Abstract
Background
Transvascular (TV-AVI) or transapical (TA-AVI) aortic valve implantation (TAVI) is a treatment option for patients with aortic stenosis being at high or prohibitive risk for surgical aortic valve implantation (SAVR). Randomized data demonstrated that these subgroups can safely been treated with TAVI. However, a comparison of SAVR and TAVI in intermediate and low-risk patients is missing. Therefore, the aim of the analysis was to compare TAVI and SAVR in all patients who were treated for aortic valve stenosis in Germany throughout 1 year.
Methods
The mandatory quality assurance collects data on the in-hospital outcome from all patients (n = 20,340) undergoing either SAVR or TAVI in Germany. In order to compare the different treatment approaches patients were categorized into four risk groups using the logistic EuroScore I (ES). In-hospital mortality and peri- and postprocedural complications were analyzed.
Results
The in-hospital mortality did not differ between TV-AVI and SAVR in the low risk group (ES <10 %: TV-AVI 2.4 %, SAVR 2.0 %, p = 0.302) and was significantly higher for SAVR in all other risk groups (ES 10–20 %: TV-AVI 3.5 %, SAVR 5.3 %; p = 0.025; ES 20–30 %: TV-AVI 5.5 %, SAVR 12.2 %, p < 0.001; ES >30 %: TV-AVI 6.5 %, SAVR 12.9 %, p = 0.008). TA-AVI had a significantly higher mortality in all risk groups compared to TV-AVI. In comparison to SAVR, TA-AVI had a higher mortality in patients with ES <10, comparable mortality in ES 10–20 %, and lower mortality in patients with an ES >20 %. The overall stroke rate was 2.3 %. It occurred more frequently in patients with an ES <10 % treated with a transapical approach (SAVR 1.8 %, TV-AVI 1.9 %, TA-AVI 3.1 %, p < 0.01). There were no statistically significant differences in all other comparisons.
Conclusions
This study demonstrates that TAVI provides excellent outcomes in all risk categories. Compared with SAVR, TV-TAVI yields similar in-hospital mortality among low-risk patients and lower in-hospital mortality among intermediate and high-risk patient populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aortic valve stenosis is one of the most prevalent acquired valve diseases. It most often becomes relevant in elderly patients and is associated with an adverse prognosis after the onset of clinical symptoms, with a mortality of 50 % if left untreated [1]. Currently, the standard treatment is surgical aortic valve replacement (SAVR) given the absence of medical treatment options [2, 3]. Approximately one-third of all patients presenting with aortic valve stenosis, however, have not been treated in the past due to the inherent risk profile of this elderly patient population with its typical comorbidities [4]. Transcatheter aortic valve implantation (TAVI) has evolved as a viable treatment option for these patients in the past decade. TAVI can be performed using different access options. The transapical access (TA-AVI) is an antegrade approach whereas the transvascular routes (TV-AVI) via the femoral or subclavian artery or the direct aortic access are retrograde approaches [5–7]. TAVI has been proven to be superior to medical therapy or balloon valvuloplasty in a patient cohort deemed inoperable [8] and was shown to be equivalent to surgical aortic valve replacement in a ‘high surgical risk’ cohort [9]. These results have been confirmed by large-scale registries [10–12]. Furthermore, TAVI has been demonstrated to be a good treatment option for degenerated surgical valve prostheses [13–15].
The superiority of TAVI over SAVR was recently demonstrated in a randomized clinical trial with a cohort of 795 ‘high surgical risk’ patients [16]. It remains unclear, however, how these results can be translated into daily clinical practice, firstly due to the still relative low number and secondly in light of the fact that these patients have been highly selected. More importantly, comparative data between the two treatment approaches are completely lacking for low- and intermediate-risk patients.
TAVI was approved in Germany in 2006 and was rapidly adopted into clinical routine. 2013 was the first year in which more TAVI procedures were performed than SAVR procedures (without concomitant coronary artery bypass surgery). All centers are obliged by law to report the in-hospital outcome as well as several other parameters to an independent institute for quality assurance (AQUA-Institut, Göttingen, Germany).
The aim of the present analysis was to provide a comparison of TA-AVI and TV-AVI with SAVR in a complete dataset of all patients treated for aortic valve stenosis in Germany throughout 2013.
Methods
Data collection
All centers performing and invoicing for cardiac surgery or TAVI in Germany are obliged to report clinical, peri-procedural, and in-hospital outcome data to an independent quality assurance organization (AQUA Institute, Göttingen, Germany) according to German law (§108 and 137 SGB). The dataset consists of 125 parameters including baseline patients’ characteristics and peri- and postprocedural details. The submission of these data is mandatory for reimbursement. This dataset forms the basis of the present analysis and comprises virtually all procedures performed during the year 2013. Altogether, all 20,340 cases of isolated aortic valve procedures (without concomitant bypass surgery) were evaluated, of which 20,197 (99.3 %) were complete with regard to all variables necessary to calculate the ES.
The data are quality controlled on different levels, including on-site visits and structured interviews of the institutions. Furthermore, the underlying control mechanisms include testing for plausibility, completeness, concordance, and accuracy using a well-validated system. The data validation procedures are documented in the yearly publication of the Federal Joint Committee of Germany (‘Gemeinsamer Bundesausschuss G-BA’).
Patient groups
In order to allow comparisons between the different treatment approaches patients were categorized into four risk groups using the logistic EuroScore I (ES). The ES consists of 18 variables including age, sex, and different general risk factors as well as cardiac risk factors [17, 18]. This scoring system was originally generated to predict the outcome after aorto-coronary bypass surgery but proved to be a good discriminator for general risk assessment.
Comparisons were made between the following four risk groups: ES <10, 10–20, 20–30, and >30 %. For comparison of the in-hospital outcome, the lowest-risk group (ES <10 %) was further restricted to the lowest matching ES values; therefore, in the ES <10 % group mortality between SAVR and TV-AVI was evaluated in patients with an ES from 1.505 to 10 % (SAVR n = 6710, TV-AVI n = 1368), whereas comparisons with TA-AVI was restricted to patients with an ES from 2.083 to 10 % (SAVR n = 5909; TV-AVI n = 1397, TA-AVI n = 423).
Additional analyses were performed with a subgroup of SAVR patients, excluding emergency cases. Urgent cases—for example, aortic valve endocarditis—are usually handled by SAVR and are typically associated with a worse outcome that may therefore influence the overall results.
Statistical analysis
The statistical analysis was performed by the AQUA Institute using SPSS for Windows Version 21.0. Comparisons between the three treatment approaches were performed pairwise (SAVR vs. TA-AVI, SAVR vs. TV-AVI, TA-AVI vs. TV-AVI). For continuous variables Student’s t test was used. Categorical variables were compared with either Fisher’s exact test or the Chi square test with Yates correction, as appropriate. No adjustment for multiplicity was performed.
Results are presented for continuous variables as mean ± standard deviation. For categorical variables the frequency share is given in percent. A p value <0.05 was considered statistically significant.
Results
Patients and baseline characteristics
Between January 1 and December 31, 2013, a total of 20,340 patients underwent isolated aortic valve interventions in Germany. More than half of all patients (10,441; 51 %) were treated with a transcatheter approach (TV-AVI, 7620 patients; TA-AVI, 2821 patients). The baseline characteristics of all groups examined are given in Table 1. There were significant differences in all major baseline criteria between the three groups, with the SAVR group having the lowest rates of risk factors.
The reasons for performing a transcatheter approach in the formally low- and intermediate-risk groups (ES <20 %) included patients’ age (71.4 %), frailty (49.4 %), patients’ wish (28.5 %), prognosis-limiting comorbidities (10.2 %), and porcelain aorta (5.8 %).
The mean duration of the hospital stay after the index procedure was 10.7 ± 8.2 days after TV-AVI, 12.4 ± 8.7 days after TA-AVI, and 12.1 ± 8.4 days after SAVR (TV-AVI vs. SAVR and TA-AVI p < 0.01).
In-hospital mortality
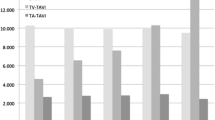
The in-hospital mortality across all risk categories was 3.0 % in SAVR, 4.7 % in TV-AVI, and 8.3 % in TA-AVI (p < 0.01 for all comparisons). After categorization there were obvious differences in the in-hospital mortality between the three treatment approaches (Fig. 1). The in-hospital mortality did not differ significantly between SAVR and TV-AVI in the lowest risk group with ES 1.505–10 % (SAVR 2.0 %, TV-AVI 2.4 %; p = 0.302). This result holds true when emergency cases were excluded (SAVR 1.9 %, TV-AVI 2.4 %; p = 0.251). However, the mortality was significantly higher for SAVR in comparison to TV-AVI in all other risk groups (ES 10–20 %: TV-AVI 3.5 %, SAVR 5.3 %; p = 0.025; ES 20–30 %: TV-AVI 5.5 %, SAVR 12.2 %, p < 0.001; ES >30 %: TV-AVI 6.5 %, SAVR 12.9 %, p = 0.008).
In-hospital mortality of patients undergoing aortic valve procedures with either surgical aortic valve replacement (SAVR, blue line); transapical aortic valve implantation (TA-AVI, red line); transvascular aortic valve implantation (green line). Patients were categorized according to the logistic EuroScore I. Statistical significance (p < 0.05) between groups are marked with asterisk for TV-AVI vs. SAVR; hash symbol for TV-AVI vs. TA-AVI; section sign for TA-AVI vs. SAVR
A comparison of TA-AVI with SAVR reveals a significantly higher mortality for the transcatheter approach in the lowest risk group with ES between 2.083 and 10 % (SAVR 2.1 %, TA-AVI 5.9 %; p < 0.001). There was no difference in mortality in those with ES 10–20 % (5.9 % for both approaches; p = 1.0) and a significantly better in-hospital outcome for TA-AVI compared with SAVR in the risk groups ES 20–30 % and ES >30 % (p = 0.001 and p = 0.002, respectively). When emergency cases were excluded, the benefit of TA-AVI vs. SAVR in the higher risk groups is no longer statistically significant (ES 20–30 %: SAVR 12.2 %, TA-AVI 7.9 %; p = 0.07 and ES >30 %: SAVR 12.9 %, TA-AVI 11.5 %; p = 0.670).
The in-hospital mortality was significantly higher for all risk groups in patients treated with TA-AVI in comparison to TV-AVI.
Intra-procedural complications
Transcatheter aortic valve implantation is associated with an inherent risk for several severe complications, including coronary obstruction, aortic dissection, annular rupture, pericardial tamponade, device embolization, and vascular complications as detailed in Fig. 2. There was a significantly higher rate of vascular complications in patients who underwent TV-AVI in comparison to TA-AVI (5.8 vs. 0.5 %, p < 0.01). Likewise, pericardial tamponade occurred more often in TV-AVI than in TA-AVI (1.2 vs. 0.3 %, p < 0.01). Other complications occurred with very low frequency (<0.5 %) in all approaches.
Post-procedural complications
Post-operative low cardiac output, requiring medical therapy, intra-aortic balloon pumping, or another mechanical assist device, were more frequent in both SAVR and TA-AVI in comparison to TV-AVI across all risk groups (p < 0.01). Low cardiac output did not differ between TA-AVI and SAVR in the ES <10 % group, but the incidence was higher in SAVR patients with an ES >10 % (p < 0.01; Fig. 3a).
Comparison of periprocedural complications between patients undergoing aortic valve procedures with either surgical aortic valve replacement (SAVR, blue line); transapical aortic valve implantation (TA-AVI, red line); transvascular aortic valve implantation (green line). Patients were categorized according to the logistic EuroScore I. Statistical significance (p < 0.05) between groups are marked with asterisk for TV-AVI vs. SAVR; hash symbol for TV-AVI vs. TA-AVI; section sign for TA-AVI vs. SAVR. a Occurrence of low cardiac output requiring intervention. b Need for revision due to bleeding, hematoma, low cardiac output, pericardial tamponade, myocardial ischemia, infection, aortic dissection, prosthesis dysfunction, instable sternum, chylothorax, local revision at site of access. c Occurrence of postoperative delirium. d Intra-hospital occurrence of stroke
The need for re-intervention after the index procedure was lowest in the TV-AVI group (p < 0.01). While there was no difference between TA-AVI and SAVR in the ES <10 % group, re-interventions occurred significantly more often in SAVR in all groups with an ES >10 % (Fig. 3b).
Postoperative delirium occurred significantly more often in both SAVR and TA-AVI compared with TV-AVI (p < 0.01). It was nearly doubled in the lowest risk group with an ES <10 % (SAVR 10.8 %, TA-AVI 11.1 %, TV-AVI 6.5 %, p < 0.01). This difference becomes even more obvious in the higher risk groups, with the highest incidence occurring in SAVR followed by TA-AVI (p < 0.01; Fig. 3c).
The overall stroke rate during hospitalization was 2.3 %. It occurred more frequently in patients with an ES <10 % who were treated with a transapical approach (SAVR 1.8 %, TV-AVI 1.9 %, TA-AVI 3.1 %, p < 0.01). There were no statistically significant differences in all other comparisons (Fig. 3d).
Discussion
The present report evaluates the in-hospital outcome of all patients who underwent isolated SAVR or TAVI for aortic valve stenosis in Germany during the year 2013. The main findings of this analysis of 20,340 patients are as follows: First, there is no difference in the in-hospital mortality in low-risk patients (ES <10 %) between SAVR and TV-AVI procedures. In all higher risk groups, TV-AVI is associated with a significantly lower mortality than SAVR. Second, TA-AVI has a significantly higher mortality than TV-AVI in all risk groups. Third, the rate of severe intra-procedural complications was highest in TV-AVI mainly due to vascular complications. Fourth, post-procedural complications such as low cardiac output, the need for a re-intervention or postoperative delirium occurred significantly more often in SAVR than in TV-AVI, especially in higher risk groups. Fifth, the overall rate of stroke was acceptably low and there were no marked differences between SAVR, TV-AVI, and TA-AVI in the different risk groups.
This analysis provides for the first time an up-to-date and complete dataset on a substantial number of patients treated for aortic valve stenosis beyond clinical studies. Given the mandatory character of the quality assurance, the profound experience with this system, and the well-developed control mechanisms, the assumption appears justified that the data presented are robust and therefore comparable to that of controlled clinical trials. Beyond most other registries, this dataset also includes complete information on surgical procedures.
The risk stratification was performed using the logistic EuroScore I. This score was originally developed more than a decade ago mainly for patients undergoing coronary artery bypass surgery [17, 18], and it has repeatedly and reproducibly been shown to overestimate the real risk of patients undergoing both SAVR or TAVI [19, 20]. Nevertheless it allows accurate risk stratification and is therefore well suited for the categorization of patients [21]. Moreover, the ES is still one of the accepted criteria used in decision-making for SAVR vs. TAVI in the guidelines on valvular heart disease [2].
The 2012 European guidelines and the 2014 AHA/ACC guidelines recommend SAVR as the standard of care for treatment of severe aortic stenosis (class IA); transcatheter approaches are recommended only in patients with a prohibitive risk for SAVR (class IB) and may only be considered in patients with either a high risk score or severe comorbidities (class IIaB) [2, 3]. Our analysis demonstrates that more than 50 % of all patients treated with TAVI did not meet the formal risk score criteria. Comorbidities, anatomical impediments, and patient frailty, which do not influence ES but were considerably more frequent and severe in TAVI than in SAVR patients, probably triggered the selection of TAVI by the heart teams. In other words, the actual risk of TAVI patients in the “lower risk groups” may well be underestimated by the ES.
Nonetheless, our analysis revealed that in-hospital mortality in the lowest risk group (ES <10 %) was equal between SAVR and TV-AVI and that TV-AVI, with significantly lower in-hospital mortality, was superior in all other risk groups. This finding clearly challenges the current guideline recommendations and calls for controlled studies in lower risk groups. The rate of intra-procedural complications, which occurred more often in TV-AVI must be considered but has no influence on mortality [22, 23]. In addition, the incidence of major vascular complications has declined over the past few years from 16.2 % in PARTNER B and 11.0 % in PARTNER A to 5.8 % in our analysis [8, 9]. This value matches the recently published result (5.9 %) of the CoreValve High Risk Study [16]. Most vascular complications can be treated immediately using percutaneous methods.
Not unexpectedly, the rate of post-operative delirium was much higher after SAVR compared with TV-AVI, most probably due to the mandatory use of general anesthesia and cardiopulmonary bypass in SAVR. Post-operative delirium has may contribute to the longer hospital stay that was observed in our study and has been linked to later cognitive impairment and increased morbidity and mortality [24–26].
The risk of stroke due to manipulation in the aorta has long been a major concern in TAVI [27]. Indeed, the overall rate was as high as 2.3 % in our study but not different between the treatment approaches. Probably, the risk associated with the large-bore devices and wires in the aorta is outbalanced by the inherent risk of cardiopulmonary bypass during SAVR.
A major finding of our study was the higher mortality of TA-AVI compared with TV-AVI across all risk groups. This may be explained in part by the higher invasiveness of the transapical approach. However, this result may not necessarily be interpreted as an inferiority of TA-AVI given the fact that the majority of centers consider TV-AVI as the method of first choice and use TA-AVI only if TV-AVI is technically not possible. In these patients, TA-AVI remains a viable if not better alternative to SAVR in the higher risk groups.
Our study has a number of limitations. First, the data are not 100 % controlled, and this may influence the frequency of complications since they were not independently adjudicated. The well-established control mechanisms of the statutory quality assurance, however, minimize this bias. Furthermore, the focused endpoint of our study is mortality, which is not questionable by nature. Second, we report only the in-hospital results. Recently published randomized trials suggest that mortality after discharge from hospital is similar if not higher in SAVR compared with TAVI [9, 16]. Nevertheless, our study cannot provide data from a longer follow-up period.
In conclusion, our study demonstrates excellent outcome of TV-AVI for high-risk patients with aortic stenosis. TV-AVI performs similar as SVAR even in lower-ES patients with lower ES and outperforms SAVR in patients with high ES.
References
Turina J, Hess O, Sepulcri F, Krayenbuehl HP (1987) Spontaneous course of aortic valve disease. Eur Heart J 8(5):471–483
Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology, European Association for Cardio-Thoracic Surgery, Vahanian A et al (2012) Guidelines on the management of valvular heart disease (version 2012). European Heart Journal. 33(19):2451–2496
Nishimura RA, Otto CM, Bonow RO et al (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63(22):e57–185
Iung B, Cachier A, Baron G et al (2005) Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 26(24):2714–2720
Walther T, Mollmann H, van Linden A, Kempfert J (2011) Transcatheter aortic valve implantation transapical: step by step. Semin Thorac Cardiovasc Surg 23(1):55–61
Webb JG, Chandavimol M, Thompson CR et al (2006) Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 113(6):842–850
Cribier A, Eltchaninoff H, Bash A et al (2002) Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106(24):3006–3008
Leon MB, Smith CR, Mack M et al (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363(17):1597–1607
Smith CR, Leon MB, Mack MJ et al (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364(23):2187–2198
Hamm CW, Mollmann H, Holzhey D et al (2014) The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J 35(24):1588–1598
Mohr FW, Holzhey D, Mollmann H et al (2014) The German Aortic Valve Registry: 1-year results from 13 680 patients with aortic valve diseasedagger. Eur J Cardiothoracc Surg 46(5):808–816
Gilard M, Eltchaninoff H, Iung B et al (2012) Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 366(18):1705–1715
Milburn K, Bapat V, Thomas M (2014) Valve-in-valve implantations: is this the new standard for degenerated bioprostheses? Review of the literature. Clin Res Cardiol 103(6):417–429
Kim WK, Kempfert J, Walther T, Mollmann H (2015) Transfemoral valve-in-valve implantation of a St. Jude Medical Portico in a failing trifecta bioprosthesis: a case report. Clin Res Cardiol 104(4):363–365
Greif M, Lange P, Mair H et al (2012) Transcatheter Edwards Sapien XT valve in valve implantation in degenerated aortic bioprostheses via transfemoral access. Clin Res Cardiol 101(12):993–1001
Adams DH, Popma JJ, Reardon MJ et al (2014) Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 370(19):1790–1798
Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R (1999) European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 16(1):9–13
Roques F, Nashef SA, Michel P et al (1999) Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 15(6):816–822 (discussion 822–813)
Osswald BR, Gegouskov V, Badowski-Zyla D et al (2009) Overestimation of aortic valve replacement risk by EuroSCORE: implications for percutaneous valve replacement. Eur Heart J 30(1):74–80
Wendt D, Osswald BR, Kayser K et al (2009) Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 88(2):468–474
Rosenhek R, Iung B, Tornos P et al (2012) ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J 33(7):822–828, 828a, 828b
Kim W-K, Liebetrau C, van Linden A et al (2015) Myocardial injury associated with transcatheter aortic valve implantation (TAVI). Clin Res Cardiol. doi:10.1007/s00392-015-0949-6
Walther T, Hamm CW, Schuler G et al (2015) Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the GARY registry. J Am Coll Cardiol 65(20):2173–2180
Saczynski JS, Marcantonio ER, Quach L et al (2012) Cognitive trajectories after postoperative delirium. N Engl J Med 367(1):30–39
Koster S, Hensens AG, van der Palen J (2009) The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg 87(5):1469–1474
Sockalingam S, Parekh N, Bogoch II et al (2005) Delirium in the postoperative cardiac patient: a review. J Card Surg 20(6):560–567
Eggebrecht H, Schmermund A, Voigtlander T, Kahlert P, Erbel R, Mehta RH (2012) Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 8(1):129–138
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Möllmann and K. Bestehorn contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Möllmann, H., Bestehorn, K., Bestehorn, M. et al. In-hospital outcome of transcatheter vs. surgical aortic valve replacement in patients with aortic valve stenosis: complete dataset of patients treated in 2013 in Germany. Clin Res Cardiol 105, 553–559 (2016). https://doi.org/10.1007/s00392-016-0962-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-0962-4