Abstract
Background
The second-generation cryoballoon (CB) is increasingly used for treatment of persistent atrial fibrillation (AF). Data regarding the clinical outcome and mechanism of arrhythmia recurrence following persistent AF ablation using CB is sparse. In this study, we aimed to assess the efficacy of CB and mechanisms of atrial tachyarrhythmia (ATA) recurrence in patients with persistent AF.
Methods and results
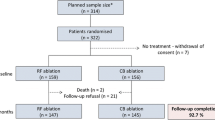
A total of 133 patients (66 ± 10 years, 60% male) with symptomatic persistent AF, who were scheduled for PVI using the second-generation CB were enrolled. Follow-up included 24 h Holter recording at 3, 6 and 12 months. Any documented episode of ATA lasting more than 30 s was considered as a recurrent arrhythmic event. All targeted veins were isolated (100%). Phrenic nerve palsy with recovery during follow-up occurred in six patients (4.5%), no patient experienced tamponade or a cerebrovascular event. During 12.6 ± 5.4 months of follow-up, 89/133 (67%) patients were free of ATA recurrences. Multivariable analysis revealed recurrence in the blanking period (HR 11.46, 0.95 CI 3.92–33.49, p < 0.001), presence of cardiomyopathy (HR 2.75, 0.95 CI 1.09–6.96, p = 0.032) and PV abnormality (HR 3.56, 0.95 CI 1.21–10.43, p = 0.021) as predictors for late recurrence.
Conclusion
In patients with persistent AF, second-generation cryoballoon use is associated with an excellent safety profile and favorable outcomes. Arrhythmia recurrence during the blanking period, presence of cardiomyopathy and PV abnormality were independent predictors of long-term AF recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common sustained chronic arrhythmia. Catheter ablation of symptomatic patients by targeting the pulmonary veins (PV) is widely used as recommended by current guidelines [1]. In recent years, cryoballoon (CB) based isolation of the PVs has an increasing trend with successful outcome especially in patients with paroxysmal AF patients [2, 3]. In patients with persistent AF, the substrate is more complex resulting in heterogeneous ablation strategies such as PV isolation as the sole ablative strategy, substrate-based ablation strategies and novel strategies such as rotor guided strategies [4,5,6,7]. PV isolation (PVI) as the sole ablative strategy using radiofrequency (RF) energy has been demonstrated to be non-inferior to more extensive ablation in persistent AF [8].
The second-generation CB (Arctic Front Advance, Medtronic) has been released with technical developments resulting in a larger and more homogeneous zone of freezing on the balloon surface, translating into significant improvements in procedural and clinical outcomes as compared with its predecessor [9, 10]. Although an increasing interest has already developed regarding the efficacy of AF ablation in patients with persistent AF, follow-up data in those patients using novel CB is limited [11,12,13]. In this study, we aimed to assess the efficacy of second-generation CB in patients with persistent AF and to analyze predictors of arrhythmia recurrence.
Methods
Between July 2015 and March 2017, 133 patients underwent second-generation cryoballoon ablation at the University Heart Center Luebeck for the treatment of symptomatic persistent AF. Persistent AF was defined as, continuous AF lasting for more than 7 days but less than 1 year [1]. Patients with left atrial (LA) thrombus, uncontrolled thyroid dysfunction, contraindication to anticoagulation, pregnancy, and previous AF ablation, severe valvular disease and a LA size > 60 mm were excluded. Severity of symptoms was recorded according to European Heart Rhythm Association (EHRA) score. Informed consent was taken from each patient before the procedure. The study was in compliance with the principals outlined in the Declaration of Helsinki and approved by the local Ethics Committee.
Preprocedural management
Transesophageal echocardiography was performed in all patients prior to the procedure. Apart from echocardiography, no additional preprocedural imaging was performed. In patients on vitamin K antagonists, anticoagulation was continued throughout the procedure aiming at an INR of 2–3. In patients treated with novel oral anticoagulants (NOACs), the drug was discontinued ≥ 24 h prior to the procedure and and re-initiated 6 h post-ablation at half the regular dose, and at full dose the following day.
Procedural management
All procedures were performed under deep sedation using midazolam, fentanyl, and propofol. Cryoballoon ablation procedure was performed as previously described [3]. Cavotricuspid isthmus ablation (CTI) using an open irrigated RF catheter (Celsius ThermoCool or ThermoCool SF, Biosense Webster Inc.) was solely performed in patients with documented or induced common type atrial flutter.
Postprocedural management
Following ablation, all patients underwent transthoracic echocardiography to rule out a pericardial effusion. All patients were treated with proton-pump inhibitors twice daily for 6 weeks. Anticoagulation was continued for at least 3 months and thereafter based on the individual CHA2DS2-VASC score. To prevent early recurrence, an antiarrhythmic drug was administered throughout the three months blanking period. Discontinuation of the antiarrhythmic drug was strongly recommended thereafter. Follow-up was performed either by the outpatient clinic or the referring cardiologist at 3, 6, and 12 months as well as in case of symptoms suggestive of arrhythmia recurrence and included a ≥ 24 h Holter recording and interrogations of implanted devices, if present. Symptoms suggestive of recurrent atrial tachyarrhythmia (ATA) prompted additional outpatient clinic visits. Any documented episode of ATA lasting more than 30 s were considered as recurrent arrhythmic event. Repeat ablation was offered to the patients in case of symptomatic ATA recurrence after the blanking period or symptomatic drug refractory recurrent ATA within the blanking period that could not be managed without intervention.
The primary endpoint of this study was ATA recurrence including common type flutter after a 3 months blanking period or triggering a redo ablation within the blanking period.
Secondary endpoints were complications related to the procedure, such as pericardial effusion/tamponade, PNP, cerebrovascular events, and groin complications.
Statistical analysis
Continuous data are presented as mean ± standard deviation, skewed continuous parameters were expressed as median (interquartile range defined as Q1–Q3). Categorical data were summarized as frequencies and percentages and were compared using χ2 test. Comparisons between baseline characteristics were performed by independent Student’s t test, Mann–Whitney rank-sum, Fisher exact, or χ2 tests where appropriate. To analyze the association between baseline and procedural parameters on AF recurrence, binary logistic regression analysis was used. Parameters that were found to be univariately associated with the outcome and those that show a slight association with the outcome with p < 0.20 are included in the multivariable analysis. Kaplan–Meyer and Cox regression analysis was performed to describe ATA free survival. Statistical analyses were performed using SPSS statistical software (version 22.0; SPSS Inc., Chicago, IL, USA). A 2-tailed p < 0.05 was considered statistically significant.
Results
A total of 133 patients (66 ± 10 years, 60% male) with persistent AF including 14 patients (10.5%) with concomitant documented typical atrial flutter were included in this study. Baseline characteristics are summarized in Table 1. Cardiomyopathy (CMP) was diagnosed in 34 patients [25.6%, (23/34 nonischemic CMP, 9/34 ischemic CMP, 2/34 hypertrophic CMP)] and 19 patients (14.3%) had an implanted electrical cardiac device including pacemaker (PM, n = 9) or implantable cardioverter defibrillator (n = 10).
All targeted veins were isolated. Mean number of CB applications per PV and mean temperature during the procedure per PV as well as procedural characteristics are presented in Table 1. PV abnormality was observed in 21 patients (15.7%) with a left common ostium in 13 patients (9.7%) and a right middle PV in 9 patients (6.8%). Total procedural and fluoroscopy time were 107.5 ± 22.3 and 22.8 ± 10.1 min, respectively. In 14 (10.5%) patients, cryoballoon application converted atrial fibrillation to sinus rhythm and 70 (52.6%) patients required DC cardioversion.
Adverse events
Complications requiring intervention occurred in 2 (1.5%) patients (arterioveneous fistula requiring surgery).
PNP occurred in 6 (4.5%) patients during the ablation of the right sided veins. PNP resolved in 4/6 (66.6%) during the procedure, 2 days later in 1/6 (16.6%) and 12 months later in remaining 1/6 (16.6%) patient.
Other minor adverse events with spontaneous sequel occurred in four (3.0%) patients and included hematoma (n = 3) and pericardial effusion (n = 1) without hemodynamic compromise. No other adverse events such as significant pericardial effusion, pericardial tamponade, symptomatic PV stenosis, cerebral embolism, or atrioesophageal fistula were noted.
Clinical follow-up
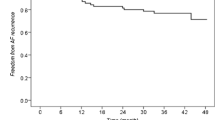
Twenty-five (19%) patients had early recurrence of ATA within the first 3 months after index CB ablation. After a mean follow-up period of 12.6 ± 5.4 months, single procedure success rate including a 3 months blanking period was 67% (Fig. 1). Our institutional approach strongly recommends discontinuation of AAD after blanking period. After blanking period, in 125/133 (93.9%) patients, AAD was discontinued. 3/44 (6.8%) patients in the ATA recurrence group and 5/89 (5.6%) patients with no ATA recurrence continued to take AAD, due to either patient’s preference or referring physician’s preference (p = 0.78). Freedom of AAD at 12th month follow-up was 111/133 (83.4%). 29/44 (65.9%) of patients with ATA recurrence and 82/89 (92.1%) of patients without recurrence (p = 0.001) were free of AAD at 12-month visit.
Univariate analysis demonstrated that in patients with ATA recurrence, the LA diameter was larger (44.6 ± 5.7 vs 41.2 ± 6.5 mm, p = 0.004), LVEF was lower (48.6 ± 10.0 vs 51.8 ± 7.7, p = 0.04), LIPV diameter was larger (14.6 ± 2.6 vs 13.6 ± 2.7 mm, p = 0.04) as compared to the remaining patients. Also, presence of cardiomyopathy (17/44 vs 17/89, p = 0.02), PV abnormality (11/44 vs 10/89, p = 0.047) and arrhythmia recurrence during blanking period (19/44 vs 6/89, p < 0.001) were more common in patients with ATA recurrence (Table 1). After multivariate analysis, presence of early recurrence within the blanking period (HR 11.46, 0.95 CI 3.92–33.49, p < 0.001), presence of cardiomyopathy (HR 2.75, 0.95 CI 1.09–6.96, p = 0.032) and PV abnormality (HR 3.56, 0.95 CI 1.21–10.43, p = 0.021) solely remained as independent predictors of late recurrence (Fig. 2, Supplement).
A total 29 of 44 (66%) patients underwent redo ablation for recurrent ATA. In 8 (27%) patients, the repeat procedure was performed during the blanking period due to need for multiple and/or failure of electrical CV despite antiarrhythmic drug therapy. PV re-connection was found in 18/29 (62.0%) patients and included one vein (n = 12), two veins (n = 3) or four veins (n = 3). The reconnected PVs were the LSPV (n = 9), LIPV (n = 5), RSPV (n = 7) or the RIPV (n = 9). After PV re-isolation, 15 patients received additional ablation lines. Sites of additional lesions were roof line in three, anterior line in five, CTI line in seven and ostial potential ablation in three individuals. In 11/29 (37.9%) patients with recurrence despite isolated PVs, ostial potential ablation was performed in six, anterior line in six, roof line in four, posterior line in two and CTI line were ablated in four patients, respectively.
Discussion
The main findings of the study are, (I) the use of second-generation 28 mm CB in persistent AF patients results in 100% isolation of targeted veins, with 1-year clinical success rate of 67%. (II) Recurrence during blanking period, presence of cardiomyopathy and PV abnormality were independent predictors of recurrence after CB PVI. (III) In addition, in the majority of patients undergoing repeat ablation PV re-connection was observed.
Currently, the best ablative strategy to treat patients with persistent AF is controversial, due to complex substrate. Besides PVI, different ablative strategies such as ablation of complex fractionated atrial electrograms, rotors or low voltage areas or linear ablation are performed aiming at improved outcomes [5,6,7, 14].
Results from the STAR-AF II study suggest that outcome after PVI only in patients with persistent AF, using RF ablation in conjunction with a 3D mapping-system, is not inferior to more extensive ablation strategies such as ablation of complex fractionated atrial electrograms (CFAE) or placement of linear lesions in addition to PVI [8]. These results re-emphasize the importance of durable PVI even in patients with persistent AF.
PVI is still the primary ablative approach for AF, especially in paroxysmal and persistent AF [2, 3, 11]. The randomized “fire and ice” trial proved non-inferiority of ablation with the second-generation CB compared to RF-based PVI with regard to efficacy and safety for the treatment of patients with PAF [2]. After this study, the number of CB-based PVI procedures increased. Although previously published data suggests lower recurrences with second-generation CB in paroxysmal AF patients, there is limited data in persistent AF concerning success rates. Aytemir et al. [15] reported 50% success rate with first-generation CB in patients with persistent AF at a median 18 (6–27) months follow-up. However, second-generation CB was found to have better outcomes compared with first-generation CB among patients with both paroxysmal and persistent AF [9, 16]. Freedom from ATA following persistent AF ablation with RF and second-generation CB was comparable at 1-year follow-up after a single procedure [11]. Guhl et al. [17]. analyzed the results of 69 s generation CB procedures for persistent AF and ATA recurrence-free rate at 1-year was 58.5%. Koektuerk et al. [12] evaluated 1-year outcome after second-generation CB for persistent AF patients. After mean follow-up of 10.6 ± 6.3 months, 67 of 100 patients were in sinus rhythm. Recurrence during blanking period was the only independent predictor of late recurrence. In a study by Lemes et al. [13], 69% of persistent AF patients were recurrence free after a follow-up of 416 + 178 (range 188–744) days. In a recent meta-analysis [18], after a mean follow-up of 16.7 ± 3.0 months, 68.9% were free from recurrences.
In our study, the freedom from ATA recurrence at a mean follow-up period of 12.6 ± 5.4 months was comparable to previous studies. Similar to the study by Koektuerk et al. [12], recurrence during the blanking period was an independent predictor of late recurrence.
Due to failure of AADs or need for multiple cardioversions, eight patients (27.5%) underwent a redo ablation during the blanking period. This amount is higher than previously described, e.g., in the STOP-AF [19] trial, in which 19% of patients underwent repeat ablation during the blanking period. However, this study included only paroxysmal AF patients with a suggested less complex arrhythmic substrates compared to our cohort with persistent AF and a considerable amount of patients with cardiomyopathy.
In our study, presence of cardiomyopathy and PV abnormality also emerged as independent predictors of late recurrence. This might be attributed to the characteristics of the study population enrolled in our analysis. Patients with cardiomyopathy have a more diseased atrium that may predispose to develop AF. There are several explanations for the higher recurrence rate in patients with anatomical variants of the PVs. The anatomical variant may pose technical challenges during the procedures, thus potentially compromising effective lesion formation. However, the authors believe, that the most likely mechanism of the higher recurrence rate is the more distal PV isolation in common PV ostia which results in a smaller substrate modification of the LA and PV antrum. Variant PV- anatomy as assessed by preprocedural evaluation via a multi-detector computed tomography was found to be a predictor of AF recurrence after PVI by remote magnetic navigation [20]. Heeger et al. [21] reported high acute success rates and procedural safety for the treatment of left common pulmonary veins (LCPV). Freedom from ATA recurrence was 64% after 2 years of follow-up with no statistical difference compared to patients of the control group with normal PV anatomy (66%). On the contrary, Shigeta et al. [22]. found that, presence of LCPV was associated with poor clinical outcome of AF ablation in patients undergoing second-generation CB-based PVI. We used PV angiography to assess PV anatomy and in our study, while the presence of right middle PV, and/or LCPV was linked to ATA recurrence.
Furthermore, CTI ablation was performed during index cryoballoon ablation, if atrial flutter was documented or induced. This additional ablation approach might have an impact on the recurrence of ATA during follow-up. However, in our cohort, none of the patients undergoing CTI ablation during redo ablation had previous CTI ablation and performance of CTI in patients with and without ATA recurrence was equally distributed. Furthermore, one has to acknowledge that atrial flutter and atrial fibrillation frequently coexist. Correspondingly, 4.5% of paroxysmal AF patients in the FIRE and ICE study underwent CTI ablation due to coexisting CTI dependent atrial flutter. We therefore think, that our results are comparable to other studies and results should not be biased by the fact that CTI ablation was performed [2].
Several significant improvements became evident in clinical practice with the use of second-generation CBs, including better acute and chronic success rate and decreased fluoroscopy time [2, 3]. Besides ostial PV isolation, application of second-generation CB results in large atrial lesion formation causing substrate modification, especially in the posterior LA wall. The spherical shape of second-generation CBs may cause a mismatch between the balloon and PV ostia during optimal contact causing additional atrial wall ablation that may eliminate components responsible for persistent AF such as CFAEs [23], rotors [24], and vagal ganglia [25] as well. Despite technological improvements, recurrences still occur in around one-third of patients and etiology of recurrences are supposed to relate to (1) lack of durable pulmonary vein isolation and (2) need for addressing non-pulmonary vein triggers. In our study patients, analysis of redo procedures revealed re-connection of the PVs in 62% of patients with recurrence, suggesting the importance of electrical linkage of the LA with the PVs. Moreover, in 6 of 11 patients with isolated PVs and ATA recurrence ostial potential ablation was performed due to distal PV isolation. Future techniques aiming at durable PV isolation and larger ostial atrial lesions might probably increase the success rates.
Our study findings with 67% clinical success rate have some important clinical implications. First, our results are in line with recently published studies with a higher rate of freedom from ATA. Moreover, our study expands the literature in terms of a larger patient population included. The complication rate was also low without any pericardial tamponade or cerebrovascular events and with only two femoral arteriovenous fistula necessitating surgery. PNP was observed in only 4% of the patients, which was also comparable with other studies using cryoballoon [26, 27]. Early recurrence in blanking period, presence of cardiomyopathy and PV abnormality can be used to identify patients at risk for recurrence. PV re-connection was observed in nearly 2/3 of patients with ATA recurrence, highlighting the importance of durable PVI in the treatment of persistent AF.
Limitations
This study has some limitations. First, this is a single centre, retrospective, nonrandomized study evaluating the efficacy and safety of primarily second-generation CB ablation in persistent AF patients without preprocedural cardiac imaging. Due to our institutional standard is cryoballoon for initial ablation approach of AF, our study has no control group. Because some of the patients were scheduled for cryoballoon ablation after cardioversion, more than one-third of patients were in sinus rhythm during the procedure, despite having persistent AF. Although our study has the highest patient group reported, large-scale randomized studies are needed both to confirm and expand our findings. Second, our follow-up did not include routine continuous monitoring with implanted devices or 7 day-Holter recording. Nevertheless, follow-up included 24 h Holter monitoring, and/or device interrogations (if present), at 3, 6 and 12 months.
No systematic oesophagoscopy was performed in this study. Therefore, no data about the incidence of esophageal injury is available.
Conclusion
In patients with persistent AF, second-generation cryoballoon use is associated with an excellent safety profile and favorable outcomes. Arrhythmia recurrence during the blanking period, presence of cardiomyopathy and PV abnormality were independent predictors of long-term AF recurrence.
Abbreviations
- ATA:
-
Atrial tachyarrhythmia
- AF:
-
Atrial fibrillation
- CB:
-
Cryoballoon
- CMP:
-
Cardiomyopathy
- CTI:
-
Cavotricuspid isthmus
- EHRA:
-
European Heart Rhythm Association
- ICD:
-
Implantable cardioverter defibrillator
- INR:
-
International normalized ratio
- LA:
-
Left atrium
- LCPV:
-
Left common pulmonary vein
- LIPV:
-
Left inferior pulmonary vein
- LSPV:
-
Left superior pulmonary vein
- LVEF:
-
Left ventricular ejection fraction
- PM:
-
Pacemaker
- PNP:
-
Phrenic nerve palsy
- PV:
-
Pulmonary vein
- PVI:
-
Pulmonary vein isolation
- RIPV:
-
Right inferior pulmonary vein
- RMPV:
-
Right middle pulmonary vein
- RSPV:
-
Right superior pulmonary vein
- RF:
-
Radiofrequency
- SR:
-
Sinus rhythm
References
Paulus Kirchhof, Authors/Task Force Members; Stefano Benussi, Authors/Task Force Members; Dipak Kotecha, Authors/Task Force Members; Anders Ahlsson, Authors/Task Force Members; Dan Atar, Authors/Task Force Members; Barbara Casadei, Authors/Task Force Members; Manuel Castella, Authors/Task Force Members; Hans-Christoph Diener, Authors/Task Force Members; Hein Heidbuchel, Authors/Task Force Members; Jeroen Hendriks, Authors/Task Force Members; Gerhard Hindricks, Authors/Task Force Members; Antonis S. Manolis, Authors/Task Force Members; Jonas Oldgren, Authors/Task Force Members; Bogdan Alexandru Popescu, Authors/Task Force Members; Ulrich Schotten, Authors/Task Force Members; Bart Van Putte, Authors/Task Force Members; Panagiotis Vardas, Authors/Task Force Members; Stefan Agewall, Document Reviewers; John Camm, Document Reviewers; Gonzalo Baron Esquivias, Document Reviewers; Werner Budts, Document Reviewers; Scipione Carerj, Document Reviewers; Filip Casselman, Document Reviewers; Antonio Coca, Document Reviewers; Raffaele De Caterina, Document Reviewers; Spiridon Deftereos, Document Reviewers; Dobromir Dobrev, Document Reviewers; José M. Ferro, Document Reviewers; Gerasimos Filippatos, Document Reviewers; Donna Fitzsimons, Document Reviewers; Bulent Gorenek, Document Reviewers; Maxine Guenoun, Document Reviewers; Stefan H. Hohnloser, Document Reviewers; Philippe Kolh, Document Reviewers; Gregory Y. H. Lip, Document Reviewers; Athanasios Manolis, Document Reviewers; John McMurray, Document Reviewers; Piotr Ponikowski, Document Reviewers; Raphael Rosenhek, Document Reviewers; Frank Ruschitzka, Document Reviewers; Irina Savelieva, Document Reviewers; Sanjay Sharma, Document Reviewers; Piotr Suwalski, Document Reviewers; Juan Luis Tamargo, Document Reviewers; Clare J. Taylor, Document Reviewers; Isabelle C. Van Gelder, Document Reviewers; Adriaan A. Voors, Document Reviewers; Stephan Windecker, Document Reviewers; Jose Luis Zamorano, Document Reviewers; Katja Zeppenfeld, Document Reviewers; 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR et al (2016) Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 374:2235–2245
Metzner A, Reissmann B, Rausch P, Mathew S, Wohlmuth P, Tilz R et al (2014) One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 7:288–292
Tilz RR, Chun KR, Schmidt B, Fuemkranz A, Wissner E, Koester I et al (2010) Catheter ablation of long-standing persistent atrial fibrillation: a lesson from circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol 21:869–874
Lin T, Rillig A, Bucur T, Metzner A, Mathew S, Wissner E et al (2016) Focal impulse and rotor modulation using the novel 64-electrode basket catheter: electrogram characteristics of human rotors. Europace 17:1791–1797
Rostock T, Salukhe TV, Steven D, Drewitz I, Hoffmann BA, Bock K et al (2011) Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm 8:1391–1397
Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB et al (2015) Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 8:18–24
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P (2015) STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372:1812–1822
Fürnkranz A, Bordignon S, Schmidt B, Gunawardene M, Schulte-Hahn B, Urban V et al (2013) Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovasc Electrophysiol 24:492–497
Di Giovanni G, Wauters K, Chierchia GB, Sieira J, Levinstein M, Conte G et al (2014) One-year follow-up after single procedure cryoballoon ablation: a comparison between the first and second generation balloon. J Cardiovasc Electrophysiol 25:834–839
Ciconte G, Baltogiannis G, de Asmundis C, Sieira J, Conte G, Di Giovanni G et al (2015) Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace 17:559–565
Koektuerk B, Yorgun H, Hengeoez O, Turan CH, Dahmet A, Yang A et al (2015) Cryoballoon ablation for pulmonary vein isolation in patients with persistent atrial fibrillation: one-year outcome using second generation cryoballoon. Circ Arrhythm Electrophysiol 8(5):1073–1079
Lemes C, Wissner E, Lin T, Mathew S, Deiss S, Rillig A et al (2016) One-year clinical outcome after pulmonary vein isolation in persistent atrial fibrillation using the second-generation 28 mm cryoballoon: a retrospective analysis. Europace 18:201–205
Koutalas E, Rolf S, Dinov B, Richter S, Arya A, Bollmann A, Hindricks G, Sommer P (2015) Contemporary mapping techniques of complex cardiac arrhythmias—identifying and modifying the arrhythmogenic substrate. Arrhythm Electrophysiol Rev 4:19–27
Aytemir K, Oto A, Canpolat U, Sunman H, Yorgun H, Şahiner L, Kaya EB (2013) Immediate and medium-term outcomes of cryoballoon-based pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation: single-centre experience. J Interv Card Electrophysiol 38:187–195
Metzner A, Heeger CH, Wohlmuth P, Reißmann B, Rillig A, Tilz RR, Mathew S, Lemes C, Deiß S, Maurer T, Saguner A, Ouyang F, Kuck KH, Wißner E (2016) Two-year outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon: lessons from the bonus freeze protocol. Clin Res Cardiol 105(1):72–78
Guhl EN, Siddoway D, Adelstein E, Voigt A, Saba S, Jain SK (2016) Efficacy of cryoballoon pulmonary vein isolation in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol 27:423–427
Omran H, Gutleben KJ, Molatta S, Fischbach T, Wellmann B, Horstkotte D, Körber B, Nölker G (2018) Second generation cryoballoon ablation for persistent atrial fibrillation: an updated meta-analysis. Clin Res Cardiol 107:182–192
Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG; STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713–1723
Sohns C, Sohns JM, Bergau L, Sossalla S, Vollmann D, Lüthje L et al (2013) Pulmonary vein anatomy predicts freedom from atrial fibrillation using remote magnetic navigation for circumferential pulmonary vein ablation. Europace 15:1136–1142
Heeger CH, Tscholl V, Wissner E, Fink T, Rottner L, Wohlmuth P et al (2017) Acute efficacy, safety, and long-term clinical outcomes using the second-generation cryoballoon for pulmonary vein isolation in patients with a left common pulmonary vein: a multicenter study. Heart Rhythm 14:1111–1118
Shigeta T, Okishige K, Yamauchi Y, Aoyagi H, Nakamura T, Yamashita M et al (2017) Clinical assessment of cryoballoon ablation in cases with atrial fibrillation and a left common pulmonary vein. J Cardiovasc Electrophysiol 28(9):1021–1027
Guler TE, Aksu T, Yalin K, Golcuk SE, Mutluer FO, Bozyel S (2017) Combined cryoballoon and radiofrequency ablation versus alone radiofrequency ablation for long-standing atrial fibrillation. Am J Med Sci (epub of print)
Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM (2012) Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol 60:628–636
Yorgun H, Aytemir K, Canpolat U, Şahiner L, Kaya EB, Oto A (2014) Additional benefit of cryoballoon-based atrial fibrillation ablation beyond pulmonary vein isolation: modification of ganglionated plexi. Europace 16:645–651
Guhl EN, Siddoway D, Adelstein E, Bazaz R, Mendenhall GS, Nemec J, Saba S, Schwartzman D, Voigt A, Wang NC, Jain SK (2016) Incidence and predictors of complications during cryoballoon pulmonary vein isolation for atrial fibrillation. J Am Heart Assoc 5(7). https://doi.org/10.1161/JAHA.116.003724
Metzner A, Rausch P, Lemes C, Reissmann B, Bardyszewski A, Tilz R et al (2014) The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol 25(5):466–470
Acknowledgements
K. Yalin recieved research and educational grant from Turkish Society of Cardiology. E. Lyan received travel grants and Speaker’s Bureau Honoraria from Biosense Webster, Medtronic, Boston Scientific. R. Tilz received travel grants from St. Jude Medical, Topera, Biosense Webster, Daiichi Sankyo, Sentrheart and Speaker’s Bureau Honoraria from Biosense Webster, Biotronik, Pfizer, Topera, Bristol-Myers Squibb; Bayer, Sanofi Aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yalin, K., Abdin, A., Lyan, E. et al. Safety and efficacy of persistent atrial fibrillation ablation using the second-generation cryoballoon. Clin Res Cardiol 107, 570–577 (2018). https://doi.org/10.1007/s00392-018-1219-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1219-1