable ages and body mass index (BMI). Disease severity in patients with BD was evaluated by using the BD current activity form (BDCAF) and Krause disease severity score [16, 17]. The study was approved by the Ethics Committee of Necmettin Erbakan University, Meram Medical Faculty, and all participants gave written and verbal informed consent consistent with the Helsinki Declaration.
Inclusion criteria
The patients who had been diagnosed with BD according to International BD Study Group criteria were enrolled in the study (International study group for BD; Evaluation of diagnostic [“classification”] criteria in BD disease-towards internationally agreed criteria [18]). Healthy subjects with no regular use of medications, having no history of smoking or of alcohol consumption, and no known disease were involved as the control group. Detailed physical examinations and laboratory tests for individuals in the control group were made, and those with disease symptoms and/or findings were excluded.
Exclusion criteria
Patients and controls with concomitant systemic diseases such as cardiac disease, dyslipidemia, diabetes mellitus, chronic obstructive lung disease, cancer, thyroid function disorder, hematological disorders, acute or chronic liver and renal diseases, acute or chronic infections, a history of smoking or alcohol consumption, and disrupted biochemical parameters were excluded. Patients using drugs such as antihypertensive agents, statins, steroids, and other drugs that can effect cIMT and AIP values were not included in this study. Patients with TG levels of 400 mg/dl and above were not included in the study.
cIMT measurement
Patients were laid on a routine examination table by elevating their head at a 45° angle in a supine position. The cIMT was measured on the right and left carotid arteries by a single investigator who was blinded to the patient assignment group. cIMT was obtained by longitudinal B‑mode images of the left and right common carotid arteries, immediately proximal to the carotid bifurcation. Three-lead ECG monitoring was made simultaneously with B‑mode image records. The carotid arteries were evaluated with the Vivid 7 echocardiography device (General Electrics, Horten, Norway) by using a 10 MHz multifrequency linear probe. Researchers recorded image sequences for 15 s from both the right and left common carotid artery by using three standard probe angles (posterior, lateral, and anterolateral). The acquired images were recorded for playback analysis and sent to an outside lab for standardized measurements. The intima–media thickness (IMT) of the carotid arteries was measured in the distal common carotid artery at a level 10–20 mm proximal to the carotid bulb. The two bright echogenic lines in the arterial wall were identified as the intima and the media. Three measurements (right and left common carotid artery, bifurcation, and the first 2–3 cm of internal carotid artery) were made for each side of the body; separate means were calculated and recorded as the right and left IMT [19]. Then, the average value of right and left IMT values was taken, and cIMT was obtained.
Biochemical analysis
Venous blood samples were obtained from all participants after 10- to 12-h fasting. Fasting serum glucose (FPG), creatinine, alanine aminotransferase (ALT), TC, TG, HDL, and C‑reactive protein (CRP) levels were analyzed on an Abbot Architect 16000 system with the original reagents. HDL levels were detected by a direct enzymatic method without precipitation. Complete blood counts were measured by the method of laser-based flow cytometric impedance, using an automated blood cell counter (Mindray BC-6800, Shenzhen, PR China). The erythrocyte sedimentation rate (ESR) was determined by iSed (Alcor Scientific).
Calculation of LDL and AIP
Low-density lipoprotein cholesterol (LDL-C) levels were computed using the Friedewald formula (TC = LDL + HDL + TG/5). AIP was calculated with the log10 TG/HDL formula.
Statistical analysis
For statistical analysis, an SPSS package program (version 18, Chicago, IL, USA) was used. Results were given as mean ± standard deviation. First of all, the homogeneous distribution of groups was evaluated with the Kolmogorov–Smirnov test. It was found that the groups had homogeneous distribution. After that, the comparison of groups was made with a Student’s t‑test. For the correlation analysis, Pearson correlation analysis was used. By using a stepwise linear regression analysis, parameters that were independently associated with a dependent variable of cIMT were analyzed. In the subgroup analysis, the Mann–Whitney U test was used for comparison of groups which had 30 or fewer individuals, and a Student’s t‑test was used for comparison of groups which had more than 30 individuals. A p value <0.05 was considered as significant.
Results
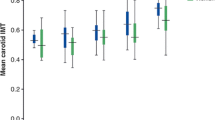
Demographic characteristics of BD patients are given in Table 1. The cIMT (p < 0.001), AIP (p < 0.001; Fig. 1), TG (p = 0.003), CRP (p = 0.003), and ESR (p = 0.002) values of BD patients were significantly higher than those of the control group, and the HDL (p = 0.002) value of BD patients was significantly lower than that of the control group. All biochemical results are shown in Table 2.
In the correlation analysis, there was a positive relation between cIMT and age (r = 0.233, p = 0.003), BMI (r = 0.162, p = 0.036), disease duration (r = 0.254, p = 0.006), BDCAF disease activity score (r = 0.369, p < 0.001), Krause disease severity score (r = 0.363, p < 0.001), AIP (r = 0.261, p = 0.001), TG (r = 0.219, p = 0.004), LDL (r = 0.177, p = 0.022), and CRP (r = 0.221, p = 0.004). There was a negative relation between cIMT and HDL (r= −0.198, p = 0.010). There was a positive correlation between AIP and age (r = 0.278, p = 0.001), BMI (r = 0.177, p = 0.022), disease duration (r = 0.254, p = 0.006), BDCAF disease activity score (r = 0.228, p = 0.003), Krause disease severity score (r = 0.252, p < 0.001), TC (r = 0.246, p = 0.001), TG (r = 0.884, p < 0.001), LDL (r = 0.155, p = 0.044), and ALT (r = 0.189, p = 0.014). There was a negative relation between AIP and HDL (r = −0.664, p < 0.001). The correlation relation between cIMT and AIP is shown in Fig. 2. All correlation analysis results are presented in Table 3.
In a stepwise multiple regression analysis, a strongly independent relation was found between cIMT with AIP (beta [β] = 0.232, p = 0.018), male gender (β = 0.370, p < 0.001) and Krause disease severity score (β = 0.348, p = 0.001).
In the subgroup analysis, cIMT, AIP, and TG values of female control patients were significantly lower than in the other three groups (all p < 0.001). The HDL value of female control patients was significantly higher than in the other three groups (all p < 0.001). The male control cIMT value was higher than the BD male cIMT value (p < 0.001). The AIP value of female BD patients was lower than that of male BD patients (p = 0.015), and the HDL value of female BD patients was higher than that of male BD patients (p = 0.006). The subgroup comparison of cIMT and AIP is shown in Table 4.
Discussion
We found that AIP, cIMT, TG, ESR, and CRP values of BD patients were higher than those in the control group, and the HDL value was lower than that in the control group. In the correlation analysis, there was a positive correlation between the cIMT value with disease duration and disease activity score, AIP, TG, LDL, and CRP. There was a negative relation between the cIMT with HDL. Regression analysis showed strong independent relationships between cIMT and either AIP, disease duration, disease activity score, or male gender.
BD is a vasculitic disease which progresses with chronic inflammation [9]. Chronic inflammation can cause atherosclerosis. Measurement of cIMT is a strong marker of subclinical atherosclerosis and heart disease risk [20]. In various studies, there were different threshold values for cIMT. In a study using a Turkish population, the limiting cIMT value for the age group of 30–40, which was the same age group we studied, was found to be 0.46 mm [21]. But in extensive work in the UK, the cIMT cutoff value for ages 30–39 was reported as 0.60 mm [22]. In several studies conducted with BD patients, the cIMT value was found to be significantly higher than in the control group [9, 23]. In a meta-analysis, the cIMT cutoff value for BD patients was given as 0.54 mm [24]. In our study, cIMT values of both male and female BD patients were higher than the cutoff values of this meta-analysis and the control group. In our study, the cIMT values of healthy male controls was significantly higher than those of healthy women. BD is severe, particularly in male patients [25]. Also, the cardiovascular disease risk was higher in males when compared with females [26]. In BD patients, atherosclerotic heart disease risk increases markedly. Therefore, male BD patients have high cardiovascular disease risk. It was detected that cIMT values were higher in male patients than in female patients, and the increase of cIMT values with age was more pronounced in male patients [27]. But it was reported that the sensitivity and specificity of cIMT values for determining the risk of cardiac disease in women are higher than in men [28]. In our study, cIMT values of female BD patients were markedly higher than in healthy women. These results might show that there can be an increased cardiac risk and atherosclerosis risk in female BD patients.
High TG and low HDL levels are strong risk factors for cardiac disease. Even a high TG level is a better marker than LDL for cardiac risk [29]. TG particles contain very low density lipoprotein (VLDL) and small dense LDL particles [30]. It is a known fact that an increase of TG levels causes an increase in the small dense LDL level and finally causes an increase of cardiovascular risk [31]. This is because small dense LDL particles have strong atherogenic characteristics, and they can cause atherosclerosis by increasing lipid peroxidation and reactive oxygen radicals [32]. HDL allows the use of peripheral cholesterol by transporting it to the liver. Also, it includes antioxidant enzymes such as paraoxonase [33]. Low HDL levels are strongly related to increased cardiac disease risk and cIMT levels [34]. Recently, it was reported that AIP is a strong predictor of atherosclerosis and cardiac disease risk, and AIP is calculated by finding the logarithm value of the ratio of TG value to HDL value [35]. It was reported that an AIP value of 1.0–0.24 shows medium cardiac risk [36]. In our study, AIP values of BD patients were significantly higher than the control group, and our patients with BD have medium cardiac risk. When the subgroup analysis was made according to gender, we found that AIP values of male BD patients were significantly higher than those of female BD patients. In our study, the HDL level of female individuals in the control group was significantly higher than in the other three groups. The HDL level of female BD patients was also slightly lower than that of male individuals in the control group. In male BD patients, the HDL level was significantly lower than in the other three groups. Thus, the AIP value was found to be higher in male BD patients than in the control group. Another reason was the significantly high TG level in male BD patients when compared with other groups.
Obese women have less visceral adipose tissue compared to obese men, and it is a known fact that the TG level is higher in women when compared with men, especially during the postmenopausal period in which there is excess subcutaneous fat tissue. However, TG levels under the age of 45 years is similar for both genders [25, 37]. All female patients in our study were premenopausal. Also, the average age distribution of all the patients was under 45 years, and BMI was within normal limits. Therefore, we thought that TG levels were not affected by age and gender. Female BD patients also had cardiac risk, despite medium cardiac risk in male BD patients, whereas AIP was high in healthy male controls and very low in healthy female individuals. The results of our study suggest that female BD patients can also have low cardiac risk.
TG level is also a strong risk factor for cIMT and cardiac disease, but according to results of a meta-analysis, it was reported that TG level is a cardiac risk factor in men rather than women, and it is a strong marker for cIMT [38]. Also in our study, the TG level of female BD patients was significantly higher than that of healthy female individuals. The results of our study suggest that cardiac risk and the incidence of atherosclerotic heart disease increased due to elevated TG levels and decreased HDL levels in female BD patients when compared with healthy women. However, female BD patients have low cardiac risk than male BD.
We found a strong relationship between AIP and cIMT in the correlation analysis. These findings may suggest that TG and HDL levels in BD patients can be strong markers both for atherosclerotic heart disease and cardiac disease risk. Also, we found a strong relationship between AIP and BDCAF and Krause disease severity scores in the correlation analysis. The AIP in BD patients may be a cheap and easy marker for predicting the disease activity score and subclinical atherosclerosis.
In our study, we found a strong relationship between LDL value and cIMT. Especially small dense and oxidized LDL subtypes of LDL can easily cause lipid peroxidation and can cause the formation of reactive oxygen radicals and atherosclerotic heart disease. In our study, LDL values in the BD and control groups were similar. But CRP, which is a good marker of inflammation, was significantly higher in BD patients, and CRP elevation can be evidence of chronic inflammation in BD patients. Increased inflammation in BD patients may facilitate the entry of LDL into lipid peroxidation. Thus, the atherosclerotic process can be accelerated in BD patients.
Conclusion
AIP and cIMT in BD patients were significantly higher than in healthy controls, and there was strong positive correlation between AIP and cIMT values. AIP and cIMT values can be good markers for increased subclinical atherosclerosis and cardiac risk in BD patients, especially in male BD patients. Male BD patients have a medium risk of cardiac disease; therefore, we recommend that patients with BD be monitored frequently by clinicians.
References
Desbois AC, Wechsler B, Cluzelc P et al (2014) Cardiovascular involvement in Behcet’s disease. Rev Med Inter 35:103–111
Kural-Seyahi E, Fresko I, Seyahi N et al (2014) The long-term mortality and morbidity of Behcet syndrome: a 2‑decade outcome survey of 387 patients followed at a dedicated center. Cardiology 129:203–206
Moran AE, Forouzanfar MH, Roth GA et al (2014) The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation 129:1493–1501
Di Angelantonio E, Sarwar N, Perry P (2009) Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302:1993–2000
Sacks FM, Ridker PM (1999) Lipid Lowering and beyond: results from the CARE study on lipoproteins and inflamamation. Cholesterol and Recurrent Events. Herz 24:51–56
Seyahi E, Ugurlu S, Cumali R et al (2008) Atherosclerosis in Behçet’s Syndrome. Semin Arthritis Rheum 38:1–12
Ozuguz P, Karabulut AA, Tulmac M et al (2014) Markers of endothelial dysfunction and evaluation of vascular reactivity tests in Behcet disease. Angiology 65:937–943
Keser G, Aksu K, Tamsel S et al (2005) Increased thickness of the carotid artery intima-media assessed by ultrasonography in Behçet’s disease. Clin Exp Rheumatol 23:71–76
Icli A, Cure E, Cure MC et al (2016) Novel myokine: irisin may be an independent predictor for subclinic atherosclerosis in Behçet’s disease. J Investig Med 64:875–881
Nwagha UI, Ikekpeazu EJ, Ejezie FE et al (2010) Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr Health Sci 10:248–252
Mudhaffar SK (2013) Atherogenic index of plasma (AIP) as a parameter in predicting cardiovascular risk in males compared to the conventional dyslipidemic indices (cholesterol ratios). Karbala J Med 6:1506–1513
Grover SA, Levington C, Paquet S (1999) Identifying adults at low risk fora significant hyperlipidemia: a validated clinical index. J Clin Epidemiol 52:49–55
Han SH, Nicholls SJ, Sakuma I et al (2016) Hypertriglyceridemia and cardiovascular diseases: revisited. Korean Circ J 46:135–144
Shapiro MD, Fazio S (2016) From lipids to inflammation: new approaches to reducing atherosclerotic risk. Circ Res 118:732–749
Onat A, Can G, Kaya H et al (2010) Atherogenic index of plasma (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol 4:89–98
Bhakta BB, Brennan P, James TE et al (1999) Behcet’s disease: evaluation of a new instrument to measure clinical activity. Rheumatol 38:728–733
Krause I, Mader R, Sulkes J et al (2001) Behçet’s disease in Israel: the influence of ethnic origin on disease expression and severity. J Rheumatol 28:1033–1036
International Study Group for Behcet’s Disease (1990) Criteria for diagnosis of Behcet’s disease. Lancet 335:1078–1080
Benedetto FA, Mallamaci F, Tripepi G et al (2001) Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol 12:2458–2464
Icli A, Cure E, Cure MC et al (2015) Endocan levels and subclinical atherosclerosis in patients with systemic lupus Erythematosus. Angiology doi:10.1177/0003319715616240
Beşir FH, Yazgan S, Celbek G et al (2015) Normal value correlates of carotid intima-media thickness and affecting parameters in healthy adults. Anadolu Kardiyol Derg 12:427–433
Lim TK, Lim E, Dwivedi G et al (2008) Normal value of Carotid intima-media thickness – a surrogate marker of atherosclerosis: quantitative assessment by B‑mode carotid ultrasound. J Am Soc Echocardiogr 21:112–116
Ozturk C, Balta S, Balta I et al (2015) Neutrophil-lymphocyte ratio and carotid-intima media thickness in patients with Behçet disease without cardiovascular involvement. Angiology 66:291–296
Merashli M, Ster IC, Ames PR (2016) Subclinical atherosclerosis in Behcet’s disease: a systematic review and meta-analysis. Semin Arthritis Rheum 45:502–510
Rodriguez-Carballeira M, Alba MA, Solans-Laque R et al (2014) REGEB investigators. Registry of the Spanish network of Behçet’s disease: a descriptive analysis of 496 patients. Clin Exp Rheumatol 32:33–39
Franklin BA, Cushman M (2011) Recent advances in preventive cardiology and lifestyle medicine: a themed series. Circulation 123:2274–2283
Zhao B, Liu Y, Zhang Y et al (2012) Gender difference in carotid intima-media thickness in type 2 diabetic patients: a 4‑year follow-up study. Cardiovasc Diabetol 11:51
Kablak-Ziembicka A, Przewlocki T, Tracz W et al (2005) Gender differences in carotid intima-media thickness in patients with suspected coronary artery disease. Am J Cardiol 96:1217–1222
Sarac I, Backhouse K, Shojaee-Moradie F et al (2012) Gender differences in VLDL1 and VLDL2 triglyceride kinetics and fatty acid kinetics in obese postmenopausal women and obese men. J Clin Endocrinol Metab 97:2475–2481
Krauss RM (2004) Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 27:1496–1504
Boizel R, Benhamou PY, Lardy B et al (2000) Ratio of triglycerides to HDL cholesterol is an indicator of LDL particle size in patients with type 2 diabetes and normal HDL cholesterol levels. Diabetes Care 23:1679–1685
Cure MC, Cure E, Tufekci A et al (2013) Low-density lipoprotein subfraction, carotid artery intima-media thickness, nitric oxide, and tumor necrosis factor alpha are associated with newly diagnosed ischemic stroke. Ann Indian Acad Neurol 16:498–503
Gaal K, Tarr T, Lorincz H, Borbas V et al (2016) High-density lipopoprotein antioxidant capacity, subpopulation distribution and paraoxonase-1 activity in patients with systemic lupus erythematosus. Lipids Health Dis 15:60
Rosvall M, Persson M, Östling G et al (2015) Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: the Malmö Diet and Cancer Study. Atherosclerosis 239:615–621
Soska V, Jarkovsky J, Ravcukova B et al (2012) The logarithm of the triglyceride/HDL-cholesterol ratio is related to the history of cardiovascular disease in patients with familial hypercholesterolemia. Clin Biochem 45:96–100
Dobiasova M (2006) AIP – atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek 52:64–71
Acartürk E, Cayli M, Akpinar O et al (2004) Relation between age and gender differences in plasma triglyceride concentrations and coronary artery disease in Southern Turkey. Clin Chim Acta 339:123–128
Stensland-Bugge E, Bonaa KH, Joakimsen O et al (2000) Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: the Tromso study. Stroke 31:574–581
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Cure, A. Icli, A. Ugur Uslu, R. Aydoğan Baykara, D. Sakiz, M. Ozucan, F. Yavuz, S. Arslan, M. Cumhur Cure, and A. Kucuk declare that they have no competing interests.
The study was approved by the Ethics Committee of Necmettin Erbakan University, Meram Medical Faculty, and all participants gave written and verbal informed consent consistent with the Helsinki Declaration.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Rights and permissions
About this article
Cite this article
Cure, E., Icli, A., Ugur Uslu, A. et al. Atherogenic index of plasma may be strong predictor of subclinical atherosclerosis in patients with Behçet disease. Z Rheumatol 76, 259–266 (2017). https://doi.org/10.1007/s00393-016-0141-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-016-0141-z
Keywords
- Behçet’s disease
- Carotid artery intima–media thickness
- Atherogenic index of plasma
- Subclinical atherosclerosis