Abstract
Renal denervation can reduce blood pressure in patients with uncontrolled hypertension. The adherence to prescribed antihypertensive medication following renal denervation is unknown. This study investigated adherence to prescribed antihypertensive treatment by liquid chromatography–high resolution tandem mass spectrometry in plasma and urine at baseline and 6 months after renal denervation in 100 patients with resistant hypertension, defined as baseline office systolic blood pressure ≥140 mmHg despite treatment with ≥3 antihypertensive agents. At baseline, complete adherence to all prescribed antihypertensive agents was observed in 52 patients, 46 patients were partially adherent, and two patients were completely non-adherent. Baseline office blood pressure was 167/88 ± 19/16 mmHg with a corresponding 24-h blood pressure of 154/86 ± 15/13 mmHg. Renal denervation significantly reduced office and ambulatory blood pressure at 6-month follow-up by 15/5 mmHg (p < 0.001/p < 0.001) and 8/4 mmHg (p < 0.001/p = 0.001), respectively. Mean adherence to prescribed treatment was significantly reduced from 85.0 % at baseline to 80.7 %, 6 months after renal denervation (p = 0.005). The blood pressure decrease was not explained by improvements in adherence following the procedure. Patients not responding to treatment significantly reduced their drug intake following the procedure. Adherence was highest for angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and beta blockers (>90 %) and lowest for vasodilators (21 %). In conclusion, renal denervation can reduce office and ambulatory blood pressure in patients with resistant hypertension despite a significant reduction in adherence to antihypertensive treatment after 6 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately, 8–18 % of all patients with high blood pressure (BP) are apparently resistant to drug treatment [1, 2]. Poor concordance with treatment is a major challenge clinicians often face in treating hypertension and non-adherence is commonly observed especially in uncontrolled hypertensive patients [3, 4]. Non-adherence to prescribed guideline-recommended medication is associated with stroke, myocardial infarction, and cardiovascular death [5]. Catheter-based renal denervation (RDN) has emerged as a new treatment option for patients with uncontrolled hypertension [6]. The clinical evidence in support of RDN as an effective interventional technique in patients with resistant hypertension is heterogeneous. A number of observational studies and three randomized, controlled trials documented significant changes in office and ambulatory BP after RDN [7–12] but some smaller studies and the single-blind, randomized, sham-controlled Symplicity HTN-3 trial did not show superiority of RDN when compared to medical therapy alone [13, 14]. It has been postulated that behavioral changes may cause or contribute to observed effects following RDN. Specifically, changes of medication adherence and the Hawthorne effect could be of major importance in patients included in a clinical trial [15]. In the conduct of earlier trials in RDN therapy, it has been questioned whether the population was truly resistant to medical therapy or merely non-adherent to antihypertensive drugs, with the caveat that adherence has neither been investigated systematically before nor following RDN. There are multiple ways of assessing drug adherence in patients but only few of them are accurate and reliable [16–18]. Urine and plasma liquid chromatography–high resolution tandem mass spectrometry (LC–HR-MS/MS) represents one of the most accurate and objective measurement of drug intake [17, 19]. The present study aimed to systematically determine changes of the individual intake of antihypertensive drugs using a robust detection method in patients with resistant hypertension undergoing RDN.
Methods
Studied patients
The study included a total of 100 consecutive patients undergoing bilateral RDN, aged ≥18 years, with resistant hypertension according to the European Society of Hypertension/European Society of Cardiology guidelines (office SBP ≥140 mmHg despite treatment with ≥3 antihypertensive drugs of different classes, including a diuretic at maximum or highest tolerated dose) and completed 6-month follow-up [1]. The analyses were performed as part of a prospective study aimed to document the long-term safety and effectiveness of RDN (NCT01888315). None of the patients included in the current study was part of the Symplicity HTN-1 or HTN-2 trials. Only patients with stable antihypertensive drug regimen were included in the study and patients with secondary, treatable causes of hypertension were excluded [20]. Stable antihypertensive drug regime was defined as no change in antihypertensive medication for at least 4 weeks prior to the baseline examination. Patients and physicians were instructed not to change antihypertensive medication during the study period except when medically required. Written informed consent was obtained from all participating patients when they entered the study. Patients were informed about the possibility of adherence measurements by urine/plasma toxicological analysis but were unaware of the timing. All patients had at least 5 contacts to the study center. The LC–HR-MS/MS analyses were processed blindly with regards to patient characteristics and timing of blood/urine sampling. The results of LC–HR-MS/MS analysis were not used as an inclusion criterion to ensure drug intake. The study was approved by the local ethic committee in accordance with the Declaration of Helsinki.
Assessment of adherence
All patients were interviewed whether they have taken their current medication as prescribed at each follow-up visit. After a positive declaration, plasma and urine samples were obtained at baseline and at 6-month follow-up. The baseline visit was 1 day prior to the RDN procedure. All visits were scheduled at 8.00 am. Adherence was defined as 100 % confirmation of all prescribed antihypertensive drugs by LC–HR-MS/MS analysis. Patients in whom the analysis confirmed the presence of less medication than prescribed (antihypertensive drug intake detected <100 %) were classified as non-adherent.
Drug screening by liquid chromatography–high resolution tandem mass spectrometry
Venous blood and urinary samples were collected at baseline and 6 months after RDN by specially trained technicians. Samples were frozen at −80 °C until analysis. For sample workup, precipitation of 100 µl urine with 500 µl acetonitrile according to Wissenbach et al. [21] and of 250 µl plasma with 750 µl ZnSO4 solution (35 mg/ml water:methanol, 70:30) was performed. The analytes were separated using a ThermoFisher (TF, Dreieich, Germany) Accucore PhenylHexyl column and gradient elution with 2 mM aqueous ammoniums formate plus 0.1 % formic acid (pH 3, eluent, A) and ammonium formate solution with acetonitrile/methanol (50:50, V:V, 1 % water) plus 0.1 % formic acid. The drugs and/or metabolites were detected by a TF Q-Exactive high resolution (HR) mass spectrometer with a HESI-II source with pos/neg switching and targeted HR-MS/MS on 108 predefined protonated ions (inclusion list) of the unchanged parent compounds and their metabolites. Data files were processed using TF TraceFinder software 3.2 based on precursor accurate masses, isotopic patterns, five most intense fragment ions, and reference library spectrum. The LC–HR-MS/MS analyses were performed blindly, i.e., without knowledge about specific patient characteristics. The results were reported qualitatively whether the prescribed drug was found in urine and/or plasma (Online supplement Fig. 1) [19, 22, 23].
Renal sympathetic denervation procedure
The RDN procedure was performed bilaterally via femoral access with a dedicated radiofrequency catheter (Symplicity™ Flex Catheter System, Ardian/Medtronic Inc., California, USA), inserted percutaneously as described elsewhere [20].
Follow-up and assessment of blood pressure
All patients underwent a 6-month follow-up visit including full history and physical examination, office and ambulatory BP measurements, and blood and urine chemistry. Office BP was measured in the morning (1 h after medication intake) in a sitting position after resting for at least 5 min at each arm. The arm with the higher BP was used for all subsequent readings [1]. Averages of triplicate measures were calculated and used for subsequent analysis. Twenty-four-hour ABPM (Mobil-O-Graph™, Medispec Deutschland GmbH, Krefeld, Germany) was performed before RDN in all patients to exclude pseudo-resistance and at 6 months. Readings were taken every 15 min during daytime (7 AM to 10 PM) and every 30 min at nighttime (10 PM to 7 AM). Patients were assessed while adhering to their usual activity and nocturnal sleep routine. Only patients with >70 % valid BP measurements of either awake or asleep were included [24]. Mean SBP and DBP were calculated as overall 24-h average. At 6-month follow-up, patients were classified based on their office and ambulatory BP into responder (office SBP reduction ≥10 mmHg or ambulatory SBP reduction ≥5 mmHg) and non-responders (office SBP reduction <10 mmHg or ambulatory SBP reduction <5 mmHg) [12].
Statistical analysis
Data are presented as mean ± standard deviation unless otherwise specified. Categorical variables are presented by absolute and relative frequencies. Statistical comparisons between groups were performed using the Pearson Chi square test for categorical variables and paired and unpaired t test or Wilcoxon rank sum test or one-way ANOVA for continuous variables where appropriate. Significance tests were two-tailed with p < 0.05 considered significant. All statistical analyses were calculated using the SPSS statistical software (version 20.0, SPSS Inc., Chicago, IL, USA).
Results
Baseline patients’ characteristics are depicted in Table 1. Patients mean age was 62.7 ± 10.6 years, 67 % were male, with a mean body mass index of 30.8 ± 5.8 kg/m2. Despite the prescription of 5.2 ± 1.4 antihypertensive drugs, office BP at baseline was 167/88 ± 19/16 mmHg with a 24-h mean blood pressure of 154/86 ± 15/13 mmHg. There were no significant differences between the baseline characteristics of adherent and non-adherent patients.
Procedural characteristics
Experienced operators, who had performed at least 15 RDN procedures, performed all 100 RDN procedures without any serious adverse events. Intravenous analgesics were administered in all patients. The mean procedural time was 73.3 ± 17.2 and 73.1 ± 31.8 ml of contrast (Imeron® 350) was used. On average 11.1 ± 2.5 complete 120-s radiofrequency ablations were delivered circumferentially along both renal arteries with special emphasis on the distal segment. Ablation attempts (prematurely interrupted, <120 s) were not counted. Hemodynamically significant renal artery stenosis following RDN was excluded by use of duplex ultrasound in all patients 1 day after RDN and at 6-month follow-up. Renal function measured by cystatin c GFR remained unchanged during follow-up. Cystatin c GFR was 74.5 ± 30.8 ml/min/1.73 m2 at baseline and 72.4 ± 28.3 ml/min/1.73 m2 (p = 0.324) at 6-month, respectively.
Office and ambulatory blood pressure
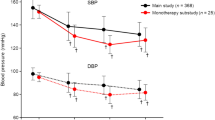
Office SBP and DBP at baseline were significantly reduced from 167 ± 19 and 88 ± 16 mmHg to 152 ± 25 mmHg (p < 0.001) and 83 ± 14 mmHg (p < 0.001) at 6-month follow-up, respectively (Fig. 1). Patients with an office SBP ≥160 mmHg (n = 63) at baseline had significant higher office SBP reduction at 6-month follow-up when compared to patients with an office SBP of 140–159 mmHg (n = 37) at baseline (−18 ± 23 mmHg versus −9 ± 15 mmHg, p = 0.038). Response to treatment, defined as a reduction in office SBP ≥10 mmHg after 6 months, was documented in 63 patients (63 %), subsequently defined as office BP responders. Twenty-four-hour SBP and DBP was significantly reduced from 154/86 ± 15/13 mmHg at baseline (n = 100) to 146/82 ± 17/13 mmHg after 6 months (n = 84), respectively. Forty-six patients (55 %) had an ambulatory SBP reduction ≥5 mmHg 6 months after treatment, subsequently defined as ABPM responders. In 13 patients, ABPM readings were excluded from the analyses because of <70 % valid BP measurements over a 24-h time period. Three patients rejected ABPM for personal reasons at 6-month follow-up.
Results of toxicological urine and plasma screening for antihypertensive drugs or metabolites
All prescribed antihypertensive agents were analyzed in urine and plasma samples by LC–HR-MS/MS. Adherence to prescribed antihypertensive drugs ranged from 0 to 100 % at baseline and 6-month follow-up (Fig. 2a, b). Fifty-two patients (52 %) were completely adherent, in 46 patients (46 %) less than the prescribed medication was detected, while 2 patients (2 %) showed a complete absence of any prescribed antihypertensive agent. There were no significant differences between adherent (52 %) and non-adherent (48 %) patients at baseline with respect to age, sex, body mass index, office and ambulatory BP, office heart rate, coronary artery disease, hypercholesterolemia, type 2 diabetes, cystatin c GFR, number of all prescribed drugs and number of antihypertensive drugs (Table 1). Adherence rates at baseline were not associated with office or ambulatory BP changes at 6 months (Fig. 3). When comparing adherence to different classes of antihypertensive drugs, patients receiving beta blockers had the highest adherence (98 %), followed by ACE inhibitors/angiotensin receptor blockers (93 %), diuretics (91 %), mineralocorticoid receptor antagonists (80 %), and centrally acting sympatholytics (80 %) (Fig. 4). Contrary, calcium-channel blockers were measured in 105 out of 163 samples (64 %) and vasodilators in 8 out of 38 samples (21 %) at baseline and 6-month follow-up, respectively. Adherence to prescribed treatment significantly decreased from 85.0 ± 21.7 % at baseline to 80.7 ± 22.7 % after 6 months (p = 0.005, Fig. 5) and remained unchanged in both, office (Fig. 6a) and ambulatory (p = 0.596) BP responder at 6-month follow-up. In office and ambulatory non-responder, adherence to antihypertensive treatment significantly decreased 6 months after RDN from 90.6 to 83.4 % (p = 0.006, Fig. 6b) and from 84.2 to 77.1 % (p = 0.005), respectively. Patients with unchanged adherence (n = 47) compared with patients with changes in adherence to antihypertensive therapy had a significant more pronounced office SBP reduction (−18 ± 24 versus −12 ± 18 mmHg, p = 0.05) and a comparable ambulatory SBP reduction (−8 ± 11 versus −8 ± 11 mmHg, p = 0.986), respectively. In 17 patients adherence increased and in 36 patients adherence decreased during the study period. Office and ambulatory SBP changes between the groups were comparable.
Medication changes
Patients and physicians were instructed not to change the antihypertensive drug regimen during the study period. However, antihypertensive medication was reduced in 22 patients (22 %) because of the development of symptoms and confirmed low BP (SBP <120 mmHg) and increased in 12 patients (12 %) who continued to have BP above target during the study period, respectively (Table 2). Even after censoring for post-procedural medication changes, no significant differences in BP reduction were documented.
Discussion
To best of our knowledge, this is the first study to assess adherence to antihypertensive drug treatment measured by LC–HR-MS/MS in plasma and urine in patients with resistant hypertension who have undergone RDN. Herein, RDN significantly lowered office and ambulatory BP, despite a worsening rather than an improvement of drug adherence at 6 month. In contrast to responders whose adherence remained stable, patients not responding to treatment significantly decreased their drug intake following the procedure.
Renal denervation evolved as a new treatment option for patients with uncontrolled hypertension [6, 25]. The available evidence suggests that RDN reduces office and ambulatory BP in open-label registries [8, 9, 11, 12] and three randomized, controlled trials [7, 10, 26]. Following the publication of the blinded, sham-controlled Symplicity HTN-3 study [13], which met its primary safety endpoint, but did not reach its efficacy endpoint, several possibilities were discussed regarding why the results were disparate compared with prior clinical trials and registries [25, 27]. It has been argued that the absence of a positive finding in Symplicity HTN-3 was mainly related to adding a control group (receiving sham procedure) and blinding of patients as well as follow-up assessors [15]. In previously published studies [7–14], drug adherence was not thoroughly monitored, either before RDN or during follow-up. This made these studies in principle vulnerable to the Hawthorne effect: Patient’s health care behavior might have changed with the inclusion in clinical trials with regular physician contacts during follow-up examinations and education to measure BP regularly at home. Indeed, patients potentially started taking their drugs following RDN, leading to a subsequent dramatic, but non-specific BP fall. An alternative behavior might be a reduction of adherence after RDN because patients have the impression that they have been treated for their hypertension. However, objective evidence to support this hypothesis is lacking. In the present study, adherence to drug treatment significantly decreased throughout the study period, thereby not explaining the observed reduction in office and ambulatory BP. In patients responding to RDN (either in terms of office or ambulatory BP) adherence remained on average unchanged, in fact although it decreased in many patients whereas in patients not responding to RDN non-adherence significantly increased. The reasons for non-response to RDN are not completely understood, but inappropriate patient selection, procedural performance, and an insufficient contribution of sympathetic nervous system activation to the etiology of the patient’s hypertension are all possible factors [28]. In light of the present findings, non-adherence needs to be considered as one contributing factor and emphasize to adherence-promoting programs and compliance assessment should be taken in account especially in non-responders to RDN. The reduction in drug adherence observed at 6 month in the overall group may be one reason why the full benefits of RDN on BP control have been underestimated. Indeed, BP control at 6 months is due to both the RDN procedure and the maintenance of an effective drug therapy. If this latter is affected by a decreasing adherence over time, the real impact of RDN on BP cannot be assessed correctly.
In the conduct of earlier trials in RDN therapy, it has been questioned whether the population was truly resistant to medical therapy or mainly non-adherent. Non-adherence to pharmacological treatment is recognized as an important barrier to successful BP control in hypertensive patients [3, 4]. In patients with resistant hypertension, monitoring of drug adherence per se can improve BP control in more than 30 % of cases [29]. In a recently published study, toxicological urine screening revealed non-adherence to prescribed drug regimen in more than 50 % of patients referred with uncontrolled hypertension to an outpatient clinic of a tertiary center in Germany [3]. Of those patients being non-adherent, 30 % were completely non-adherent and 70 % had incomplete adherence to antihypertensive therapy. Herein, the rate of completely adherent patients was comparable (52 %), whereas 46 % were partially adherent, and only 2 % were completely non-adherent. Overall, we documented higher adherence to antihypertensive drugs compared with previous reports [3, 4]. It is noteworthy to mention, that all patients were treated in a specialized setting and provided written inform consent for objective adherence measurements by LC–HR-MS/MS during the study, both factors might have positively impacted adherence. However, in order to prevent stimulation of ‘white-coat’ adherence, patients were not informed about the timing and execution of the measurements. In the present study, non-adherence was almost evenly distributed between different classes of anti-hypertensive drugs with the exception of vasodilators. This is in contrast with published evidence indicating that adherence to treatment in the general hypertensive population varies across different drug classes with lower adherence rates in diuretics and beta blockers when compared to angiotensin receptor blockers and ACE inhibitors [30]. For several reasons, these data may not be generalizable to patients with resistant hypertension, treated with ≥3 antihypertensive drug. In case side effects occur, it is nearly impossible for patients receiving multiple drugs to assign the side effect to one specific drug, causing random, non-predictable discontinuations. Further, the majority (62 %) of our patients received fixed-dose combinations and when a patient omits consecutive doses of a single-pill combination, 2 or 3 drugs are simultaneously missed [17]. This is supported by the fact that beta blockers, which were rarely used in fixed-dose combinations, showed the highest adherence. These findings are in line with a recently published study, using toxicological urine screening to assess non-adherence to the prescribed drug regimen in patients with uncontrolled hypertension [3].
One might argue that RDN represents a potential second line treatment approach for patients who remain non-compliant despite maximal efforts of a specialized hypertension clinic. In the two patients who did not take any medication treated in this study, mean office and ambulatory BP was reduced by 13/5 and 4/2 mmHg (mean office BP from 166/95 to 153/90 mmHg, mean 24-h BP from 142/91 to 138/89 mmHg), respectively. Certainly, much more research is needed to investigate patient behaviors, shared decision-making and whether RDN is able to replace antihypertensive drugs in patients with milder forms of hypertension.
Limitations
Our study may have some limitations. Drug intake was measured qualitatively but not quantitatively, therefore no information about exact drug doses can be provided. Furthermore, drug measurements can be affected by the white-coat adherence phenomenon whereby patients tend to improve their adherence before and after clinical visits [17]. This may underestimate chronic or intermittent non-adherence. However, this limitation applies to the majority of currently used adherence tests conducted in the clinic prior to scheduled appointments. The known chemical instability of the dihydropyridine calcium-channel blockers amlodipine, felodipine, lercanidipine, nifedipine, and nitrendipine could have affected the corresponding LC–HR-MS/MS analyses results [3, 31]. This might be one reason for the apparently low adherence to calcium-channel blockers documented herein. Finally, participation in a prospective study might have impacted adherence. Therefore, rigorous analyses of adherence to medication in future randomized, controlled trials are required to confirm the results of the present study.
Conclusions
Catheter-based RDN can reduce office and ambulatory BP in certain patients with uncontrolled hypertension on multiple antihypertensive drugs. In the present study, these BP changes were not explained by improvements in adherence following the procedure. However, patients without BP fall significantly decreased their adherence to drug treatment. These data support the role of RDN as an antihypertensive treatment option and emphasize the importance of measuring drug adherence not only before but also after the procedure in order to assess the true BP lowering of the intervention.
References
Mancia G, Fagard R, Narkiewicz K et al (2013) 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34(28):2159–2219. doi:10.1093/eurheartj/eht151
Judd E, Calhoun DA (2014) Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens 28(8):463–468. doi:10.1038/jhh.2013.140
Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW (2013) Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 31(4):766–774. doi:10.1097/HJH.0b013e32835e2286
Tomaszewski M, White C, Patel P et al (2014) High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 100(11):855–861. doi:10.1136/heartjnl-2013-305063
Böhm M, Schumacher H, Laufs U, Sleight P, Schmieder R, Unger T, Teo K, Yusuf S (2013) Effects of nonpersistence with medication on outcomes in high-risk patients with cardiovascular disease. Am Heart J 166(2):306–314.e7. doi:10.1016/j.ahj.2013.04.016
Mahfoud F, Lüscher TF, Andersson B et al (2013) Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 34(28):2149–2157. doi:10.1093/eurheartj/eht154
Esler MD, Krum H, Schlaich M, Schmieder RE, Böhm M, Sobotka PA (2012) Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 126(25):2976–2982. doi:10.1161/CIRCULATIONAHA.112.130880
Mahfoud F, Ukena C, Schmieder RE et al (2013) Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 128(2):132–140. doi:10.1161/CIRCULATIONAHA.112.000949
Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V (2013) Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J 34(28):2132–2140. doi:10.1093/eurheartj/eht197
Azizi M, Sapoval M, Gosse P et al (2015) Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 385(9981):1957–1965. doi:10.1016/S0140-6736(14)61942-5
Böhm M, Mahfoud F, Ukena C et al (2015) First Report of the Global SYMPLICITY Registry on effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension 65(4):766–774. doi:10.1161/Hypertensionaha.114.05010
Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD (2014) Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383(9917):622–629. doi:10.1016/s0140-6736(13)62192-3
Bhatt DL, Kandzari DE, O’Neill WW et al (2014) A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370(15):1393–1401. doi:10.1056/NEJMoa1402670
Fadl Elmula FEM, Hoffmann P, Larstorp AC et al (2014) Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension 63:991–999. doi:10.1161/HYPERTENSIONAHA.114.03246
Persu A, Jin Y, Fadl Elmula FE, Jacobs L, Renkin J, Kjeldsen S (2014) Renal denervation after Symplicity HTN-3: an update. Curr Hypertens Rep 16(8):460. doi:10.1007/s11906-014-0460-x
Waeber B, Feihl F (2013) Assessment of drug compliance in patients with high blood pressure resistant to antihypertensive therapy. EuroIntervention 9(Suppl R):R29–R34. doi:10.4244/eijv9sra6
Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J (2013) Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension 62(2):218–225. doi:10.1161/hypertensionaha.113.00687
Ewen S, Baumgarten T, Rettig-Ewen V, Mahfoud F, Griese-Mammen N, Schulz M, Böhm M, Laufs U (2015) Analyses of drugs stored at home by elderly patients with chronic heart failure. Clin Res Cardiol 104(4):320–327. doi:10.1007/s00392-014-0783-2
Maurer HH (2007) Current role of liquid chromatography-mass spectrometry in clinical and forensic toxicology. Anal Bioanal Chem 388(7):1315–1325. doi:10.1007/s00216-007-1248-5
Tsioufis C, Mahfoud F, Mancia G, Redon J, Damascelli B, Zeller T, Schmieder RE (2014) What the interventionalist should know about renal denervation in hypertensive patients: a position paper by the ESH WG on the interventional treatment of hypertension. EuroIntervention 9(9):1027–1035. doi:10.4244/EIJV9I9A175
Wissenbach DK, Meyer MR, Remane D, Weber AA, Maurer HH (2011) Development of the first metabolite-based LC-MS(n) urine drug screening procedure-exemplified for antidepressants. Anal Bioanal Chem 400(1):79–88. doi:10.1007/s00216-010-4398-9
Helfer AG, Turcant A, Boels D, Ferec S, Lelievre B, Welter J, Meyer MR, Maurer HH (2015) Elucidation of the metabolites of the novel psychoactive substance 4-methyl-N-ethyl-cathinone (4-MEC) in human urine and pooled liver microsomes by GC-MS and LC–HR-MS/MS techniques and of its detectability by GC-MS or LC-MS(n) standard screening approaches. Drug Test Anal 7(5):368–375. doi:10.1002/dta.1682
Maurer HH (2010) Analytical toxicology. EXS 100:317–337
Parati G, Stergiou G, O’Brien E et al (2014) European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 32(7):1359–1366. doi:10.1097/HJH.0000000000000221
Mahfoud F, Luscher TF (2015) Renal denervation: symply trapped by complexity? Eur Heart J 36(4):199–202. doi:10.1093/eurheartj/ehu450
Rosa J, Widimsky P, Tousek P et al (2015) Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the prague-15 study. Hypertension 65(2):407–413. doi:10.1161/hypertensionaha.114.04019
Lüscher TF, Mahfoud F (2014) Renal nerve ablation after SYMPLICITY HTN-3: confused at the higher level? Eur Heart J 35(26):1706–1711. doi:10.1093/eurheartj/ehu195
Mahfoud F, Edelman ER, Böhm M (2014) Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 64(7):644–646. doi:10.1016/j.jacc.2014.05.037
Burnier M, Schneider MP, Chiolero A, Stubi CL, Brunner HR (2001) Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens 19(2):335–341
Kronish IM, Woodward M, Sergie Z, Ogedegbe G, Falzon L, Mann DM (2011) Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation 123(15):1611–1621. doi:10.1161/CIRCULATIONAHA.110.983874
Baranda AB, Alonso RM, Jimenez RM, Weinmann W (2006) Instability of calcium channel antagonists during sample preparation for LC-MS-MS analysis of serum samples. Forensic Sci Int 156(1):23–34. doi:10.1016/j.forsciint.2004.11.014
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft (KFO 196), Deutsche Gesellschaft für Kardiologie, and Deutsche Hochdruckliga.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The institution (Klinik für Innere Medizin III, Universitätsklinikum des Saarlandes) has received scientific support from Medtronic/Ardian. CU, MB and FM received speaker honorarium from Medtronic/Ardian (Mountain View, California, US), and St. Jude Medical (Eschborn, Germany). BC has received speaker honorarium from Medtronic/Ardian (Mountain View, California, US). CU and MB are supported by Deutsche Forschungsgemeinschaft (KFO 196). SE, DL and FM are supported by Deutsche Hochdruckliga. FM is supported by Deutsche Gesellschaft für Kardiologie.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ewen, S., Meyer, M.R., Cremers, B. et al. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol 104, 1097–1105 (2015). https://doi.org/10.1007/s00392-015-0905-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0905-5