Abstract
Objectives
Evaluation of the classification of the patients with coronary procedures [CP: coronary angiography (CA) and/or percutaneous coronary intervention (PCI)] in Germany to provide valid data as a basis for the evaluation of resource planning and comparison of results from other countries.
Background
In the case of CP, most data are restricted to procedures related to acute coronary syndrome (ACS), either STEMI or NSTEMI, and does not cover the whole range of coronary angiographies and therapeutic procedures. To contribute to the clarification of this situation, our analysis was performed.
Methods
The data of all patients with CP documented according to § 137 German Social Security Code V (SGB V) by hospitals registered under the requirements of § 108 SGB V were analyzed. By cluster analysis, the most important predictors for four short-term clinical outcomes (intra-procedural and post-procedural events, death, length of hospital stay) were identified. Differences were analyzed on a national, federal state and interstate level.
Results
In 2012, 764,233 CP were performed in 733,337 patients (64.7 % males) in 890 hospitals. In 88.2 % of all patients, a cardiac disease was detected; in 11.3 %, it was excluded. 5 clusters were identified which were based on the following parameters: ACS, invasive coronary procedure, cardiac disease (i.e., hypertensive cardiac disease, cardiomyopathy, aortic aneurysm, cardiac valve disease), exclusion of CHD. There were significant differences between the five patient clusters: percentages of patients with diabetes, renal insufficiency and CHF varied between 14.1 and 32.9 %, 9.5 and 31.6 %, and 7.0 and 16.9 %, respectively; average duration of hospitalization and death rates varied between 5.5 (without ACS) and 8 days (ACS) and 0.3 and 4.5 %, respectively. The distribution of patient clusters varied significantly between the German federal states as well as within a federal state.
Conclusions
Five patient clusters with CP showed significantly differing profiles of risk, outcome and resource consumption as well as a regional distribution. This has to be considered for comparisons between hospitals as well as on a regional and international level and hospital planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to a study performed in 12 European countries of patients with acute myocardial infarction, the use of PCI differs widely between these countries [1]. Data based partly on estimations by experts suggest that only 40–60 % of patients with STEMI received PCI in Europe [2].In addition, and perhaps as a consequence, considerable differences were reported in the short-term outcome after acute myocardial infarction between two of the 12 participating countries [3]. However, in principle and for methodological reasons, it is difficult to compare health systems and their outcomes. First of all existing studies usually lack representative clinical data because they are normally based on voluntary participants who differ from the overall national population in behavior, treatment and outcome [2, 4]. In the case of coronary procedures [CP: coronary angiography (CA), percutaneous coronary intervention (PCI)], most data are restricted to procedures related to acute coronary syndrome (ACS), either STEMI or NSTEMI and not on the full range of coronary angiographies and therapeutic procedures [5]. Only a few countries, such as Sweden and the UK, have continuous clinical registries for ACS with mandatory participation and comparable datasets [2].
Based purely on the reported numbers of CP, some stakeholders have claimed a surfeit of diagnostic and treatment procedures in Germany. On the other hand, it should be clarified whether there is an unfulfilled need. The AQUA Institute, which is commissioned by the German Federal Joint Committee to analyze the mandatory hospital quality assurance, stated in its 2012 report [6], that only 4.0 % of the patients with PCI underwent the procedure without clinical or non-invasive signs of ischemia and that in 92.4 % clinical or non-invasive signs of ischemia were the indication for the coronary angiography. To define the necessary amount of a certain diagnostic or therapeutic procedure, it would be necessary to know the real need—in the case of CP, the definition and size of the relevant population and its needs are to be determined. That means that the relevant population consists not only of patients with ACS but also of patients with coronary symptoms and the need to clarify the diagnosis as a prerequisite for a targeted invasive therapy.
One could argue and assume that the official statistics or health reports such as the quality report of the AQUA Institute should be suitable for this purpose. But these reports are based on diagnosis-related groups (DRG), and the quality indicators of the official health reports do not lend themselves to detailed needs analysis for several reasons. The DRG are in a way unclear because of the high number, are clinically not plausible in every case and are adopted on a yearly basis. Data from the Disease Management Programs (DMP) are not valid for several reasons, for example, over- and under-reporting of events [7].
As a basis for a more profound analysis of possible oversupply, it is necessary to know details about the population undergoing CP, i.e., whether this population is homogeneous or could be clustered into specific groups according to the risk of intra- and post-procedural complications, death and length of stay in the hospital, with the consequence of different resource consumption. One could assume that at least patients undergoing coronary angiography would differ from those with PCI with regard to resource consumption and outcome. In case of different patient clusters, it would be of interest to know the differences between the clusters and whether these patient clusters differ between hospitals or federal states in Germany.
To contribute to clarification of these topics, all patients in Germany who underwent CP in 2012 were analyzed on the basis of the data of the AQUA Institute.
Methods
This analysis is based on the CP datasets of the German Federal Council, held by the AQUA Institute (Göttingen, Germany), which were documented in accordance with § 137 Social Security Code V (SGB V) by hospitals registered under § 108 SGB V. This data collection is compulsory for all in-patient procedures in hospitals billing CP to German statutory health insurance or private insurance companies (890 hospitals).
In a first step, factors were identified which had the most important influence on the four target parameters, i.e., intra- and post-procedural events, death, and length of hospital stay. The basis for this were all variables concerning demography, concomitant risk factors, indication and diagnosis per patient available in the dataset, such as age, gender, indication for CP, diagnosis, diagnostic procedures and former treatment (PCI, coronary artery bypass grafting).
In a second step and based on the a.m. variables, a cluster analysis was performed with IBM SPSS 20 two-step cluster analysis [8] to reveal natural clusters in the dataset. In step 1, a cluster feature tree (CFT) was calculated according to a threshold of 0.2 for the distance metric log likelihood. In step 2, based on the Schwarz Bayesian Criterion, an agglomerative algorithm was used to calculate five clusters. Analysis of the clusters showed a clear structure, which led directly to the a priori patient classification used for this analysis; the clusters are described in detail. Since it was assumed that there may be differences between patients with PCI and those with CA without PCI, these groups were analyzed separately with regard to anamnestic data, the leading indication for CA, the leading diagnosis after CA, intra- and post-procedural events and resource requirements.
Compared to use of the original clusters, the analysis of the a priori patient classification has the advantages that the methodology is transparent and reproducible (for other datasets) and can be used for comparisons over time.
In a third step and as an example for German federal states, the distribution of patient clusters within hospitals in Bavaria was analyzed with regard to resource needs. The AQUA dataset contains a number of parameters that could be used to estimate the resource consumption: mainly, the duration of hospitalization and in addition the fluoroscopy time and the amount of contrast medium used.
Results
In 2012, in Germany, 764,233 CP were performed in 733,337 patients (64.7 % male). These cases were reported by 890 hospitals. The age distribution is shown in Fig. 1.
Whereas 56.9 % of the male patients receiving the procedures were under 71 years of age, 57.3 % of females were over 70 years old at the time of the procedure.
In 60.4 % (56.2 % male, 68.3 % female), a diagnostic coronary angiography without PCI was performed; in 89.2 % of PCI procedures, stents were implanted.
As a result of the cluster analysis, the most important parameters with influence on the clinical outcome (intra-, post-procedural events, death, length of stay) were acute coronary syndrome, diagnostic coronary angiography before admission, leading diagnosis after coronary angiography and leading indication for coronary angiography.
Based on these parameters, the following patient clusters were developed; the percentage per patient cluster and numbers are given in brackets:
-
Patient cluster 1: patients without acute coronary syndrome (ACS), without preceding coronary diagnostic procedure, diagnoses: hypertensive heart disease or other cardiac disease, exclusion of coronary heart disease (CHD) (11.5 %, n = 88,207, mean age 63.1 years);
-
Patient cluster 2: patients without ACS, without preceding invasive coronary diagnostic procedure, diagnoses: CHD, cardiomyopathy, aortic aneurysm, cardiac valve disease (22.7 %, n = 173,146, mean age 68.4 years);
-
Patient cluster 3: patients without ACS, but with preceding invasive coronary diagnostics (32.8 %, n = 250,314, mean age 69.1 years);
-
Patient cluster 4: patients with ACS but without preceding invasive coronary diagnostics (20.9 %, n = 159,578, mean age 69.1 years);
-
Patient cluster 5: patients with ACS and with preceding invasive coronary diagnostics (12.2 %, n = 92,988, mean age 70.0 years).
The age distribution per cluster is presented in Fig. 2 and shows a comparable distribution for each cluster with a peak in the age group of 71–75.
For special analysis and due to the fact that the need for PCI may influence the outcome and the resource consumption, the 5 clusters were each separated into two sub-clusters according to the type of procedure (diagnostic vs. therapeutic). The sub-clusters were marked at the second digit of the cluster number. For example, cluster 1 is divided into sub-clusters 1.1 and 1.2. Sub-cluster 1.1 comprises patients of cluster 1 with coronary angiography only, while sub-cluster 1.2 consists of patients of cluster 1 with PCI either with or without coronary angiography.
In cluster 1, the mean age of patients with CA only was 62.5 years; those with PCI were a mean of 69.4 years old. The differences between these two groups within the other clusters were 0.5 years or less. For the diagnostic coronary angiography, the leading indication and resulting diagnosis are shown in Figs. 3 and 4.
In cluster 1 are 40,603 patients, who were not treated with PCI after the coronary angiography, or were not in Canadian Cardiovascular Society (CCS) class III or IV or had no signs of instrument-based ischemia or no suspicion of CHD, this corresponds to 46.0 % of this class and 5.3 % of all patients. In 21,311 of these 40,603 cases, cardiac-based dyspnea was documented. For the remaining 19,292 cases (2.5 %), there was no information in the AQUA database about the reason for coronary angiography.
Patients with ACS (patient clusters 4 and 5) have a significantly higher risk (p < 0.05) for an intra-procedural or post-procedural event (Fig. 5), and the death rate is also higher (Fig. 5). The event rates are even higher (p < 0.05) in patients with ACS and without any preceding invasive diagnostic despite a more favorable comorbidity profile (see Fig. 6). Figures 7, 8 and 9 show the results differentiated according to coronary angiography only (cluster 1.1, 2.1, 3.1, 4.1, 5.1) and PCI (cluster 1.2, 2.2, 3.2, 4.2, 5.2). The death rate varies between the clusters (0.3 % in cluster 1.1, 5.4 % in cluster 4.2, 4.4 % in cluster 5.2) and depends also on age: 0.9 % in patients younger than 51 years, 16.9 % in very old patients (>95 years).
Requirement of resources per patient cluster (statistically significant differences (p < 0.05) except post-procedure hospitalization between clusters 1.2 vs. 3.1, 4.1 vs. 5.1, contrast medium 3.1 vs. 5.1 and 4.2 vs. 5.2, duration of hospital stay 4.1 vs. 5.1 and 4.2 vs 5.2, fluoroscopy time 3.1 vs. 5.1)
The fraction of patients with congestive heart failure differs between the patient clusters: in patient cluster 1 only 7.0 %, in patient cluster 2 21.1 %, in patient cluster 3 10.4 % and in patient clusters 4 and 5 14.7 and 16.3 %. The percentages of patients with an ejection fraction of <40 %, with diagnosis of congestive heart failure, with diabetes or renal insufficiency are shown in Fig. 6. In patients without ACS—clusters 1–3—any objective indications of stress-induced ischemia were reported for 30.0/33.0/30.7 %, cardiac-induced dyspnea (rest or stress induced) was documented for 53.6/63.8/60.3 % and further symptoms such as rhythm disturbances in 37.4/36.7/30.9 %. No angina was found in 32.0/30.7/24.9 %.
In patients without ACS, the percentage with diabetes was higher in those who were treated with a PCI than patients with coronary angiography only:
-
In cluster 1, 25.6 vs. 14.1 %,
-
In cluster 2, 25.3 vs. 21.8 %,
-
In cluster 3, 30.2 vs. 27.3 %.
Whereas 43.1 % (patient cluster 5) and 51.0 % (patient cluster 4) of ACS patients remained in the hospital for 5 days or more after the procedure, the patients without ACS stayed a significantly shorter time (patient cluster 1/2/3:15.8/25.8/17.7 %).
In patient cluster 1, the smallest group, the main reason for the diagnostic CA was in 83.5 % the exclusion of a cardiac disease; in 6.4 %, the reason was not specified—by definition, these patients were hospitalized for PCI directly. In 18.9 %, a hypertensive heart disease and in 7.6 % another cardiac disease was diagnosed, in 73.5 % the suspicion of a coronary disease was not confirmed. In cluster 2 (patients without ACS and without preceding invasive coronary diagnostics) in 82.2 % a coronary disease [CHD with narrowing of lumen either ≥50 % (=relevant CHD) or less than 50 %] was detected by angiography. In 88.2 % of all patients, a cardiac disease was detected (74.7 % CHD, 2.8 % cardiomyopathy, 4.0 % valve vitium, 3.3 % hypertensive heart disease, 3.5 % other cardiac disease) and in 11.3 % excluded. Diabetes was documented in a range of between 14.1 % (cluster 1.1) and 32.9 % (cluster 5.2). In patients without ACS and without preceding invasive coronary diagnostic (cluster 1 and 2), there were 43.2 % with relevant CHD.
Figure 7 shows the consumption of resources per patient cluster, differentiated according to CA only and PCI, referring to the variables available in the AQUA dataset. The requirements differ substantially between the patient clusters. Average fluoroscopy time was 7.0 ± 9.4 and 8.7 ± 10.3 min in patients without/with ACS (median 4.2/6.1 min), Contrast medium for patients without ACS was 118.7 ml (median 100 ml) compared to 145.3 ml (median 125 ml) in patients with ACS. The differences were statistically significant (p < 0.05).
The mean duration of hospitalization per patient cluster is 1–2 days longer for patients with ACS. More than a third of patients with ACS stayed longer than 7 days in the hospital (Fig. 8).
After the CP, a substantial percentage of patients with ACS stay in hospital for more than 4 days, whereas this is true for less than a quarter of patients without ACS (Fig. 9).
Taken the mean length of stay as the parameter, the resource consumption varies by up to 2.5 days between the patient clusters.
Differences between hospitals are remarkable: in Bavaria, the mean length of stay differs between 3.2 and 10.8 days. In a similar way, the distribution of patient clusters differs considerably within Bavaria: the percentage of patients with ACS varies between 54.2 and 0 % (Fig. 10)—even in hospitals with more than 1000 cases per year. The duration of hospitalization differs accordingly (data not shown), as does the duration of post-procedural hospitalization.
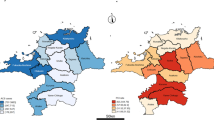
As shown for Bavaria as an example for a federal state, the distribution of patient clusters also differs significantly between the federal states. The percentage of patients with ACS varies between 23.2 % in Mecklenburg-West Pomerania and 58.7 % in Bremen (Fig. 11).
Patient clusters in Germany per federal state MV Mecklenburg-West Pomerania, BE Berlin, TH Thuringia, WL Westphalia, BB Brandenburg, NO North Rhine, BA Bavaria, SN Saxony, ST Saxony-Anhalt, SL Saarland, BW Baden-Württemberg, SH Schleswig-Holstein, NI Lower Saxony, RP Rhineland-Palatinate, HE Hessen, HH Hamburg, HB Bremen)
Discussion
This detailed analysis provides information about the risk factor profile and the short-term outcome of all patients who underwent a coronary procedure, either coronary angiography only or PCI only or combined angiography and PCI in Germany during 2012. The data allow identification of groups of patients with different risk profiles with regard to intra-procedural and post-procedural events or death during hospitalization and resource consumption. In addition, a certain assessment of the adherence to guidelines is possible.
In the smallest group (11.5 % of all cases, patient cluster 1), the leading indications for coronary angiography were suspicion of CHD and exclusion of CHD (83.5 %), and CHD was excluded in 73.5 %, corresponding to 9.6 % of all patients; in 26.5 %, a coronary disease was diagnosed. This seems to be low compared to data of the CathPCI Registry in the US [9] which described, in patients without known CHD, a prevalence of 41.0 % for obstructive CHD (stenosis >50 %). In our case, the corresponding patients originate from cluster 1 and 2 and the resulting 43.2 % correspond very well to the US data.
The exclusion rates for CHD within the other clusters varied between 0 (cluster 2, given by the definition of this cluster) and 8.6 % in cluster 4 (patients with ACS as a pre-angiography diagnosis). These data almost correspond to the 7.0 % of patients who underwent coronary angiography with the indication of vague chest pain in the Swedish SCAAR registry [10]. Considering the results of the German CPU-registry [11], 20.2 % of Troponin-positive patients had no ACS and the most frequent non-coronary diagnoses in non-revascularized patients were arrhythmias (13.4 %), pericarditis/myocarditis (4.5 %), decompensated congestive heart failure (3.7 %), Takotsubo cardiomyopathy (2.7 %) and hypertensive crisis (2.4 %), our results seem plausibly.
Whereas the procedure for patients in clusters 2–5 can be considered as conforming to ESC guidelines on the management of stable coronary artery disease, respectively, on myocardial revascularization [12, 13] by definition, in cluster 1 there are 40,603 patients with suspected CHD/exclusion of CHD/known CHD as leading indication for coronary angiography, who were not treated with PCI after the coronary angiography or were not in Canadian Cardiovascular Society (CCS) class III or IV or had no signs of instrument-based ischemia, corresponding to 46.0 % of this class and of 5.3 % of all patients. That means that 5.3 % may not be treated conforming to guidelines. As cardiac-induced dyspnea is considered as indication for coronary angiography, this percentage reduces to 2.5 %, for which no information about the reason for angiography is documented in the AQUA database. This does not mean that CA was not indicated; it is still the treating physician together with the patient who decides about indication and procedure.
Overall, the percentage of patients with an event during the procedure is relatively low. However, it is more than twice higher in patients with ACS (patient clusters 4 and 5) than in patients without ACS (patient clusters 1, 2 and 3). The higher number of intra- and post-procedural events in patients with ACS without (cluster 4) compared to those with preceding coronary diagnostic (cluster 5) cannot be explained by the data, but it can be assumed that the latter were treated for diagnosed CHD before the index event and thereby the prognosis may be positively influenced. In each cluster, the percentage of intra-procedural and post-procedural events is higher in the group with PCI. For post-procedural complications, the relation is similar, but on a higher level (1.0–6.9 %). Also, the death rates during the hospitalization period are higher in patient clusters 4 (3.7–5.4 %) and 5 (3.4–4.4 %) compared to patients without ACS (0.3–0.8 %), reflecting the higher risk of patients with ACS. These results are not surprising and correspond to data from other countries. The in-hospital mortality in the Melbourne Interventional Group (MIG) Registries was 1.4 % in patients undergoing PCI (urgent or elective) due to single or multi-vessel CHD. The MIG data did not differentiate between urgent and elective PCI and therefore the results cannot be directly compared to ours. Nevertheless, mortality rates of clusters 1 and 2 fall in the range of MIG data [14]. The Belgium STEMI registry reported an in-hospital mortality of 5.5 % after STEMI, compared to 7.4 % in octogenarians [15]. This data coincide with data from Switzerland: during 2007 and 2012, the in-hospital mortality rate was 4.5 % in younger patients with ACS and 7.4 % in octogenarians [16]. In the Swedish SWEDEHEART registry [17] in patients undergoing PCI, the in-hospital mortality varied between 0.8 and 14 % depending on age (<60, >95 years), which fits very well with our data, which show a death rate of 0.9 % in patients younger than 51 years and of 16.9 % in very old patients (>95 years); complications were reported in 1.8 %. The in-hospital death rate of around 4 % in patients with ACS (patient cluster 4 and 5) is comparable to the death rate reported from Sweden (5.8 %) and considerably lower than the 8.8 % in the UK [3]. In this comparison, it should be taken into consideration that the German population with ACS (cluster 4 and 5) has a much higher percentage of patients with congestive heart failure (14.7–16.9 %) than in Sweden and the UK (15 % vs 9.7 respectively 5.3 %). The same applies for the rate of patients with diabetes: in Germany, the percentage of diabetes as comorbidity in patients with ACS varied between 20.7 % (patient cluster 4) and 32.3 % (patient cluster 5), whereas the national outcome registries of Sweden and the UK report percentages of 22.7 and 17.4 %, respectively. [3, 14].The higher comorbidity may be one reason that the mean duration of stay of 8 days in these patients is longer than in Sweden (5 days) and the UK (6 days). Another reason may be that the time before the procedure in patients with ACS in Germany is 1.6 days. This seems to be long compared to the delay from symptom to primary PCI reported from Sweden (198 min) and the UK (199 min) [3], but the AQUA dataset does not contain this information and therefore such a comparison would be misleading.
The differences in the length of stay between the patient clusters reflect the reasons for CP. Patients with ACS are hospitalized 1–2 days longer than patients without ACS, i.e., those who do not present as emergency patients. The data about the duration of hospitalization in Germany are nearly identical with the 5.1 and 6.4 care days from the intensive heart care unit to discharge described in the SEPHIA-report [18] or the Swedish SWEDEHEART registries.
The differences between the hospitals and also between the federal states are far from negligible; in fact, they are somewhat surprising in their extent. At the level of the hospital, they may reflect regional epidemiology as well as specialization, while differences at the level of the large states may be more difficult to explain. At least we can conclude that the total demand for a hospital’s resources will depend heavily on the mixture of patients treated. This could serve as an improved estimation for resource consumption. On the other hand, the differences could be a hint as potential for improvement and optimization of care and resources. Reasons for these differences are unknown and topic of actual research. On the national level, differences in knowledge about risk factors or guidelines may be causal. Regional differences in the burden of disease and the prevalence of risk factors could play an important role too as described actually [19, 20]. These differences apply not only to medical risk factors such as hypertension, diabetes—prevalence ranged between 7.5 % and 15.8 %—or other factors of the metabolic syndrome but also to socio-demographic parameters such as unemployment rate (3.5–12.3 %) and education level, which are different in the federal states. Similar differences were reported from the SWEDEHEART registry: in Sweden, the use of CA and PCI varied by a factor of 2–3 among counties, partly owing to variations in cardiovascular sickness. But in accordance with the authors of the RIKHS-HIA report, it has to be considered that comparisons between regions or hospitals must be interpreted with great caution and that, i.e., patient characteristics have to be considered.
But the a.m. parameters do not explain the differences between hospitals. At the moment, reasons for these differences are unknown. One could speculate that some hospitals are more specialized on diagnostic procedures or selection of patients.
One of the strengths of this analysis is that it is based on the data of all hospital CP cases of the statutory and the private health insurance in Germany for the year 2012. Therefore, a selection bias can be excluded and the data describe the reality in Germany. Comparisons between hospitals and even between countries are possible.
To our knowledge, for coronary angiography and PCI, our analysis describes differences with regard to structure of patients, length of stay, etc., at the level of hospitals and federal states in Germany for the first time. The analysis provides benchmarks for patient segments which enable the comparison of specific patient segments within a hospital and between hospitals. The results could serve as basis for further research to improve quality of care. Different patient profiles should be taken into consideration: the high mortality rate of 32.5 % in a university-based intensive cardiac care unit may serve as a hint [21]. Different risk of development of acute kidney injury (AKI) may be another example—9.6 % of STEMI patients developed AKI according to a recent retrospective observational study [22]. The ALKK PCI registry [23] provided an additional hint: in patient with STEMI, the use of drug-eluting stents in the ALKK hospitals ranged between 2.3 and 58.3 % during the study period of 2006–2011. It is much more operable for a CP center with lower quality if it can identify patient segments where it is underperforming. At the regional level, this could lead to the initiation of specific prevention strategies as well as to implement structural changes. As it is well known that information and feedback about quality differences is an important motive for changes, our results may be one reason for such initiatives, i.e., on the level of hospitals.
In addition, the dataset allows to a certain extent comparison with available international data that also cover the population and not only selected samples [24]. And in contrast to the recent study by Laut et al. [1], which tried to explain the differences in utilization of PCI across 12 European countries mostly by health-care-associated economic and demographic characteristics, our study includes medical parameters such as indications for CP and some comorbidities.
The database has some limitations. One is the limited number of comorbidities documented in the database of the AQUA Institute. Another limitation is the lack of follow-up information after discharge from the hospital; information on the 30-day mortality, for example, is an important outcome indicator of medical and hospital performance. This is planned to be included from 2016 [25] and would enable comparisons with other registries such as SWEDEHEART and international databases. A principle issue and limitation may be systematic differences concerning the classification of ACS and as consequence the encoding, for example, the differentiation between NSTEMI-ACS with and without elevation of troponin. The same is true for the evaluation of CHD, which usually is not based on objective criteria derived from quantitative CA or fractional flow reserve. In addition, the AQUA dataset does not contain data necessary to calculate pre-test probability of coronary events.
Based on the identification of clusters of patients with CP, which show significantly different profiles of risk, outcome and resource consumption, we were able to confirm that considerations based on average values of a whole patient population may be misleading. This is particularly the case if the groups compared are not homogeneous in terms of the most important variables with influence on outcome or resource consumption or if the homogeneity could not be tested. For national and international comparisons, sophisticated analyses are necessary to avoid incorrect or even misleading conclusions. The ratios of the patient clusters should be considered in the planning and controlling of hospital resources.
Conclusion
Based on data of all patients with CP of one year in Germany, for the first time, analysis showed that patients with CP can be grouped into clusters according to their risk factor profile and short-term outcome. These clusters result in different resource consumption. Despite higher rate of comorbidity (diabetes, CHF) in Germany, the data correspond well to results from other countries such as Sweden referring to in-hospital mortality as well as indication for CA, and are more favorable than in the UK. Concerning patient profile and resource consumption, there are relevant differences between the German federal states and hospitals. This has to be considered for comparisons between hospitals as well as on a regional and international level and hospital planning.
References
Laut GL, Gale PG, Lash TL, Kristensen SD (2013) Determinants and patterns of utilization of primary percutaneous intervention across 12 European countries: 2003–2008. Int J Cardiol 168:2745–2753
Widimsky P, Wijns W, Fajadet J et al (2010) Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J 31:943–957
Chung S-C, Gedeborg R, Nicholas O, James S et al. (2014) Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet. doi:10.1016/S0140-6736(13)62070-X
Eagle KA, Goodman SG, Avezum A, Budaj A, for the GRACE Investigators et al (2002) Practice variation and missed opportunities for reperfusion in ST-segment –elevation myocardial infarction: findings from the global registry of acute coronary events (GRACE). Lancet 359:373–377
Jernberg T, Johanson P, Held C, Svennblad B, for the SWEDEHEART, RIKS-HIA et al (2011) Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 305:1677–1684
AQUA-Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH (2013) Qualitätsreport 2011 Göttingen
Horenkamp-Sonntag D, Linder R, Ahrens S, Verheyen (2012) Externe Validität ärztlicher Primärdaten-Angaben im Rahmen der DMP-Dokumentation. 4. AGENS-Methodenworkshop, Bremen
IBM Corp (2013) IBM SPSS Statistics for Windows, version 22.0. IBM Corp, Armonk
Patel MR, Peterson ED, Dai D, Brennan JM et al (2010) Low diagnostic yield of elective coronary angiography. N Engl J Med 362:886–895
SCAAR (2012) Annual report 2011 in SWEDEHEART annual report 2011, Stockholm
Illmann A, Riemer T, Erbel R, Giannitsis E, Hamm C, Haude M, Heusch G, Maier LS, Münzel T, Schmitt C, Schumacher B, Senges J, Voigtländer T, Mudra H (2014) Disease distribution and outcome in troponin-positive patients with or without revascularization in a chest pain unit: results of the German CPU-Registry. Clin Res Cardiol. 103(1):29–40
The task Force on the management of stable coronary artery disease of the European Society of Cardiology (2013) 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J. doi:10.1093/eurheartj/eht296
The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association of Cardio-Thoracic Surgery (EACTS) (2014) 2014 ESC/EACTS Guidelines on myocardial revasularization. Eur Heart J. doi:10.1093/eurheartj/ehu278
Yan BP, Clark DJ, Buxton B, Ajani AE et al (2009) Clinical characteristics and early mortality of patients undergoing coronary artery bypass grafting compared to percutaneous coronary intervention: insights from the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) and the Melbourne Interventional Group (MIG) registries. Heart Lung Circ 18(3):184–190
Vandecasteele EH, De Buyzere M, Gevaert S, de Meester A, Convens C, Dubois P, Boland J, Sinnaeve P, De Raedt H, Vranckx P, Coussement P, Evrard P, Beauloye C, Renard M, Claeys MJ (2013) Reperfusion therapy and mortality in octogenarian STEMI patients: results from the Belgian STEMI registry. Clin Res Cardiol 102(11):837–845. doi:10.1007/s00392-013-0600-3. Epub 2013 Jul 26. PubMed PMID: 23887831
Jaguszewski M, Ghadri JR, Diekmann J, Bataiosu RD, Hellermann JP, Sarcon A,Siddique A, Baumann L, Stähli BE, Lüscher TF, Maier W, Templin C (2014) Acute coronary syndromes in octogenarians referred for invasive evaluation: treatment profile and outcomes. Clin Res Cardiol [Epub ahead of print] (PubMed PMID: 25142902)
SWEDEHEART (2012) Annual report 2011, Stockholm
SEPHIA (2012) Annual report 2011 in SWEDEHEART annual report 2011, Stockholm
Stang A, Stang M (2014) An inter-state comparison of cardiovascular risk in Germany: towards an explanation of high ischemic heart disease mortality in Saxony-Anhalt. Dtsch Ärztebl Int 111:530–536
Gößwald A, Schienkiewitz A, Nowossadek E, Busch MA (2013) Prävalenz von Herzinfarkt und koronarer Herzkrankheit bei Erwachsenen im Alter von 40 bis 79 Jahren in Deutschland. Bundesgesundheitsbl 56:650–655
Zobel C, Dörpinghaus M, Reuter H, Erdmann E (2012) Mortality in a cardiac intensive care unit. Clin Res Cardiol 101(7):521–524. doi:10.1007/s00392-012-0421-9 (Epub 2012 Feb 9. PubMed PMID: 22318675)
Shacham Y, Leshem-Rubinow E, Steinvil A, Assa EB, Keren G, Roth A, Arbel Y (2014) Renal impairment according to acute kidney injury network criteria among ST elevation myocardial infarction patients undergoing primary percutaneous intervention: a retrospective observational study. Clin Res Cardiol 103(7):525–532. doi:10.1007/s00392-014-0680-8 (Epub 2014 Feb 13. PubMed PMID:24522799)
Härle T, Zeymer U, Schwarz AK, Lüers C, Hochadel M, Darius H, Kasper W, Hauptmann KE, Andresen D, Elsässer A (2014) Use of drug-eluting stents in acute myocardial infarction with persistent ST-segment elevation: results of the ALKK PCI-registry. Clin Res Cardiol 103(5):373–380. doi:10.1007/s00392-014-0664-8 (Epub 2014 Jan 17. PubMed PMID: 24435358)
Terkelsen CJ, Lassen JF, Norgaard BL et al (2005) Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 26:18–26
Herzstiftunge.V. Deutsche (2012) Deutscher Herzbericht 2011 24. Bericht Sektorenübergreifende Versorgungsanalyse zur Kardiologie und Herzchirurgie in Deutschland, Frankfurt
Acknowledgments
The authors thank the Federal Joint Committee that it allowed the evaluation of the data and Mrs. Gale for review of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bestehorn, K., Bauer, T., Fleck, E. et al. Coronary procedures in German hospitals: a detailed analysis for specific patient clusters. Clin Res Cardiol 104, 555–565 (2015). https://doi.org/10.1007/s00392-015-0818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0818-3