Abstract
Background
Cardiotoxicity is a leading cause of morbidity and mortality among patients receiving cancer therapy. The most commonly used definition is cancer therapy-related cardiac dysfunction (CTRCD) defined by a left ventricular ejection fraction reduction. Global longitudinal strain (GLS) has been implied to be superior in detecting early subclinical dysfunction.
Objectives
Evaluate the prevalence of reduced GLS and whether it is associated with CTRCD development among patients receiving cancer therapy.
Methods
Data were collected as part of the Israel Cardio-Oncology Registry (ICOR), a prospective registry enrolling all adult patients receiving different types of cancer therapy, who were referred to the cardio-oncology clinic. Patients were divided into two groups—reduced GLS (> − 17%) vs. preserved GLS (≤ − 17%). Multivariable analyses were adjusted for a propensity score for baseline characteristics.
Results
Among 291 consecutive patients, 48 (16%) patients were included in the reduced GLS group. Overall, 11 (5%) patients developed CTRCD at following echocardiogram evaluation. Patients with preserved GLS had a significantly lower risk for CTRCD development [odds ratio (OR) 0.11, 95% confidence interval (CI) 0.03–0.41, p = 0.001], with every 1-unit improvement of GLS the risk of CTRCD decreased by 16% (OR 0.84, 95%CI 0.73–0.95, p = 0.007). After adjustment for baseline characteristics, including cardiovascular risk factors and systolic function, preserved GLS remained significantly associated with a lower risk for CTRCD development (OR 0.11, 95%CI 0.02–0.64, p = 0.014), with every 1-unit improvement lowering the risk by 19% (OR 0.81, 95%CI 0.67–0.98, p = 0.032).
Conclusions
Reduced GLS is common among patients receiving cancer therapy and may identify patients at increased risk for CTRCD development.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last several decades, cancer therapy continues to advance, resulting on one hand with increased survival rates, but on the other hand strengthen the importance of long-term side effects from chemotherapeutic drugs [1, 2], with cardiovascular disease being a leading cause of death among patients with cancer [3]. Cancer therapy-related cardiac dysfunction (CTRCD), defined by a left ventricular ejection fraction (LVEF) reduction, is a well-documented side effect of certain therapeutic agents [4]. LVEF has been shown to be an important indicator of the outcome as a monitor of heart function [5]. The use of LVEF as a primary measurement of overall cardiac function, however, requires a substantial tissue function loss, often irreversible [6,7,8], before being clinically detectable [9,10,11]. Global longitudinal strain (GLS), a parameter of 2D speckle-tracking echocardiography (STE) has been shown to provide clinicians with information on more subtle left ventricular function changes [12] and is associated with overall CTRCD outcomes [13, 14]. GLS has been shown to be useful in the prognostication of all-cause mortality [15]; however, its ability to predict all-cause mortality in patients receiving cancer therapy has not been well documented. Routine use of GLS in patients receiving cancer therapy has not been fully adopted yet, due to limited data [16, 17]. Using GLS routinely as a measure of cardiac function, the aim of this study was to evaluate the frequency of reduced GLS among patients receiving cancer therapy, and whether it is associated with CTRCD development and all-cause mortality.
Methods
Study population
The study population is part of the Israel Cardio-Oncology Registry (ICOR)—a prospective registry enrolling all adult patients evaluated in the cardio-oncology clinic at Tel Aviv Sourasky Medical Center. All patients signed an informed consent at the first visit in the clinic and are then followed prospectively. The registry was approved by the local ethics committee and is registered in clinicaltrials.gov (Identifier: NCT02818517).
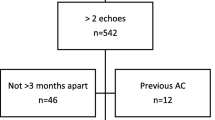
The clinic follows adult patients who are currently receiving cancer therapy. In general, the registry includes three types of populations: patients that developed cardiovascular complications during therapy; high-risk patients with baseline risk factors and as of February 2017, the clinic evaluates preventively all patients planned for anthracyclines (ANT) therapy. From October 2016 to August 2018, 419 patients receiving cancer therapy were evaluated, of which 128 patients were excluded due to not performing GLS assessment, leaving 291 patients for analysis.
Study protocol
Past medical history, cardiac risk factors, cancer type and chronic medical treatment were noted in all patients. Regarding the ongoing cancer therapy, we analyzed only therapy associated with LVEF dysfunction, according to the European Society of Cardiology position paper 2016 [4]. At least one echocardiogram, including GLS assessment, was performed for each patient in the study. Patients were divided into two groups—reduced GLS group vs. preserved GLS group. Preserved GLS was defined as ≤ − 17% adhered to the standard benchmark set by previous studies [18]. Both groups were evaluated for the parameters associated with reduced GLS; the risk of EF reduction and CTRCD development, defined as a LVEF reduction of > 10%, to a value below 53% [19] at following echocardiogram evaluation and all-cause mortality retrieved from the electronic records of the governmental population.
Echocardiography
Three standard apical views (4-chamber, 2-chamber, and apical long-axis) were recorded using a General Electric system, model Vivid S70 echocardiogram and were performed by the same vendor, technician and interpreting cardiologist in order to prevent inter-vendor variability. Left ventricle (LV) diameters were measured from the parasternal short axis, by means of a two-dimensional or a two-dimensional-guided M-mode echocardiogram of the LV, at the papillary muscle level [20]. LVEF was calculated by the biplane method.
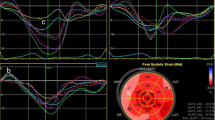
Images were acquired using high frame rate (> 50 frames/s) [21], and thereafter stored digitally for offline analysis. GLS was measured using STE software and tracking within an approximately 5 mm wide region of interest. A mid-systolic frame was used to initialize LV boundaries which were then automatically tracked throughout the cardiac cycle. Manual corrections were performed to optimize boundary tracking as needed. Optimization of images for endocardial visualization through adjustment of gain, compress, and time-gain compensation controls were done prior to acquisition (Fig. 1).
Statistical analysis
Categorical variables were reported as frequency and percentages. Continuous variables were evaluated for normal distribution using histogram and Q–Q plot. Normally distributed continuous variables were reported as mean and standard deviation (SD) while skewed data were presented as medians and interquartile ranges (IQR). Categorical variables were compared between categories using Chi-square test or Fisher’s exact test and continuous variables were compared using independent samples t test or Mann–Whitney test. Univariable logistic regressions were used to evaluate the association between baseline GLS and CTRCD development. A propensity score was modeled from baseline characteristics specified in Tables 1 and 2. A propensity score-adjusted logistic regressions were then used. Cox regressions were used to evaluate the association between baseline GLS and all-cause mortality. Cox regressions were adjusted for propensity scores which were modeled according to the above-specified parameters. A two-tailed p < 0.05 was considered statistically significant. Analyses were performed with SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
Baseline parameters
Of 291 patients evaluated consecutively, 48 (16%) patients were included in the reduced GLS group, according to the first GLS evaluation, while the remaining 243 patients were included in the preserved GLS group. Patients in the reduced GLS group were older (68[60–77] vs. 61[48–69], p < 0.001) with a male predominance (52% vs. 25%, p < 0.001) (Table 1). Among this group, cardiac morbidities were observed at a significantly higher prevalence, including ischemic heart disease (31% vs. 9%, p < 0.001), systolic dysfunction (LVEF < 50%) (46% vs. 0.4%, p = 0.001), atrial fibrillation (17% vs. 6%, p = 0.016) and chronic renal failure (13% vs. 1%, p < 0.001). However, aside from hyperlipidemia (42% vs. 20%, p = 0.001), no significant differences were noted in other cardiovascular risk factors (Table 1).
Patients with reduced GLS were more likely to be treated with beta blocker (BB) (62% vs. 23%, p < 0.001), however, no significant differences were observed regarding angiotensin converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) treatment (44% vs. 28%, p = 0.26).
At first evaluated echocardiography, patients in the reduced GLS group had as expected lower median GLS (14.7[11–16] vs. 20.7[19–22], p < 0.001) as well as lower EF (50[40–58] vs. 60[60–60], p< 0.001) and higher left ventricle end diastolic diameter (LVEDD) (51[46–54] vs. 46[43–49], p < 0.001) and left ventricle end systolic diameter (LVESD) (32[27–39] vs. 25[23–28], p < 0.001) (Table 1).
Cancer type and chemotherapeutic agents
The study population included different types of cancer (Table 2). Breast cancer was the most frequent type of cancer (55%), however, the reduced GLS group was seen to have a lower prevalence of this type of malignancy (31% vs. 60%, p < 0.001). On the other hand, lung cancer and hematologic cancer were more frequent among the reduced GLS group (15% vs. 3%, p = 0.005 and 25% vs. 12%, p = 0.013, respectively) (Table 2). Trastuzumab and pertuzumab (both recombinant humanized monoclonal antibodies against HER2) therapy were significantly used more frequently among the reduced GLS group (19% vs. 8%, p = 0.029 and 13% vs. 3%, p = 0.016, respectively). Similarly, the use of proteasome inhibitor and tyrosine kinase inhibitor therapy were more frequent among the reduced GLS group (6% vs. 1%, p = 0.033 and 8% vs. 1%, p = 0.016, respectively). No significant difference was observed regarding treatment with doxorubicin (a type of ANT) (21% vs. 18%, p = 0.657; Table 2).
Outcomes according to GLS
Overall, 45 (94%) patients in the reduced GLS and 192 (79%) patients in the preserved GLS performed follow-up echocardiography exam (Table 3). Over a median follow-up of 2.9 months (IQR 1.8–5.2) (Table 3), 11 (5%) patients developed CTRCD, with a substantially higher prevalence among the reduced GLS group (16% vs. 2%, p = 0.001) (Table 3; Fig. 1). Patients with preserved GLS had a significantly lower risk for CTRCD development (OR 0.11, 95%CI 0.03–0.41, p = 0.001), with every 1-unit improvement of GLS the risk of CTRCD decreased by 16% (OR 0.84, 95%CI 0.73–0.95, p = 0.007) (Table 4). After adjustment for baseline characteristics, including cardiovascular risk factors and systolic function, preserved GLS remained significantly associated with a lower risk for CTRCD development (OR 0.11, 95%CI 0.02–0.64, p = 0.014), with every 1-unit improvement lowering the risk by 19% (OR 0.81, 95%CI 0.67–0.98, p = 0.032). Interestingly, after adjusting for BB, ACEI/ARB treatment, GLS did not remain significantly associated to CTRCD (p = 0.166) (Table 4).
Similarly, reduced GLS was independently associated with any EF reduction (p = 0.004), remaining significant after adjustment for cardiovascular risk factors and systolic function (p = 0.049), and again, after adjustment for BB, ACEI/ARB treatment reduced GLS did not remain significant (p = 0.227) (Table 4).
One-year all-cause mortality was higher among patients with reduced GLS (17% vs. 1%, p < 0.001) (Table 5). Patients with preserved GLS had a significantly lower risk for all-cause mortality (OR 0.31, 95%CI 0.16–0.62, p = 0.001), with every 1-unit improvement of GLS, the risk decreased by 14% (OR 0.86, 95%CI 0.8–0.93, p < 0.001) (Table 4). However, after adjustment for cardiovascular risk factors and systolic function, GLS did not remain significantly associated to all-cause mortality (p = 0.132) (Table 4).
Discussion
In the present study, we emphasized the importance of using GLS assessment routinely among patients receiving cancer therapy, which may allow early cardiac dysfunction diagnosis.
According to the Expert Consensus for Multimodality Imaging Evaluation of the American Society of Echocardiography [19], GLS is the optimal parameter of deformation for the early detection of subclinical LV dysfunction among patients with cancer. However, due to the lack of large randomized control trials, currently there is no evidence to guide specific GLS surveillance among patients receiving cancer therapy [4]. In our study, we found that reduced GLS is frequent (16%) and is related to high prevalence of ischemic heart disease, systolic dysfunction, atrial fibrillation, chronic renal failure and hyperlipidemia. However, no significant differences were noted regarding smoking, hypertension and diabetes mellitus. Past studies have also noted the correlation between cardiovascular risk factors, especially chronic renal failure, and reduced GLS [22, 23].
Doxorubicin and trastuzumab are well studied and known to cause cardiotoxicity [19], expressed by LVEF and GLS reduction [8, 24, 25]. As expected, in our study trastuzumab treatment was significantly more frequent among the reduced GLS group, however, no differences were noticed regarding doxorubicin therapy. This finding may be explained by the fact that the majority of patients treated with doxorubicin in our registry, which has a dose-dependent risk of developing cardiotoxicity [4], were breast cancer patients who are exposed to lower therapeutic doses (240 mg/m2) and therefore a low correlation was noticed in our study. Similarly to trastuzumab, treatment with pertuzumab, proteasome inhibitor and tyrosine kinase inhibitor were more frequent among the reduced GLS group, which may imply the need for routine follow-up among that specific population, however; larger trials are needed to support this data.
A number of studies, including the PRADA [26] and OVERCOME [27] trials, implied that routine baseline use of BB, ARB and ACEI provides protection against early decline in global LV function. However, currently there is no evidence to guide specific cardio-protection treatment according to GLS surveillance [28]. Interestingly, in our trial we observed a significantly high BB use among the reduced GLS group. This discrepancy can be attributed to the fact that the BB treatment was administered not as a result of the reduced GLS, but rather due to the high prevalence of cardiac morbidities in the mentioned group.
Past studies [13, 17, 29] have shown an association between reduced GLS and LV dysfunction, however, most of the studies were small, retrospective and mainly included breast cancer patients. Our study is novel through evaluating prospectively a large population with diverse types of cancer. We demonstrated that preserved GLS is associated with a lower risk for CTRCD development and, furthermore, with any 1-unit improvement in GLS, the risk of CTRCD decreased. Importantly, this study’s increased strength, compared to past studies [30], comes from using multivariable analyses adjusted for a propensity score which was modeled from all baseline characteristics, showing that the association remained significant through the adjustment to cardiac risk factors and systolic function. However, adding BB, ACEI/ARB treatment to the model evoked its significance, which may imply that cardio-protective treatment may prevent CTRCD development.
Using univariable analysis, reduced GLS was associated with all-cause mortality, however, after multivariable analyses adjustment, the association did not remain significant. Regrettably, the specific causes of death for most of the patients were unknown since it occurred out of our hospital. The relation of GLS to all-cause mortality was implied in the past [30] and may be explained as GLS being a marker for severe disease, elevated inflammatory cytokines [31] and overall cardiac stress. This may also support that after adjustment to cardiac risk factors, reduced GLS did not emerge associated with all-cause mortality; however, this can also be explained by the small number of deaths.
Our study has several limitations. First, it was a single center study. Second, we evaluated the patients at different time points of their therapy and therefore outcome data may not account for patients who developed GLS reduction or CTRCD later. Finally, the relatively short period of difference between echocardiography assessments might have influenced the prevalence of CTRCD development and all-cause mortality.
In summary, our study shows that reduced GLS is frequent among patients receiving cancer therapy and specifically among patients with cardiac risk factors or cancer therapy such as trastuzumab, pertuzumab, proteasome inhibitor and tyrosine kinase inhibitor, which may imply the need for close GLS follow-up among that specific population. Moreover, we implied that reduced GLS is associated with CTRCD development, independently of comorbidities and LVEF. Interestingly, our data suggest that treatment with BB, ACEI/ARB may prevent CTRCD development among patients with reduced GLS.
Abbreviations
- CTRCD:
-
Cancer therapy-related cardiac dysfunction
- LVEF:
-
Left ventricular ejection fraction
- GLS:
-
Global longitudinal strain
- STE:
-
Speckle-tracking echocardiography
- ANT:
-
Anthracyclines
- LV:
-
Left ventricle
- SD:
-
Standard deviation
- IQR:
-
Interquartile ranges
- BB:
-
Beta blocker
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ARB:
-
Angiotensin II receptor blocker
- LVEDD:
-
Left ventricle end diastolic diameter
- LVESD:
-
Left ventricle end systolic diameter
- OR:
-
Odd ratio
- CI:
-
Confidence interval
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Tilemann LM, Heckmann MB, Katus HA, Lehmann LH, Müller OJ (2018) Cardio-oncology: conflicting priorities of anticancer treatment and cardiovascular outcome. Clin Res Cardiol 107(4):271–280
Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD (2011) Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 13(3):R64
Zamorano JL, Lancellotti P, Muñoz DR, Aboyans V, Asteggiano R, Galderisi M et al (2016) ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J 2016(37):2768–2801
Quiñones MA, Greenberg BH, Kopelen HA, Koilpillai C, Limacher MC, Shindler DM et al (2000) Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy: studies of left ventricular dysfunction. J Am Coll Cardiol 35:1237–1244
Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 91:2869–2879
Sawaya H, Seba IA, Plana JC, Januzzi JL, Ky B, Cohen V et al (2011) Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 107:1375–1380
Yoon HJ, Kim KH, Kim HY, Park H, Cho JY, Hong YJ, Park HW, Kim JH, Ahn Y, Jeong MH, Cho JG, Park JC (2019) Impacts of non-recovery of trastuzumab-induced cardiomyopathy on clinical outcomes in patients with breast cancer. Clin Res Cardiol 10:15. https://doi.org/10.1007/s00392-019-01417-x[Epub ahead of print] PMID: 30737527
Santoro C, Aripino G, Esposito R, Lembo M, Paciolla I, Cardalesi C et al (2017) 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: a balance with feasibility. Eur Heart J Cardiovasc Imaging 18:930–936
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, DeGiacomi G et al (2010) Anthracycline induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 55:213–220
Wadhwa D, Fallah-Rad N, Grenier D, Krahn M, Fang T, Ahmadie R et al (2008) Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res Treat 117:357–364
Buckert D, Cieslik M, Tibi R, Radermacher M, Rasche V, Bernhardt P, Hombach V, Rottbauer W, Wöhrle J (2018) Longitudinal strain assessed by cardiac magnetic resonance correlates to hemodynamic findings in patients with severe aortic stenosis and predicts positive remodeling after transcatheter aortic valve replacement. Clin Res Cardiol 107(1):20–29
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC et al (2012) Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 5:596–603
Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F et al (2010) Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23:351–369
Stanton T, Learno R, Marwick TH (2009) Prediction of all-cause mortality from global longitudinal speckle strain comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2(5):356–364
Sawaya H, Sebag IA, Plana JC, Januzzi JC, Ky B, Tan TC et al (2012) Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 5:596–603
Negishi K, Negishi T, Hare JL, Haluksa BA, Plana JC, Marwick TH (2013) Independent and incremental value of deformation indices for prection of trastuzumab induced cardiotoxicity. J Am Soc Echocardiogr 26:493–498
Dalen J, Thorstensen A, Aase SA, Ingul CB, Topr H, Vatten LJ et al (2010) Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr 11(2):176–183
Plana JC, Galderisi M, Barac A et al (2014) Expert Consensus for Multimodality Imaging Evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 27(9):911–939
Lang RM, Badano LP, Mor-avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1–39):E14
Charbonnel C, Convers-Domart R, Rigaudeau S (2017) Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging 18:392–401
Fujikura K, Peltzer B, Tiwari N, Shim HG, Dinhofer AB, Shitole SG et al (2018) Reduced global longitudinal strain is associated with increased risk of cardiovascular events or death after kidney transplant. Int J Cardiol 272:323–328
Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Burrage M, Leano R et al (2015) Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS One 10(5):e0127044
Laufer-Perl M, Derakhshesh M, Milwidsky A, Mor L, Ravid D, Amrami N et al (2016) Usefulness of global longitudinal strain for early identification of subclinical left ventricular dysfunction in patients with active cancer. Am J Cardiol 122(10):1784–1789
Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH (2014) Use of speckle strain to assess left ventricular response to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 15(3):324–331
Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW et al (2016) Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 37(21):1671–1680
Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM et al (2013) Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (prevention of left ventricular dysfunction with enalapril and carvedilol in patients submitted to intensive chemotherapy for the treatment of malignant hemopathies). J Am Coll Cardiol 61(23):2355–2362
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH (2014) Use of myocardial strain imaging for the early detection of cardiotoxic patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63(25 Pt A):2751–2768
El-Sherbeny WS, Sabry NM, Sharbay RM (2019) Prediction of trastuzumab-induced cardiotoxicity in breast cancer patients receiving anthracycline-based chemotherapy. J Echocardiogr 17(2):76–83
Rhea IB, Uppuluri S, Sawada S, Schneider BP, Feigenbaum H (2015) Incremental prognostic value of echocardiographic strain and its association with mortality in cancer patients. J Am Soc Echocardiogr 28(6):667–673
Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW (2013) Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol 31(13):1656–1661
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Laufer-Perl, M., Arnold, J.H., Mor, L. et al. The association of reduced global longitudinal strain with cancer therapy-related cardiac dysfunction among patients receiving cancer therapy. Clin Res Cardiol 109, 255–262 (2020). https://doi.org/10.1007/s00392-019-01508-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-019-01508-9