Abstract
Introduction
Mild induced hypothermia (MIH) is indicated for comatose survivors of sudden cardiac arrest (SCA) to improve clinical outcome. In this study, we compared the efficacy of two different cooling devices for temperature management in SCA survivors.
Methods
Between April 2008 and August 2009, 80 patients after survived in-hospital (IHCA) and out-of-hospital cardiac arrest (OHCA) were included in this prospective, randomized, single center study. Hypothermia was induced after randomization by either invasive Coolgard® cooling or non-invasive ArcticSun® surface cooling at 33.0 °C core body temperature for 24 h followed by active rewarming. The primary endpoint was defined as the efficacy of both cooling systems, measured by neuron-specific enolase (NSE) levels as a surrogate parameter for brain damage. Secondary efficacy endpoints were the clinical and neurological outcome, time to start of cooling and reaching the target temperature, target temperature-maintenance and hypothermia-associated complications.
Results
NSE at 72 h did not differ significantly between the 2 groups with 16.5 ng/ml, interquartile range 11.8–46.5 in surface-cooled patients versus 19.0 ng/ml, interquartile range 11.0–42.0 in invasive-cooled patients, p = 0.99. Neurological and clinical outcome was similar in both groups. Target temperature of 33.0 °C was maintained more stable in the invasive group (33.0 versus 32.7 °C, p < 0.001). Bleeding complications were more frequent with invasive cooling (n = 17 [43.6 %] versus n = 7 [17.9 %]; p = 0.03).
Conclusion
Invasive cooling has advantages with respect to temperature management over surface cooling; however, did not result in different outcome as measured by NSE release in SCA survivors. Bleeding complications were more frequently encountered by invasive cooling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Even though nowadays more patients achieve return of spontaneous circulation (ROSC) after sudden cardiac arrest (SCA), and succeed to be admitted to an intensive care unit (ICU) after successful cardiopulmonary resuscitation, the majority does not survive to hospital discharge. The rate of mortality still remains high [11]. It has been shown that approximately 80 % of the SCA survivors persist in coma for variable lengths of time [18] and most of the patients present themselves with neurological sequels [4]. Mild induced hypothermia (MIH) improves both survival and neurological outcome after SCA [12], and has been endorsed by guidelines for neuroprotection [23].
Surface cooling devices are non-invasive and range from simple cooling blankets or ice packs to sophisticated machines with automatic feedback control offering an estimated cooling rate of about 0.3–0.9 °C/h [29]. For invasive methods—i.e. intravascular cooling catheters, administration of intravenous ice-cold fluids, body cavity lavage, extra-corporeal circuits or selective brain cooling—cooling rates between 1.4 and 6.6 °C/h have been reported [29]. Tømte et al. showed that the cooling method (invasive vs. surface cooling) used in nonrandomized patients after survived out-of-hospital cardiac arrest (OHCA) made no difference on survival to hospital discharge or the neurologic function at follow-up [31].
The aim of this study was to compare the efficacy of invasive and non-invasive cooling after SCA as measured by neuron-specific enolase (NSE) levels, the overall clinical and neurological outcome, and to assess temperature control as well as the incidence of cooling-associated complications.
Methods
This trial was performed at a single tertiary care center between April 2008 and August 2009. Consecutive patients admitted to our ICU with ROSC after in-hospital cardiac arrest (IHCA) or OHCA were eligible for this study. Inclusion criteria were ROSC after SCA with a presumed cause of cardiac origin, persisting coma after successful resuscitation and patient age of >18 years. Patients were excluded if they were responding to verbal comments, had a proven pregnancy, coagulation disorder, intractable cardiogenic shock, despite adequate fluid resuscitation and inotropic support, known severely limited life expectancy or terminal illness. We also excluded patients with neoplastic diseases known to increase NSE levels, stroke (ischemic and/or hemorrhagic) or traumatic brain injury in the previous 30 days, and patients subjected to extracorporeal circulation in the previous 30 days.
The screening process and the data collection according to the Utstein style [16] are shown in Fig. 1. Eligible patients were randomly assigned in a 1:1 ratio to receive invasive cooling (Coolgard® [CG], Zoll Medical Corporation, Chelmsford, MA, USA) or surface cooling (ArcticSun® [AS], Medivance Inc., Louisville, CO, USA) using computer-generated random numbers.
The study protocol and consent procedure were approved by the local institutional review board and have therefore been performed in accordance with the ethics standards laid down in the 1964 Declaration of Helsinki and its later amendments. To start as early as possible with the treatment, informed consent was waived in accordance with ethical standards of the local institutional review board and guidelines for Good Clinical Practice. Patients’ relatives were informed about the trial as soon as possible. Later during hospitalization, informed consent was signed by patients regaining their consciousness or by legal representatives in charge.
Personnel involved in the care of patients during the time of MIH could not be blinded with respect to treatment assignments. However, the physicians responsible for assessing the neurological outcome during follow-up at hospital discharge were unaware of treatment assignments as well as the core laboratory for NSE assessment.
Patients underwent routine initial assessment and standard institutional intensive care treatment, including adequate sedation, paralysis to prevent shivering, mechanical ventilation, medical therapy for stabilization of circulation and—when indicated—invasive coronary angiography and intervention. MIH was initiated as soon as possible after admission.
Patients randomized to invasive cooling were supplied with an endovascular catheter (Icy®, Zoll Medical Corporation, Chelmsford, MA, USA) via the femoral vein, connected to the thermal regulation system CG via a closed-loop tubing system.
The non-invasive AS thermoregulatory control system features a temperature controlled circulation of distilled water through a series of biocompatible hydrogel-coated energy transfer pads applied to the patient’s skin.
All patients had a catheter to drain urine along with a bladder temperature probe inserted to continuously measure core temperature and provide feedback to the cooling device. The automatic mode was set to 33.0 °C at the maximum cooling rate. The target temperature was maintained for 24 h and re-warming was performed at 0.2–0.3 °C/h. Once the core temperature reached 35.0 °C paralysis and sedation were stopped. Devices continued to operate for an additional 24–48 h to avoid hyperthermia.
Patients were evaluated in terms of demographic variables and circumstances of cardiac arrest. The APACHE II Score was measured to assess the severity of disease and to describe the morbidity of a patient when comparing the outcome with other patients [15]. Patient temperature was documented hourly during the induction and maintenance of the cooling period and during rewarming. After termination of MIH temperature was documented three times daily. Neurological examinations were carried out at admission using the Glasgow Coma Scale [30]. Neurologic examinations by attending physicians were performed at hospital discharge using the Glasgow-Pittsburgh Cerebral Performance Categories (CPC) [14]. The categories were defined as follows: CPC 1, conscious and alert with mild or no disability (return to work possible), CPC 2, conscious and alert with moderate disability (independent living possible, but not return to work), CPC 3, conscious with severe disability (unable to live independently), CPC 4, comatose or persistent vegetative state, CPC 5, death/brain death. For the purpose of this study, outcomes were dichotomized into two groups: group 1 included all patients with good (CPC 1/2) and group 2 with impaired neurological outcome (CPC 3–5).
To discover early-onset infections or bleeding complications, markers of systemic inflammation and blood counts were analyzed at specific time points before (0 h) and after initiation of cooling (6, 12, 24, 36, 48 and 72 h). NSE examinations were performed at 72 h only for study purposes. Because of the lack of data regarding the outcome procedures in reference to NSE levels, the attending physicians were not informed about the NSE values. Processing of these blood samples included centrifugation for 10 min at 3.500 rpm at 20 °C and subsequent storage of the cell-free suspension at −75 °C. Hemolyzed samples were considered non-analyzable because of the relatively high content of NSE in red blood cells and platelets. NSE values were determined using an immunoassay test kit (Kryptor, Brahms, Hennigsdorf, Germany).
The primary study endpoint was the neurological outcome assessed by NSE values at 72 h as a surrogate parameter for brain damage.
Secondary endpoints were defined as the efficacy of both cooling systems; i.e. time to the start of cooling after SCA and reaching the target temperature during the induction phase and target temperature maintenance (any recorded temperature > or <33.0 °C) during the 24 h cooling maintenance phase. In addition, the clinical and neurological outcome was assessed between both treatment groups.
Safety endpoints were defined as hypothermia-associated adverse events (AE) and included bleeding events, renal failure, infections and therapy requiring arrhythmias. Standard definitions were applied, such as the Thrombolysis in Myocardial Infarction (TIMI) classification for bleeding assessment in major and minor bleeding [5]. Sepsis was defined as systemic inflammatory response syndrome (SIRS) plus infection. Acute renal failure was present if within 48 h an abrupt increase in creatinine ≥0.3 mg/dl (26.4 µmol/l) or ≥50 % from baseline was registered. Pneumonia was diagnosed if new and persisting X-ray positive infiltrations were present plus leukocytosis (12 × 109/l) or leukopenia (<4 × 109/l); body temperature >38.3 °C or <36 °C and positive purulent bronchial secretion.
As therapy requiring arrhythmias the occurrence of ventricular tachycardia, ventricular fibrillation, supraventricular tachycardia including atrial fibrillation or bradycardia during the ICU stay and within 7 days after cardiac arrest were counted.
Owing to the exploratory nature of the study, no formal sample size calculation was performed. Baseline data of all randomized patients were analyzed. With respect to outcome analysis, only patients undergoing cooling after randomization were analyzed (“as-treated-principle”). Continuous data with normal or asymmetric distribution are described by mean ± standard deviation (SD) or median and interquartile range (IQR), respectively. For continuous data with normal distribution, an independent samples t test was used. Otherwise the nonparametric Mann–Whitney U test was used. For proportions and dichotomous, categorical variables, differences between hypothermia groups were analyzed by Fisher’s exact test. For outcome prediction sensitivity and specificity of 72 h NSE values were calculated. A p < 0.05 was considered statistically significant.
Statistical analyses were performed with SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA).
Results
Eighty patients were randomized to CG or AS (Fig. 1). Clinical and demographic characteristics of the patients are shown in Table 1. The mean age for the overall cohort was 62 years with 74 % male patients. The overall APACHE II Score of 27.5 reflects a critically ill population with a prognosticated in-hospital mortality rate between 60.5 and 63.9 %. In 45 patients (56 %) preceding symptoms had been present before SCA (e.g. syncope, chest pain, palpitations, dyspnea). Ventricular tachycardia or fibrillation as initial rhythm was detected in 66 % of patients. The rate of eye-witnessed arrest was 89 %, and in 56 % bystander resuscitation was performed. The time until ROSC was comparable in both groups. No differences were found between treatment groups except for a higher prevalence of acute myocardial infarctions and therefore percutaneous coronary interventions in the CG group (p = 0.02).
NSE values of 59 (73.8 %) patients were measured at 72 h. In the remaining patients, samples were not collected due to death before 72 h or were non-analyzable due to hemolysis. NSE values did not differ significantly between the 2 groups (16.5 ng/ml, IQR: 11.8–46.5 in AS-cooled patients versus 19.0 ng/ml, IQR: 11.0–42.0 in CG-cooled patients, p = 0.99). The cut off value predicting unfavorable outcome with 100 % specificity in our patients was 29.0 ng/ml at 72 h.
The overall survival (53.8 versus 61.5 %, p = 0.65) and survival with good neurological outcome during hospitalization (35.9 versus 35.9 %, p = 0.99; Table 2) was similar for AS- and CG-cooled patients.
Time frames of the different phases after SCA to target temperature are displayed in Fig. 2. Start of cooling ranged from 15 to 765 min after ROSC. One patient in each group was not immediately treated with MIH after randomization because of temporary circulatory instability before the onset of cooling in one and anisocoria that led to cerebral computed tomography to exclude intracranial bleeding before starting MIH in another patient.
Time frames from sudden cardiac arrest to target temperature in ArcticSun® surface- and Coolgard® invasive-cooled patients. AS non-invasive ArcticSun® surface cooling, CG invasive Coolgard® cooling, IQR interquartile range, MIH Mild induced hypothermia, ROSC return of spontaneous circulation, SCA sudden cardiac arrest
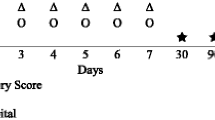
Cooling with AS was started numerically slightly earlier after SCA than with CG (180 min; IQR 155–245 min versus 242 min; IQR 165–275 min, p = 0.13). The cooling rate by CG was similar to AS (1.3 °C/h; IQR 0.7–1.6 versus 1.0 °C/h, 0.6–1.3, p = 0.29, Fig. 3) as well as the time to target temperature after the start of MIH (180 min; IQR 150–330 min in the CG group versus 240 min, IQR 180–390 in the AS group; p = 0.29). Target temperature maintenance was more stable in the CG group than in AS-cooled patients (33.0 °C; IQR 32.9–33.0 versus 32.7 °C; IQR 32.4–32.9, p < 0.001, Fig. 4). Neither cooling system showed a deviation from the proposed temperature range of 32.0–34.0 °C.
The proportion of AE is shown in Table 2. Driven by a higher rate of major bleedings—mostly mild mucosal bleedings—overall bleeding complications were more frequent with invasive cooling (Table 2). Non-bleeding related vascular complications associated with the Icy® catheter were not reported in any patient. Regarding the remaining pre-mentioned cooling-assisted complications, we did not observe any difference between both groups. There were no patients in which cooling had to be terminated due to complications.
No relevant problems occurred in placing or removing the AS pads on the patients’ skin. Reddish skin coloration observed in most patients relieved after a few minutes without resulting in any persistent harm to the skin.
Discussion
Despite a large body of resuscitation research, the optimal cooling method after SCA remains controversial. In the current trial, the first randomized comparison of invasive CG- and surface AS-hypothermia treatment in humans, devices were similarly effective in cooling comatose SCA survivors showing a similar neurological outcome by the measured NSE levels as surrogate marker of brain damage. In addition, both devices were similarly safe.
Even if it was not a randomized trial, the study by Tømte et al. compared invasive and surface cooling in patients after survived OHCA. Similar to our results, no difference between the two methods has been proven. Patients developed cooling-associated complications (pneumonia, sepsis, arrhythmias) without any statistical significance between the two groups (invasive vs. surface). Furthermore, invasive and surface cooling made no difference on survival with good neurologic function at both hospital discharge and at follow-up [31]. Although our study was not powered for clinical events, survival during hospitalization and survival in good neurological condition was similar.
NSE is a neuronal form of the intracytoplasmatic glycolytic enzyme enolase and is found in neurons and neuroectodermal cells. Owing to the limitation of neurological examination in patients treated with MIH within the first days after cardiac arrest because of sedation and muscle paralysis, determining NSE levels and taking into consideration other factors proves to correlate better with outcome. Several previous studies, mostly performed in the pre-MIH era, have shown NSE to be a reliable predictor of survival [2], regaining consciousness [19, 20] and good neurological outcome [27, 28, 33] after SCA and global cerebral ischemia [24]. The guidelines of the American Academy of Neurology for predicting outcome in comatose survivors of SCA state that serum NSE levels at days 1–3 after cardiopulmonary arrest accurately predict prognostication [32]. We chose a sampling time point of 72 h for NSE measurements. In a previous study NSE values significantly increased up to day 3 after SCA (in particular in patients with impaired neurological outcome) and rose only marginally afterwards [6, 24]. In another trial the predictive power of good neurological outcome was best at 72 h compared with 24 and 48 h [27, 28]. Similar results were presented by Rundgren et al. [26] with the help of the ROC analyses, which indicated that NSE values at 48 and 72 h were superior in comparison to earlier time points for predicting patient outcome. Furthermore, peak values of the NSE on the 3rd day after cardiac arrest were also recorded by Rosén et al. [25] and Fogel et al. [7] in their studies.
Cooling with AS could be started slightly earlier than with CG due to the ease of use of cooling mattresses and the possibility of cooling down the surface material before the patient arrives at hospital. However, this difference was not statistically different. The time frame to start of MIH in our patients was similar to previous studies [9, 13] and faster as observed in one randomized study comparing AS with standard blankets and ice packs [10]. Nevertheless, we observed some delay of cooling in our patients. An essential number of patients was first admitted to and then referred from external hospitals (35 %) on the same day. A high percentage of patients (76 %) underwent coronary angiography during the initial hospital phase in our center and cooling was halted for the time patients were in the catheterization laboratory because both feedback-controlled machines are not supplied with an independent power generator.
Overall, the time to reach the target temperature after SCA was similar between both systems. The duration of 240 min from the beginning of MIH to target temperature with a cooling rate of 1.0 °C/h in the noninvasively cooled patients is consistent with several other trials. In the HACA study, the target temperature was reached after a median of 8 h [34] while in 2 other trials using AS cooling in 27 and 64 patients after SCA, it took 137 and 190 min to reach the target temperature after cooling started [9, 10], respectively. In the current trial AS cooling achieved better cooling rates than previously published [29], possibly due to paralysis of patients that had not been implemented as standard procedure in previous studies. If paralysis is applied, it is associated with a decrease in the time to reach the target temperature [1].
According to published guidelines [22], the target temperature should be handled at 32.0–34.0 °C. In one of the milestone cooling trials by the HACA group, 20 % of patients never reached the target temperature [34]. In previous studies, the rate of inadequate AS cooling (below 32.0 °C or over 34.0 °C) ranged between 24 and 31 % [8, 10]. In the current trial, both cooling systems were able to reach the desired target temperature of 33.0 °C. In contrast to previous reports, the temperature-range in AS- or CG cooling never left the proposed range of 32.0–34.0 °C. However, CG showed slightly superior efficacy in keeping the target temperature stable at this level. The clinical significance of this finding remains, however, unclear.
Patients cooled with CG experienced more bleeding complications. Other safety endpoints, such as infections or therapy requiring arrhythmia did not differ significantly.
Hypothermia results in significant derangement of the coagulation system [3, 21, 34]. The overall 23 % major bleeding rate observed in the present trial is in line with the previous hypothermia observations reporting bleeding rates between 4 and 26 % [10, 12, 21, 34]. There may be several reasons for the higher bleeding rate with CG cooling as compared to AS-MIH, one being a possible local component of the invasive catheter (e.g. by affecting the coagulation cascade in the blood floating along the cooling catheter or by vascular access site bleeding induced by the catheter). Otherwise in our patients, local bleeding complications at the catheter insertion site always went along with mucosal bleedings. At admission, CG-cooled patients presented with more acute myocardial infarctions. Therapy with anti-coagulatory substances did not differ between both groups. Nevertheless, the activation of the coagulation system is known in connection with acute coronary syndromes and may induce bleeding complications [17].
In the current trial, two-thirds of patients developed pneumonia which is a higher rate than in previously published studies [3, 21, 34]. This might in part be explained by the fact that none of our patients received antibiotic prophylaxis during hypothermia treatment and might have also been influenced by pneumonia definitions.
Arrhythmias are common in the post-resuscitation period and are inherently related to the nature of the underlying cardiac disease, post-cardiac arrest myocardial dysfunction [22] and/or hypothermia treatment [23]. One-third of the patients in the present study developed arrhythmias, which is comparable to patients in the hypothermic treatment arm or normo-thermic control group of previous studies [3, 34].
Limitations
There are several study limitations. First, the study is not powered to detect differences in clinical outcome. Second, in some patients, the accuracy of intervals, such as time to ROSC, cooling onset or target temperature might be unreliable since information on the time when the patient collapsed relies solely on witnesses. Although care was taken to identify exact time points of relevant events by interviewing paramedics and emergency physicians, these may be subject to reporting or measurement errors. Third, our institution is a tertiary care center for patients with cardiac diseases. It is therefore possible that some SCA survivors presumed not to be of cardiac origin were referred to other hospitals. The randomized cohort might therefore not be entirely representative for the whole population of patients with ROSC following SCA.
Conclusion
Invasive and external MIH showed similar efficacy in cooling comatose patients after survived in- and out-of-hospital cardiac arrest with respect to NSE levels as a surrogate parameter for brain damage. Invasive cooling has advantages with respect to temperature stability over surface cooling, but is associated with more bleeding complications.
References
Abou-Chebl A (2004) Technical refinements and drawbacks of a surface cooling technique for the treatment of severe acute ischemic stroke. Neurocrit Care 1(2):131–143
Auer J (2006) Ability of neuron-specific enolase to predict survival to hospital discharge after successful cardiopulmonary resuscitation. CJEM 8(1):13–18
Bernard SA (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346(8):557–563
Booth CM (2004) Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 291(7):870–879
Chesebro JH (1987) Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 76(1):142–154
Einav S (2012) Modeling serum biomarkers S100 beta and neuron-specific enolase as predictors of outcome after out-of-hospital cardiac arrest: an aid to clinical decision making. J Am Coll Cardiol 60(4):304–311
Fogel W (1997) Serum neuron-specific enolase as early predictor of outcome after cardiac arrest. Crit Care Med 25(7):1133–1138
Gillies MA (2010) Therapeutic hypothermia after cardiac arrest: a retrospective comparison of surface and endovascular cooling techniques. Resuscitation 81(9):1117–1122
Haugk M (2007) Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation 75(1):76–81
Heard KJ (2010) A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 81(1):9–14
Herlitz J (1995) Hospital mortality after out-of-hospital cardiac arrest among patients found in ventricular fibrillation. Resuscitation 29(1):11–21
Holzer M (2005) Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med 33(2):414–418
Jarrah S (2011) Surface cooling after cardiac arrest: effectiveness, skin safety, and adverse events in routine clinical practice. Neurocrit Care 14(3):382–388
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1(7905):480–484
Knaus WA (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Langhelle A (2005) Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: the Utstein style. Resuscitation 66(3):271–283
Libby P (2002) Inflammation and atherosclerosis. Circulation 105(9):1135–1143
Madl C, Holzer M (2004) Brain function after resuscitation from cardiac arrest. Curr Opin Crit Care 10(3):213–217
Martens P (1998) Serum S-100 and neuron-specific enolase for prediction of regaining consciousness after global cerebral ischemia. Stroke 29(11):2363–2366
Meynaar IA (2003) Serum neuron-specific enolase predicts outcome in post-anoxic coma: a prospective cohort study. Intensive Care Med 29(2):189–195
Nielsen N (2011) Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med 39(1):57–64
Nolan JP (2010) European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 81(10):1219–1276
Peberdy MA (2010) Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122(18 Suppl 3):S768–S786
Pfeifer R (2005) Outcome after cardiac arrest: predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation 65(1):49–55
Rosén H (2001) Serum levels of the brain-derived proteins S-100 and NSE predict long-term outcome after cardiac arrest. Resuscitation 49(2):183–191
Rundgren M (2009) Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation 80(7):784–789
Schoerkhuber W (1999) Time course of serum neuron-specific enolase. A predictor of neurological outcome in patients resuscitated from cardiac arrest. Stroke 30(8):1598–1603
Shinozaki K (2009) S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care 13(4):R121
Sterz F (2003) Hypothermia after cardiac arrest: a treatment that works. Curr Opin Crit Care 9(3):205–210
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2(7872):81–84
Tømte Ø (2011) A comparison of intravascular and surface cooling techniques in comatose cardiac arrest survivors. Crit Care Med 39(3):443–449
Wijdicks EFM (2006) Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the quality standards subcommittee of the american academy of Neurology. Neurology 67(2):203–210
Zandbergen EGJ (2006) Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 66(1):62–68
Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 346, 8, 549–556 (2002)
Acknowledgments
This study was supported in part by a research grant from the University of Leipzig-Heart Center. The final manuscript has been approved by all authors. The authors would like to thank Goetz Gelbrich, PhD, for his contribution to the design of this study. We also would like to thank the staff of the Intensive Care Unit of the University of Leipzig-Heart Center.
Conflict of interest
Undine Pittl, MD, and her co-authors have no conflict of interest. On behalf of all authors, the corresponding author states that there is no conflict of interest. None of the authors received any financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov NCT: 00843297.
Rights and permissions
About this article
Cite this article
Pittl, U., Schratter, A., Desch, S. et al. Invasive versus non-invasive cooling after in- and out-of-hospital cardiac arrest: a randomized trial. Clin Res Cardiol 102, 607–614 (2013). https://doi.org/10.1007/s00392-013-0572-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-013-0572-3