Abstract
Objective
Increased transmitral flow velocity (E) to the early mitral annulus velocity (e′) ratio (E/e′), signifying increased cardiac filling pressure, was previously found to be associated with deterioration of renal function in patients with congestive heart failure. No study, however, included patients with acute myocardial ischemia. We hypothesized that elevated E/e′ ratio would be associated with an increased risk of acute kidney injury (AKI) in ST elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PCI).
Study design and methods
We conducted a retrospective study of 804 consecutive STEMI patients between June 2012 and December 2015 who underwent primary PCI and had a comprehensive echocardiographic examination performed within 72 h of hospital admission. Patients were stratified according to E/e′ ratio above and ≤15, and assessed for AKI using the KDIGO criteria, defined as either a serum creatinine rise >0.3 mg/dl, or an increase in serum creatinine ≥1.5 times baseline.
Results
Patients with E/e′ ratio >15 had lower left ventricular (LV) ejection fraction, higher systolic pulmonary artery pressures, as well as right atrial pressures, and demonstrated worse in-hospital outcomes. Patients with E/e′ ratio >15 had more AKI complicating STEMI (27 vs. 7 %; p < 0.001). In multivariate logistic regression model, E/e′ ratio >15 was independently associated with AKI (OR = 1.87, 95 % CI 0.99–3.52; p = 0.05). Other variables associated with AKI included diabetes, LV ejection fraction, and glomerular filtration rate.
Conclusions
Among STEMI patients undergoing primary PCI, the early E/e′ ratio >15 was associated with increased risk for AKI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Echocardiographic indices of elevated left ventricular (LV) filling pressures are associated with adverse outcomes following acute myocardial infarction (MI) [1–4].
The ratio of the early transmitral flow velocity (E) to the early diastolic septal or lateral mitral annulus velocity (e′) has been shown to be the most reliable non-invasive marker of elevated LV filling pressure [5]. An elevated E/e′ ratio, especially one >15, reportedly predicted poorer prognosis following MI [6, 7]. An elevated E/e′ ratio was previously found to be associated with the deterioration of renal function among hypertensive patients [8] and following renal transplantation [9]. However, its possible relation to renal function in the setting of acute ischemia was not assessed. The occurrence of acute kidney injury (AKI) following MI has complex and multifactorial pathogenesis [10–15]. We previously suggested that among this specific patient population, AKI may represent a special type of cardio-renal syndrome [15] due to the adverse hemodynamic state related both to decreased LV systolic function [16] and elevated right heart pressures [17], resulting in reduced renal perfusion [15]. We hypothesized that among ST elevation MI (STEMI) patients undergoing reperfusion with primary percutaneous coronary intervention (PCI), echocardiographic correlates of increased left ventricular pressure would be associated with an increased risk of AKI.
Materials and methods
We performed a retrospective, single-center observational study at the Tel-Aviv Sourasky Medical Center, a tertiary referral hospital with a 24/7 primary PCI service.
Included were all 930 consecutive patients admitted between June 2012 and February 2015 to the Cardiac Intensive Care Unit (CICU) with the diagnosis of acute STEMI. Patients who were treated either conservatively or by thrombolysis were excluded (n = 9), as were 26 patients, whose final diagnosis on discharge was other than STEMI (e.g., myocarditis or Takotsubo cardiomyopathy). We also excluded patients who died within 24 h of admission (n = 17), since we presumed that there was insufficient time for AKI to occur, as well as patients requiring chronic peritoneal dialysis or hemodialysis (n = 6) treatment. Finally, 68 patients whom echocardiography assessment did not include calculation of the E/e′ ratio or failed to demonstrate adequate tricuspid regurgitation flow were also excluded. The final study population included 804 patients whose baseline demographics, cardiovascular history, clinical risk factors, treatment characteristics, and laboratory results were all retrieved from the hospital electronic medical records. Diagnosis of STEMI was established in accordance with the published guidelines, including a typical chest pain history, diagnostic electrocardiographic changes, and serial elevation of cardiac biomarkers [18]. The study protocol was approved by the local institutional ethics committee.
Primary PCI was performed on patients with symptoms ≤12 h in duration as well as in patients with symptoms lasting 12–24 h in duration if the symptoms persisted at the time of admission. Time to coronary reperfusion was defined as the time from symptom onset (usually chest pain or discomfort), recorded upon admission, to the restoration of thrombolysis in myocardial infarction (TIMI) grade 3 flow in the infarct artery, as reported in the catheterization laboratory report. Following coronary interventional procedures, physiologic (0.9 %) saline was given intravenously at a rate of 1 ml/kg/h for 12 h after contrast exposure. In patients with overt heart failure, the hydration rate was reduced at the discretion of the attending physician. The contrast medium used in procedures was iodixanol (Visipaque, GE healthcare, Ireland) or iohexol (Omnipaque, GE healthcare, Ireland). The diagnosis of clinical heart failure during the acute and subacute phases of STEMI was based on typical symptoms, such as dyspnoea, signs, such as sinus tachycardia, a third heart sound or pulmonary rales, and some objective evidence of cardiac dysfunction, such as LV dilatation, and reduced ejection fraction. The serum creatinine level was determined upon hospital admission, prior to primary PCI, and at least once a day, during the CICU stay, and was available for all analyzed patients. The estimated glomerular filtration rate (eGFR) was estimated using the abbreviated Modification of Diet in Renal Disease equation (MDRD) [19]. AKI was determined using the KDIGO criteria [20], and defined as either a serum creatinine rise >0.3 mg/dl or more, or an increase in serum creatinine ≥1.5 times baseline or more within 7 days of hospital admission, compared with admission serum creatinine.
All patients underwent a screening echocardiographic examination within 3 days of admission. Echocardiography was performed by Philips IE-33, GE, or Vivid 3 models equipped with S5-1 transducers (Philips Healthcare, Andover, MA, USA). Left ventricular (LV) ejection fraction was calculated by the Biplane method. The 16-segment model was used for scoring the severity of segmental wall-motion abnormalities according to the American Society of Echocardiography [21].
Early transmitral flow velocity (E) and late atrial contraction (A) velocity were measured in the apical four-chamber view to provide an estimate of LV diastolic function [22]. The early diastolic mitral annular velocity (e′) was measured using spectral tissue Doppler imaging in both septal and lateral positions. The ratio of peak E to peak e′ (mitral E/e′ ratio) was calculated from the average of at least three cardiac cycles, using the average value of septal and lateral e′. Right atrial (RA) pressure (representing central venous pressure) was estimated by the inferior vena cava (IVC) diameter as well as its response to inspiration, as previously described [23]. Briefly, expiratory and inspiratory IVC diameters and percent collapse were measured in subcostal views within 2 cm of the RA. IVC diameter <2.1 cm that collapsed >50 % with a sniff suggested normal RA pressure (assigned as 5 mm Hg), whereas an IVC diameter >2.1 cm that collapsed <50 % with a sniff suggested high RA pressure (15 mm Hg). In patients with IVC diameter >2.1 cm and no collapse (<50 %) with a sniff, RA pressure was upgraded to 20 mm Hg. In indeterminate cases, in which the IVC diameter and collapse did not fit this paradigm, secondary indices of elevated RA pressure were integrated. If uncertainty remained, RA pressure was left as intermediate value of 10 mm Hg. Peak systolic pulmonary artery pressure (SPAP) was estimated using the modified Bernoulli formula (4 × TRVmax2) + RAP, where TRV max is the peak systolic tricuspid regurgitation velocity at end expiration, and RAP is the RA pressure [24]. Prior data have demonstrated that E/e′ ratio >15 was associated with adverse outcomes and LV remodeling after AMI [6, 7, 25]. For these reasons, the cohort was dichotomized on the basis of E/e′ value ≤ and above 15.
All data were summarized and displayed as mean ± standard deviation for continuous variables, as median and interquartile range for variables not equally distributed, and as number (percentage) of patients in each group for categorical variables. The p values for the categorical variables were calculated with the Chi-square test. Continuous variables were compared using the independent sample t test or the Mann–Whitney U test. A two-tailed p value of <0.05 was considered significant for all analyses. The influence of E/e′ ratio >15 on the risk for AKI was evaluated using multivariate logistic regression. We adjusted for age, gender, hypertension, diabetes mellitus, LV ejection fraction, eGFR, and E/e′ ratio >15. A cutoff of E/e′ > 13 as well as the use of E/e′ as a continuous variable were used for the sensitivity analysis. All analyses were performed with the SPSS software (SPSS Inc., Chicago, IL).
Results
A total of 804 patients were included in the study. Mean age was 61 ± 11 years and 657 (81 %) were males. Patients were divided into two groups according to their admission E/e′ ratio: group 1 (n = 703) with E/e′ ratio ≤15 and group 2 (n = 101) with E/e′ ratio >15. Baseline characteristics for each group are shown in Table 1. Patients with E/e′ ratio >15 were more likely to be older, of female gender, had more co-morbidities, longer time to culprit vessel reperfusion, higher Killip class, and higher admission C-reactive protein levels. Table 2 presents the key echocardiographic findings according to the E/e′ ratio < or above 15. Patients having E/e′ ratio >15 had lower LV ejection fraction (42 ± 8 % vs. 47 ± 7 %, p < 0.001) and higher SPAP (38 ± 10 vs. 29 ± 8 mm/hg, p < 0.001), and were more likely to have right atrial pressure >10 mm/Hg (25 vs. 13 %, p = 0.002) and >15 mm/Hg (12 vs. 6 %, p = 0.03). Patients having E/e′ ratio >15 had longer hospitalization, with more complications and higher 30-day mortality (Table 3).
E/e′ ratio and AKI
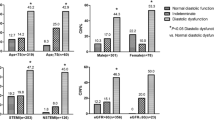
Patients having E/e′ ratio >15 were more likely to develop AKI complicating the course of STEMI (27 vs. 7 %; p < 0.001). In the univariate analysis, E/e′ ratio >15 was independently associated with AKI regardless of LV systolic function (Fig. 1). Patients having both LV ejection fraction <45 and E/e′ ratio >15 had an incidence of AKI fivefold higher compared with patients with LV >45 and E/e′ ratio <15 (30 vs. 6 %). In the multivariate logistic regression model, E/e′ ratio >15 was independently associated with AKI (OR = 1.87, 95 % CI 0.99–3.52; p = 0.05). Other variables associated with AKI included diabetes, LV ejection fraction, and eGFR (Table 4). Sensitivity analysis using a cutoff of E/e′ > 13 as well as the use of E/e′ as a continuous variable failed to demonstrate an independent association with AKI.
Discussion
In this cohort of STEMI patients undergoing primary PCI, echocardiography assessed correlates of elevated LV filling pressure were independently associated with AKI.
The occurrence of AKI, in STEMI patients undergoing primary PCI, has a complex and multifactorial pathogenesis which goes beyond the administration of contrast volume during catheterization and related to the acute cardiac pump failure resulting in reduced renal perfusion [10–16].
Acute (or type I) Cardio-Renal syndrome (CRS) described to date in patients with acutely decompensated heart failure is characterized by a rapid worsening of cardiac function, leading to AKI [26]. Renal dysfunction in CRS type I is attributable to a combination of low cardiac output, which consequently causes reduction in blood flow and renal perfusion pressure and/or venous congestion [26]. Recently published studies on heart failure patients explained the role of venous congestion on renal dysfunction and suggested central venous pressure and right atrial pressure rather than cardiac output as the main predictors of worsening renal function [27, 28]. Increased venous congestion causes an increase in renal interstitial pressure, which might lead to a hypoxic state of the renal parenchyma [29]. Increased oxidative stress and inflammation in the tubulointerstitium following venous congestion may also play a role in renal dysfunction [30]. In accordance with those findings, we previously demonstrated that STEMI patients developing AKI had both reduced LV systolic function [16] and elevated systolic pulmonary artery as well as right atrial (representing central venous) pressures [17].
Only limited data are present on the possible relation of echocardiographic parameters of diastolic dysfunction and renal impairment. E/e′ ratio >15 was found to be associated with renal impairment, manifested as both albuminuria and mild reductions in eGFR in hypertensive patients [8]. Similarly, E/e′ ratio >15 was also independently associated with progression to hemodialysis, graft failure, and overall mortality among following kidney transplantation [9]. In our cohort, E/e′ ratio >15 was associated with higher risk for AKI. The finding that patients with an E/e′ ratio >15 had both reduced LV ejection fraction and elevated right heart pressures may point to the importance of an acute LV diastolic dysfunction, resulting in elevated right heart and central venous pressures. This finding may support a plausible explanation for a pathomechanism, in which the worsening of renal function in STEMI patients is related to a combination of pump failure and reduced perfusion as well as venous congestion, thus fulfilling the criteria for type I CRS.
Our study bares some important clinical implications. As the treatment of AKI in STEMI patients following primary PCI is rather limited, the main strategy is the early identification of those at risk to develop this complication, and offers a better opportunity to apply appropriate preventive management. The addition of simple and early echocardiographic measurements (LV ejection fraction and E/e′ ratio >15) to other established clinical risk factors may be useful for the early identification of those at high risk for post-PCI AKI. In these high-risk patients, some novel biomarkers offer the opportunity to diagnose AKI proactively. Markers of tubular injury, such as urinary/plasma neutrophil gelatinase-associated lipocalin (NGAL), urinary kidney injury molecule-1 (KIM-1), and urinary IL-18 [31, 32], may help in the early detection of cellular injury in response to various renal toxins, including contrast induced nephropathy. This identification, 48–72 h prior to the actual loss of renal function (manifested as serum creatinine elevation), may allow the early interventions for renal protection and more frequent monitoring of urinary output.
We acknowledge several important limitations of our study. This was a single-center retrospective and non-randomized observational study, and may have been subject to bias, even though we included consecutive patients and attempted to adjust for confounding factors using the multivariate regression model. Patients having E/e′ ratio >15 were older, having more severe extent of coronary artery disease, longer time to culprit vessel reperfusion, heart failure, and higher admission C-reactive protein levels. In addition, they had larger infarct sizes. It is possible, thus, that the development of AKI in that group was not related to elevated E/e′ and may simply be reflective of higher degree of underlying pathology. As none of the patients in our study had the previous echocardiography with assessment of E/e′, it is possible that at least some of the patients who demonstrated the worst diastolic function post-STEMI (as indicated by the E/e′ ratio) had worse LV diastolic function prior to their infarct. Finally, as data regarding the concomitant use of Renin/Angiotensin blockers and diuretics throughout hospitalization were not present in many patients, their effect on AKI development, early left ventricular remodeling, as well as preload and afterload could not be assessed. Moreover, given the higher incidence of hypertension and heart failure in E/e′ ratio >15 group, it is conceivable that AKI group had higher percentage of patients on these medications.
Conclusions
Among STEMI patients undergoing primary PCI, an E/e′ ratio >15 was associated with increased risk for AKI.
References
Nijland F, Kamp O, Karreman AJ, van Eenige MJ, Visser CA (1997) Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: a serial Doppler echocardiographic study. J Am Coll Cardiol 30(7):1618–1624
Moller JE, Sondergaard E, Seward JB, Appleton CP, Egstrup K (2000) Ratio of left ventricular peak E-wave velocity to flow propagation velocity assessed by color M-mode Doppler echocardiography in first myocardial infarction: prognostic and clinical implications. J Am Coll Cardiol 35(2):363–370
Moller JE, Sondergaard E, Poulsen SH, Egstrup K (2000) Pseudonormal and restrictive filling patterns predict left ventricular dilation and cardiac death after a first myocardial infarction: a serial color M-mode Doppler echocardiographic study. J Am Coll Cardiol 36(6):1841–1846
Cerisano G, Bolognese L, Carrabba N, Buonamici P, Santoro GM, Antoniucci D, Santini A, Moschi G, Fazzini PF (1999) Doppler-derived mitral deceleration time: an early strong predictor of left ventricular remodeling after reperfused anterior acute myocardial infarction. Circulation 99(2):230–236
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794
Hillis GS, Moller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, Reeder GS, Oh JK (2004) Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 43(3):360–367. doi:10.1016/j.jacc.2003.07.044
Iwahashi N, Kimura K, Kosuge M, Tsukahara K, Hibi K, Ebina T, Saito M, Umemura S (2012) E/e′ two weeks after onset is a powerful predictor of cardiac death and heart failure in patients with a first-time ST elevation acute myocardial infarction. J Am Soc Echocardiogr 25(12):1290–1298. doi:10.1016/j.echo.2012.09.010
Yang Y, Wang Y, Shi ZW, Zhu DL, Gao PJ (2013) Association of E/E′ and NT-proBNP with renal function in patients with essential hypertension. PLoS One 8(1):e54513. doi:10.1371/journal.pone.0054513
Bang JY, Lee JB, Sang BH, Hoon Kim Y, Han DJ, Song JG, Hwang GS (2016) High left ventricular filling pressure on Doppler echocardiography is associated with graft failure and overall mortality following kidney transplantation. J Cardiothorac Vasc Anesth 30(3):585–591. doi:10.1053/j.jvca.2015.10.006
Shacham Y, Leshem-Rubinow E, Steinvil A, Assa EB, Keren G, Roth A, Arbel Y (2014) Renal impairment according to acute kidney injury network criteria among ST elevation myocardial infarction patients undergoing primary percutaneous intervention: a retrospective observational study. Clin Res Cardiol 103(7):525–532. doi:10.1007/s00392-014-0680-8
Marenzi G, Assanelli E, Campodonico J, De Metrio M, Lauri G, Marana I, Moltrasio M, Rubino M, Veglia F, Montorsi P, Bartorelli AL (2010) Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med 38(2):438–444. doi:10.1097/CCM.0b013e3181b9eb3b
Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Markiewicz W, Aronson D (2005) Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J 150(2):330–337. doi:10.1016/j.ahj.2004.09.055
Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM (2008) Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 168(9):987–995. doi:10.1001/archinte.168.9.987
Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, Masoudi FA (2010) The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J 160(6):1065–1071. doi:10.1016/j.ahj.2010.08.007
Shacham Y, Leshem-Rubinow E, Gal-Oz A, Arbel Y, Keren G, Roth A, Steinvil A (2015) Acute cardio-renal syndrome as a cause for renal deterioration among myocardial infarction patients treated with primary percutaneous intervention. Can J Cardiol 31(10):1240–1244. doi:10.1016/j.cjca.2015.03.031
Shacham Y, Leshem-Rubinow E, Gal-Oz A, Topilsky Y, Steinvil A, Keren G, Roth A, Arbel Y (2015) Association of left ventricular function and acute kidney injury among ST-elevation myocardial infarction patients treated by primary percutaneous intervention. Am J Cardiol 115(3):293–297. doi:10.1016/j.amjcard.2014.11.002
Shacham Y, Gal-Oz A, Topilsky Y, Keren G, Arbel Y (2016) Relation of pulmonary artery pressure and renal impairment in ST segment elevation myocardial infarction patients. Echocardiography. doi:10.1111/echo.13206
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61(4):e78–e140. doi:10.1016/j.jacc.2012.11.019
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130(6):461–470
Acute Kidney Injury Work Group (2012) Kidney disease: improving global outcomes (KDIGO)—clinical practice guideline for acute kidney injury. Kidney Inter 2:1–138
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G, American Society of Echocardiography’s G, Standards C, European Association of E (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133. doi:10.1016/j.echo.2008.11.023
Kircher BJ, Himelman RB, Schiller NB (1990) Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol 66(4):493–496
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. doi:10.1016/j.echo.2010.05.010 (quiz 786–688)
Alam M, Wardell J, Andersson E, Samad BA, Nordlander R (2000) Effects of first myocardial infarction on left ventricular systolic and diastolic function with the use of mitral annular velocity determined by pulsed wave doppler tissue imaging. J Am Soc Echocardiogr 13(5):343–352
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R (2008) Cardiorenal syndrome. J Am Coll Cardiol 52(19):1527–1539. doi:10.1016/j.jacc.2008.07.051
Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA (2008) Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51(13):1268–1274. doi:10.1016/j.jacc.2007.08.072
Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, Shannon RP, Kirkpatrick JN (2010) Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol 105(4):511–516. doi:10.1016/j.amjcard.2009.10.020
Hamza SM, Kaufman S (2007) Effect of mesenteric vascular congestion on reflex control of renal blood flow. Am J Physiol Regul Integr Comp Physiol 293(5):R1917–R1922. doi:10.1152/ajpregu.00180.2007
Tanaka M, Yoshida H, Furuhashi M, Togashi N, Koyama M, Yamamoto S, Yamashita T, Okazaki Y, Ishimura S, Ota H, Hasegawa T, Miura T (2011) Deterioration of renal function by chronic heart failure is associated with congestion and oxidative stress in the tubulointerstitium. Intern Med 50(23):2877–2887
Coca SG, Yalavarthy R, Concato J, Parikh CR (2008) Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 73(9):1008–1016. doi:10.1038/sj.ki.5002729
Koyner JL, Parikh CR (2013) Clinical utility of biomarkers of AKI in cardiac surgery and critical illness. Clin J Am Soc Nephrol 8(6):1034–1042. doi:10.2215/CJN.05150512
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Rights and permissions
About this article
Cite this article
Flint, N., Kaufman, N., Gal-Oz, A. et al. Echocardiographic correlates of left ventricular filling pressures and acute cardio-renal syndrome in ST segment elevation myocardial infarction patients. Clin Res Cardiol 106, 120–126 (2017). https://doi.org/10.1007/s00392-016-1031-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1031-8