Abstract
Purpose
In patients >80 years (octogenarians), there is an increased incidence of bradycardia due to age-related alteration of the sinus node, AV node, and the conduction system. Therefore, the treatment of angina pectoris with beta-blockers may be limited by bradycardia. The REDUCTION multicenter study evaluated the efficacy of ivabradine in stable angina pectoris in everyday practice. This subgroup analysis evaluated the efficacy and safety of ivabradine in octogenarians.
Methods
A total of 4,954 patients were included in the REDUCTION study for treatment of stable angina pectoris. This group included 382 octogenarians (mean age 83 ± 2.9 years) who were followed up over 4 months. Patients were treated with ivabradine in flexible doses (2.5, 5, or 7.5 mg bid). Heart rate (HR), angina pectoris attacks, nitrate consumption, overall efficacy, and tolerance were evaluated.
Results
After 4 months of treatment with ivabradine, HR was reduced by 12.0 ± 12.0 bpm from 83.0 ± 15.4 to 71.0 ± 10.1 bpm (p < 0.0001). Angina pectoris attacks were reduced from 3.0 ± 4.6 to 0.8 ± 1.8 per week (p < 0.0001). Consumption of nitrates decreased from 4.2 ± 5.1 to 1.2 ± 2.7 (p < 0.0001). Four patients reported suspected adverse drug reactions. In one patient a syncope occurred. There was no symptomatic bradycardia reported. Efficacy and tolerance were assessed as ‘very good/good’ for 95 and 99%.
Conclusions
The results demonstrate that ivabradine efficiently reduces HR, number of angina attacks, and nitrate consumption in octogenarian patients. The treatment was safe and well tolerated without relevant bradycardia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A high heart rate (HR) can provoke myocardial ischemia and angina in patients with coronary artery disease [1, 2]. Therefore, HR should be reduced to 55–60 bpm for treatment of angina [3–5]. Various studies demonstrated that a low resting HR was associated with a low cardiovascular mortality [5–9].
Ivabradine reduces HR by a selective effect on the sinus node [10]. In comparison to other HR-lowering agents, ivabradine has an extremely selective mode of action. Nearly all other drugs which reduce HR, such as beta-blockers or calcium channel blockers, act on the sinus node, the AV node, and the conduction system. This may lead to deleterious side effects, such as AV block or severe bradycardia [11]. These effects have particular importance in elderly patients over 80 years (octogenarians) because in this group a high incidence of age-related degeneration of the conduction system can be expected. If octogenarians receive negative chronotropic drugs, they often react more sensitive than younger people. For instance, it is a common clinical observation that elderly people often react sensitive to small doses of beta-blockers, including the induction of AV blocks and syncope.
Since HR reduction in the elderly is associated with these drawbacks, it appears to be particularly attractive to target their sinus node as selective as possible.
Octogenarians are usually not included or they are underrepresented in controlled clinical trials [12–16]. Reasons for this include poor compliance due to cerebrovascular disease or Alzheimer’s disease, comorbidities such as advanced renal or liver failure, inability to perform exercise tests, or insufficient mobility to attend follow-up visits. Octogenarians also appear to be underrepresented in different controlled studies on the effects of ivabradine [7, 17–20]. However, octogenarians are increasingly represented in common practice of general and internal physicians.
Clinical trials strictly select patients [21]. Because of selection and more thorough follow-up, patients in controlled trials do not represent everyday populations [22]. For this reason, there is a need to analyze whether favorable data from clinical trials translate into clinical practice. This was done with the open-label REDUCTION study [23]. It showed a high degree of efficacy and safety of the treatment of patients with coronary artery disease with ivabradine in a patient population of 4,954 patients under everyday practice conditions [23].
In this subgroup analysis from the REDUCTION study, we evaluated whether treatment with ivabradine is safe and efficient in everyday routine practice in octogenarians.
Methods
Design
The study was conducted in a multi-center, prospective, open-label, non-interventional design. 4,954 patients who received ivabradine were included in the study. They were prospectively followed by 1,503 cardiologists, internal medicine physicians, and general practitioners in private practice in Germany. All of these filled out a standardized questionnaire during the treatment and follow-up.
The trial was in accordance with the regulations of the Declaration of Helsinki and was conducted conform to the ethical guidelines of the European Independent Ethics Committee.
Inclusion and exclusion criteria
The inclusion criterion was the necessity for medical treatment of chronic stable angina pectoris in patients with stable sinus rhythm.
The following indications were given for treatment with ivabradine:
-
Initial medical treatment for recurrent stable angina pectoris.
-
Modification of the medical therapy of the patient during treatment for chronic stable angina pectoris, induced by drug intolerance/side effects, insufficient efficacy, or contraindications.
-
Expansion of the ongoing therapy for chronic stable angina pectoris with or without progressive disease.
In the octogenarians, it was recommended to start therapy with ivabradine 2.5 mg twice daily before a gradual up-titration to the target dose of 7.5 mg twice daily. The up-titration of dosage was recommended after 2–4 weeks if the drug was tolerated and if HR was above the target HR of 55–60 bpm for the treatment of chronic stable angina pectoris.
Clinical examinations
The patients underwent three clinical controls: at baseline and after 1 and 4 months of treatment with ivabradine. At the baseline visit, the general and cardiac history was taken. Concomitant disease, concomitant therapy, and cardiac risk factors were evaluated. Blood pressure and HR were documented. The number of angina pectoris episodes within the last week and the application of short-acting nitrates (including nitrate hubs and capsules) were registered.
The first follow-up examination took place after 2–4 weeks. At this control, the following parameters were documented: HR, blood pressure, number of angina pectoris attacks within the previous week, and the consumption of short-acting nitrates. The dose of ivabradine was increased if it was appropriate, and the patient was asked for suspected adverse drug reactions (ADR). At the second follow-up visit after 4 months, the same controls as after 1 month were repeated. The change of co-medication was documented, and a final evaluation of efficacy and tolerance of ivabradine was performed by the physician. For this purpose, a scale including ‘very good’, ‘good’, ‘moderate’, or ‘poor’ was used.
Statistical analysis
The statistical analysis included a descriptive analysis which was performed using SAS® software, version 9.1. Patients were included if they had valid data from baseline and all follow-up visits. The changes of the HR and blood pressure were analyzed with the one-sample t test. Changes of the occurrence of angina pectoris attacks and the need for the use of short-acting nitrates were evaluated by the Wilcoxon signed-rank test. Data are expressed as mean ± SD. A p value <0.05 was considered as significant.
Results
Study population
Of the 4,954 patients 382 were octogenarians. The following data refer to this group of octogenarians: 58% females, 42% males (whereas in the entire REDUCTION population there were 59% males). Their mean age was 83 ± 3 years. 96.6% were aged between 80 and 90 years, 3.4% between 90 and 100 years. 319 patients (84%) had arterial hypertension and 268 (70%) hypercholesterolemia. 123 (32%) suffered from overweight, and 108 (28%) had diabetes mellitus. 32 (8%) were smokers or ex-smokers.
History revealed a mean duration of coronary artery disease in the octogenarian group of 9.0 ± 6.7 years (whereas this was 5.2 ± 4.9 years in the entire REDUCTION group). The duration of recurrent angina pectoris was with 5.6 ± 5.2 years longer than in the total group (3.6 ± 3.9 years). In 12% of the patients, the duration of angina pectoris was short (<6 months). The CCS classification of the degree of angina pectoris of the patients is shown in Table 1 [24].
Compared to the general REDUCTION population, there were less patients with angina grade CCS I, but more with grade CCS III and IV.
Concomitant diseases, medication, and procedures
There were 129 patients (35%) who had undergone a percutaneous coronary intervention (PCI) previously. 75 patients (20%) had been treated with coronary artery bypass surgery (CABG). A PCI or CABG surgery or both had been performed in 171 of the patients (45%). 132 patients (35%) had a previous myocardial infarction. Concomitant diseases are listed in Table 2.
Before the start of therapy with ivabradine, beta-blockers were discontinued in 170 patients [in 110 patients (65%) for side effects, in 25 (15%) for contraindications, and in 48 (28%) for insufficient effect]. The mean doses of beta-blockers besides the ivabradine therapy during the observation period of the study were metoprolol 78 ± 46 mg/day, bisoprolol 5 ± 2 mg/day, carvedilol 19 ± 7 mg/day, and nebivolol 5 ± 0 mg/day. Calcium channel blockers were discontinued in 17 patients [side effects in 8 patients (47%), insufficient effect in 12 patients (71%), and contraindications in 3 patients (18%)], and glycosides in 15 patients [side effects in 4 patients (27%), insufficient effect in 12 patients (80%), and glycosides were applied in standard doses]. Co-medication during the observation period is shown in Tables 3 and 4.
The dose of ivabradine applied during follow-up is shown in Table 5.
The dose of ivabradine was increased in 31.6% of the patients during follow-up (mean 1.7 mg). In one patient (0.3%) it was reduced. After the observation period, ivabradine therapy was continued in 354 (94.4%) of the patients and discontinued in 21 (5.6%).
In four patients, ivabradine was discontinued for intolerance or side effects, in 17 patients for other reasons, such as insufficient efficacy (4 patients), insufficient compliance (2 patients), or due to independent termination of therapy by the patient (the reasons include freedom of symptoms).
Heart rate
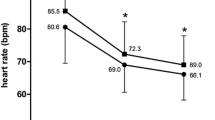
Between baseline and the third visit after 4 months, the mean HR was significantly reduced by 12.0 ± 12.0 bpm from 83.0 ± 15.4 to 71.0 ± 10.1 bpm (p < 0.0001) (Fig. 1). HR reduction in the group with concomitant beta-blocker therapy was 14.0 ± 10.6 bpm (p < 0.0001), and in the group with concomitant glycoside therapy, it was 16.6 ± 14.8 bpm (p = 0.0078). In the group with combined beta-blocker and glycoside therapy (n = 4), it was 23.5 ± 11.1 bpm (no p value in n = 4 patients).
Angina pectoris and nitrate consumption
Eighty-one percent of the patients had one or more angina pectoris attacks per week prior to the start of treatment with ivabradine. At follow-up after 4 months, the frequency of angina pectoris attacks had been markedly reduced during the treatment with ivabradine (3.0 ± 4.6 to 0.8 ± 1.8 attacks per week, p < 0.0001) (Fig. 2). In the group with concomitant beta-blocker therapy, angina pectoris attacks were reduced from 2.3 ± 2.2 to 0.6 ± 1.3 attacks per week, p < 0.0001), in the group with concomitant glycosides from 2.4 ± 2.2 to 0.2 ± 0.4 attacks per week (p = 0.0078). In the group with concomitant combined beta-blocker and glycoside therapy, angina pectoris attacks were reduced from 2.7 ± 3.8 to 0.3 ± 0.6 attacks per week (no p value calculated in n = 4 patients).
The need for nitrates (hubs/capsules) between baseline and the follow-up visit after 4 months was significantly reduced during the treatment with ivabradine (4.2 ± 5.1 to 1.2 ± 2.7, p < 0.0001) (Fig. 3). In the group with concomitant beta-blocker therapy, the need for nitrates was reduced from 3.1 ± 3.6 to 1.0 ± 2.1 applications, p < 0.0001), in the group with concomitant glycosides from 2.0 ± 2.4 to 0.3 ± 0.5 applications (p = 0.06). In the group with concomitant combined beta-blocker and glycoside therapy, the need for nitrates was reduced from 2.3 ± 4.0 to 0.3 ± 0.6 applications (no p value calculated in n = 4 patients).
Blood pressure was lowered from 138.0 ± 18.0 to 131.0 ± 13.5 mmHg (systolic −6.9 ± 13.6 mmHg) and from 81.0 ± 11.4 to 77.3 ± 8.5 mmHg (diastolic −3.8 ± 10.0 mmHg) between baseline and the fourth month of treatment including ivabradine and co-medication.
The efficacy of ivabradine was graded as ‘very good’ in 56.6% of the patients, as ‘good’ in 38.1%, as ‘moderate’ in 4.2%, and as ‘poor’ in 1.1% of the patients.
Adverse events/adverse drug reactions
Four patients (1%) experienced suspected adverse drug reactions (ADR). One was classified as severe. This was a syncope in a 92-year-old female 29 days after start of therapy with ivabradine. The event was classified as possibly related to ivabradine. The three other suspected ADR included dizziness (n = 2), classified as probably and possibly related to ivabradine, and tachycardia (n = 1), unlikely related to ivabradine. There were no clinical sequelae from any event. Luminous phenomena (phosphenes) were not reported. There were no bradycardia or AV block reported. There was no suspected ADR in the group of patients with a combined therapy with beta-blockers or glycosides.
Physicians graded the patients’ tolerance of ivabradine as ‘very good’ in 65.0% of the patients, as ‘good’ in 33.7%, as ‘moderate’ in 0.5%, and as ‘poor’ in 0.8% of the patients.
Discussion
The main findings from this study are that treatment with ivabradine is efficient and safe in the complex patient group with an age beyond 80 years. In this group, which is naturally prone to bradycardia, ivabradine reduced the HR in a controlled way and without any critical bradycardia. This was evident when ivabradine was part of a rate lowering monotherapy and also in combination with beta-blockers or glycosides. Along with the reduced HR, angina pectoris episodes and nitrate consumption were significantly reduced, whereas side effects, such as ophthalmic symptoms, occurred less frequent than expected from data of published randomized trials [7, 8, 17–19].
The octogenarian patient group seen in this study, with its mean age of 83 years, multiple co-morbidities, and co-medications, can be regarded as a more complex patient group than that typically seen in clinical trials and in average in everyday practice. Compared with the total REDUCTION group, there was a lower number of patients with angina pectoris grade CCS I and a higher proportion of those with grade CCS III [23]. History of angina pectoris was lasting longer, and the rate of myocardial infarctions was higher [23]. This suggests that the octogenarian group is a more symptomatic patient group, and that there is more potential and need to reduce angina symptoms in this group than in younger patients. In addition, there is a known reservation of cardiologists to treat octogenarians interventionally because of the widespread perception that PCI entails an unfavorable risk–benefit ratio [25, 26]. Therefore, medical treatment often remains the only therapy and has a special importance in the treatment of octogenarians. However, there is a common fear to induce bradycardia or AV block with beta-blockers, which exert action on both nodes. In this study, there was no evidence for relevant bradycardia under ivabradine. Therefore, the substance appears to be an appropriate therapeutic tool to lower HR in octogenarians. HR reduction in this age group was pronounced (approximately 12 bpm), but still comparable to that seen in previous trials and other populations [7, 17–19, 27, 28]. Concomitant beta-blocker therapy did not affect the efficacy or safety of ivabradine. Thus, the high efficacy and safety of ivabradine in combination with beta-blockers in this octogenarian group are comparable to that seen previously in younger patients treated with this combination [7, 8, 19]. The high efficacy of ivabradine in combination with beta-blockers is plausible because HR reduction by beta-blockers and ivabradine is mediated via different pathways [10].
Angina pectoris attacks and nitrate consumption were significantly reduced in the octogenarians. The reduction of symptoms and the need for antianginal treatment was comparable to that seen in other trials [17, 18]. In this study, there was a small reduction of mean blood pressure at follow-up. This can be explained by a change of the cardiovascular co-medication such as ACE inhibitors in a small group of patients during the observation period. Ivabradine is not expected to lower the blood pressure [10]. Reduction of blood pressure is a known cause for reduction of angina pectoris [29]. Therefore, blood pressure reduction in this study may have contributed to the reduction of angina pectoris and may have been another cause for relief of angina pectoris beyond the ivabradine effect. However, since blood pressure reduction in this study was only 7/4 mmHg, the possible antianginal effect due to reduction of blood pressure appears small and of minor clinical importance.
The mean ivabradine dose after 4 months of treatment in this group was only 9.4 mg per day, whereas the maximum approved dose is 15 mg per day. Therefore, there may be additional potential to further reduce symptoms in the octogenarian group by using higher doses. The moderate dosage in this study may reflect the natural caution of physicians with the handling of novel drugs and the reservations using negative chronotropic agents in the very elderly in fear of iatrogenic bradycardia.
Suspected adverse events were unexpectedly rare in the octogenarian population compared to that in other controlled trials with predominantly younger patients [7, 17–19]. This suggests an excellent tolerance of the drug under everyday conditions in octogenarians. Suspected adverse drug reactions occurred in 1.0% of the patients despite a wide range of concomitant medical therapy, possible drug interference, and potentially changed pharmacodynamics in the advanced age [30]. Adverse reactions may have been underrecognized because they may present atypically or hypo-symptomatically in the elderly patients [30]. In addition, a blunted appreciation of symptoms becomes more common in the very elderly patients. This may lead to underreporting of adverse events. Interestingly, there were no ophthalmic events, such as phosphenes, reported in the octogenarians. This difference to other trials may be explained by the kind of questionnaire used in the present study. Patients were interviewed in an open mode for side effects and not specifically for visual sensations. A previous study revealed that individuals report visual symptoms four times more often if they are aware of the possibility that they can occur [31]. It may be assumed that patients in clinical trials were more aware of phosphenes than those in everyday practice and therefore had a higher sensitivity to detect and report them.
Considering the low rate of adverse reactions, it is comprehensible that the tolerance of ivabradine was graded as ‘very good’ or ‘good’ in the majority of patients. Therefore, the favorable tolerance seen in other trials was maintained in this complex patient group.
Study limitations
The major limitation is the open label, observational, non-interventional study design. This design may lead to an overestimation of the antianginal effects of ivabradine. However, it most closely follows the conditions of everyday practice. An additional limitation is the lack of a placebo control group. However, regarding the recent subanalyses from the BEAUTIFUL trial, it appears to be unethical to perform a study with placebo in the subgroup with severe angina and high HR [32].
It was almost impossible to exclude severe nocturnal bradycardia without the performance of a Holter ECG. Holter ECGs were not routinely performed in these patients because this study was performed in an everyday practice setting, and there was no clinical indication to perform Holter ECGs in all patients.
There was a large group of patients who was treated previously with a beta-blocker and had the treatment stopped. Therefore, a selection bias due to forced withdrawal of beta-blockers and the prescription of ivabradine as a new treatment option cannot be completely ruled out. However, the study also shows that another group of octogenarians can receive beta-blockers without problems and can be treated with the combination of beta-blockers and ivabradine with additional clinical benefit.
Since the maximum dose of 7.5 mg bid was only reached in 13% of the patients, and the mean HR after 4 moths was still at 71 bpm, there appears to be further potential to increase the efficacy of the treatment. This suggests that the full therapeutic potential of the drug is underestimated in this trial. However, with the low dose used in this trial, side effects, which rather occur at higher doses, may also have been underestimated.
Conclusions
Ivabradine significantly reduces HR in the complex octogenarian patient group. This is associated with a reduction of angina pectoris episodes and nitrate consumption under everyday practice conditions. Treatment was safe without any critical bradycardia. That was also the case together with beta-blocker or glycoside co-medication. Adverse reactions were rare. Therefore, ivabradine appears to be a favorable treatment option for the complex octogenarian patients with angina pectoris.
References
McLenachan JM, Weidinger FF, Barry J, Yeung A, Nabel EG, Rocco MB, Selwyn AP (1991) Relations between heart rate, ischemia, and drug therapy during daily life in patients with coronary artery disease. Circulation 83:1263–1270
Heusch G, Schulz R (2007) The role of heart rate and the benefits of heart rate reduction in acute myocardial ischemia. Eur Heart J 9(Suppl F):F8–F14
Dietz R, Rauch B (2003) Leitlinie zur Diagnose und Behandlung der chronischen koronaren Herzkrankheit der Deutschen Gesellschaft für Kardiologie—Herz- und Kreislaufforschung. Z Kardiol 92:501–521
Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, Marco J, Morais J, Pepper J, Sechtem U, Simoons M, Thygesen K (2006) Guidelines on the management of stable angina pectoris. Eur Heart J 27:1341–1381
Fraker TD, Fihn SD (2007) Chronic Angina Focused Update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina. Circulation 116:2762–2772
Fox K, Borer J, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M (2007) Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50:823–830
Fox K, Ford I, Steg PG, Tendera M, Ferrari R, BEAUTIFUL Investigators (2008) Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet 372(9641):807–816
Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R (2008) Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 372(9641):817–821
Reil JC, Böhm M (2007) The role of heart rate in the development of cardiovascular disease. Clin Res Cardiol 96:585–592
DiFrancesco D, Camm JA (2004) Heart rate lowering by specific and selective I(f) current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs 64:1757–1765
Damman K, de Boer RA, van Veldhuisen DJ (2008) Heart failure, aging and beta-blockers: the need of more data on tolerability and efficacy. Clin Res Cardiol 97:575–577
Gurwitz JH, Col NF, Avorn J (1992) The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA 268:1417–1422
Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED (2001) Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA 286:708–713
Dauermann HL, Bhatt DL, Gretler DD, French PA, Smyth SS, Becker RC (2010) Bridging the gap between clinical trials of antiplatelet therapies and applications among elderly patients. Am Heart J 159:508.e1–517.e1
Ostad MA, Nick E, Paixao-Gatinho V, Schnorbus B, Schiewe R, Tschentscher P, Münzel T, Warnholz A (2010) Lack of evidence for pleiotropic effects of clopidogrel on endothelial function and inflammation in patients with stable coronary artery disease; results of the double-blind, randomized CASSANDRA study. Clin Res Cardiol. doi:10.1007/s00392-010-0199-6
Brehm M, Ebner P, Picard F, Urbien R, Turan G, Strauer BE (2009) Enhanced mobilization of CD34+ progenitor cells expressing cell adhesion molecules in patients with STEMI. Clin Res Cardiol 98:477–486
Borer JS, Fox K, Jaillon P, Lerebours G (2003) Antianginal and antiischemic effects of ivabradine, an If inhibitor, in stable angina A randomized, double-blind, multicentered, placebo-controlled trial. Circulation 107:817–823
Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K, INITIATIVE Investigators (2005) Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 26:2529–2536
Tardif JC, Ponikowski P, Kahan T, ASSOCIATE Study Investigators (2009) Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J 30(5):540–548
Tendera M, Borer JS, Tardif CL (2009) Efficacy of I(f) inhibition with ivabradine in different subpopulations with stable angina pectoris. Cardiology 114:116–125
Pocock SJ, Elbourne DR (2000) Randomized trials or observational tribulations? N Engl J Med 342:1907–1909
Concato J, Shah N, Horwitz RI (2000) Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 342:1887–1892
Köster R, Kaehler J, Meinertz T, REDUCTION Study Group (2009) Treatment of stable angina pectoris by ivabradine in every day practice: the REDUCTION study. Am Heart J 158(4):e51–e57
Campeau L (1976) Grading of angina pectoris. Circulation 54:522–523
Kähler J, Lütke M, Weckmüller J, Köster R, Meinertz T, Hamm CW (1999) Coronary angioplasty in octogenarians. Eur Heart J 20:1791–1798
Schuler J, Maier B, Behrens S, Thimme W (2006) Present treatment of acute myocardial infarction in patients over 75 years. Clin Res Cardiol 95:360–367
Zhang R, Haverich A, Strüber M, Simon A, Pichlmaier M, Bara C (2008) Effects of ivabradine on allograft function and exercise performance in heart transplant recipients with permanent sinus tachycardia. Clin Res Cardiol 97:811–819
Koester R, Kaehler J, Ebelt H, Soeffker G, Werdan K, Meinertz T (2010) Ivabradine in combination with beta-blocker therapy for the treatment of stable angina pectoris in every day clinical practice. Clin Res Cardiol. doi:10.1007/s00392-010-0172-4
Robinson BF (1967) Relation of heart rate and systolic blood pressure to the onset of pain in angina pectoris. Circulation 35:1073–1083
Podrazik PM, Schwarz JB (1999) Cardiovascular pharmacotherapy of aging. Cardiol Clin 17:17–34
Siegel RK (1977) Hallucinations. Sci Am 237:132–140
Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R, BEAUTIFUL investigators (2009) Relationship between ivabradine treatment and cardiovascular outcomes in patients with stable coronary artery disease and left ventricular systolic dysfunction with limiting angina: a subgroup analysis of the randomized, controlled BEAUTIFUL trial. Eur Heart J 30(19):2337–2345
Acknowledgments
The authors thank Dieter Schremmer from the ‘Gesellschaft für Therapieforschung’ in Munich, Germany, for his substantive support of the statistical analysis, and all investigators for their contributions to the study. The investigators participated were J. Taggeselle, L. Feß, R. Aubele, N. Hassler, K. Hofmann, V. Adelberger, T. Arnold, B. Holz, M. Hwaidi, H.-D. Kombächer, R. Meysing, S. Appel, J. Bazowski, R. Bernauer, H. Böneke, M. Braun, E. Daelmann, M. Deißner, S. Duddy, M.-A. Eisenbarth, H. Fissan, C. Freese, G. Gölz, M. Gutting, K. Hallbaum, M. Hilgedieck, J.-A. Hintze, H. Hohensee, T. Hohenstatt, O. Khan, H.-H. Knäbchen, A. Krämer, K. Krämer, R. Lange, A. Levertov, H. Littwitz, U. Meyer, K. Müller, L. Rokitzki, C. Ruhnau, K. Rybak, R. Schmitt, A. Spingler, H. Stellmach, R. Tietze, W. Türk, R. Vormann, T.-A. Wiegmann, G. Will, E. Wüstenberg, J. Zivojinovic. The list of the further investigators is available from the corresponding author.
Conflict of interest
RK’s and TM’s participation at scientific congresses has been supported by Servier Munich, Deutschland. TM is member of the advisory board. JK has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the REDUCTION Study Investigators.
Rights and permissions
About this article
Cite this article
Koester, R., Kaehler, J. & Meinertz, T. Ivabradine for the treatment of stable angina pectoris in octogenarians. Clin Res Cardiol 100, 121–128 (2011). https://doi.org/10.1007/s00392-010-0220-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-010-0220-0