Abstract
Objectives
The present study aimed to investigate the clinical and echocardiographic determinants of plasma NT-pro-BNP levels in patients with atrial fibrillation (AF) and preserved left ventricular ejection fraction (LVEF).
Methods
NT-pro-BNP levels were measured in 45 patients with paroxysmal AF, 41 patients with permanent AF and 48 controls.
Results
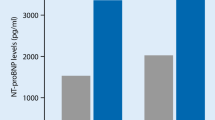
NT-pro-BNP levels were found significantly elevated in patients with paroxysmal (215 ± 815 pg/ml) and permanent AF (1,086 ± 835 pg/ml) in relation to control population (86.3 ± 77.9 pg/ml) (P < 0.001). According to the univariate linear regression analysis, age, hypertension, β-blocker use, left atrial diameter (LAD), LVEF and AF status (paroxysmal or permanent or both) were significantly associated with NT-pro-BNP levels (P < 0.05). In multiple linear regression analysis, LVEF (B coefficient: −53.030; CI: −95.738 to −10.322; P: 0.015) and LAD (B coefficient: 285.858; CI: 23.731–547.986; P: 0.033) were significant and independent determinants of NT-pro-BNP levels.
Conclusions
Plasma NT-pro-BNP levels were significantly higher in patients with paroxysmal and permanent AF compared to those with sinus rhythm in the setting of preserved left ventricular systolic function. LVEF and LAD were independent predictors of NT-pro-BNP levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natriuretic hormone axis is the best characterized cardiac endocrine pathway. The family of natriuretic peptides (NP) share several actions, including natriuresis, vasodilation, and renin inhibition [2, 12]. In the setting of volume expansion or pressure overload, the resulting wall stress initiates synthesis of pre-pro-B-type natriuretic peptide (BNP) in the ventricular myocardium. Subsequently, the peptide is cleaved first to pro-BNP, and then to the biologically active BNP and the inactive amino-terminal fragment (NT-pro-BNP) [2, 12]. NP are now considered the most popular and cost-effective biomarker for the non-invasive diagnosis and prognosis of heart failure [23]. Furthermore, plasma BNP levels are considered independent predictors of mortality or cardiovascular events in several populations, including community-based samples [11, 14, 19].
Atrial fibrillation (AF), the most common arrhythmia in clinical practice, is considered an important source of morbidity and mortality [7]. Furthermore, there is a well-documented relationship and a complex interaction between AF and heart failure [4]. AF displays significant effects on mechanical and endocrine cardiac functions [17]. AF has been associated with atrial and ventricular remodeling, even in patients without structural heart disease [1, 20]. Previous studies have demonstrated that plasma BNP levels are increased in patients with AF compared to controls, and are significantly decreased following restoration of sinus rhythm (SR) [6, 15, 21]. Limited data regarding the determinants of BNP levels in patients with AF and preserved left ventricular (LV) systolic function exist in the current literature. The present study aimed to investigate the plasma NT-pro-BNP levels in patients with isolated AF. The clinical and echocardiographic determinants of NT-pro-BNP levels were evaluated.
Patients and methods
In the present study, we recruited consecutive patients with AF, either paroxysmal or permanent, who were seen at the emergency department or at the outpatient clinic of our hospital. As control group, we enrolled consecutive individuals with no history of arrhythmias who were undergoing regular routine clinical examination for health certificate. The arrhythmia diagnosis required an electrocardiographic documentation whereas its classification was based on authoritative international consensus statements [13]. Αll participants consented to the study and the local research and ethics committee approved the protocol.
The presence of structural heart disease was evaluated by detailed medical history, physical examination, and transthoracic echocardiography. Patients with known structural heart disease (coronary artery disease, heart failure, valvular disease, congenital heart disease, left ventricular hypertrophy, ejection fraction <50%) or underlying extracardiac conditions affecting the NT-pro-BNP levels [renal failure (serum creatinine > 1.2 mg/dl), liver dysfunction] were excluded from the study. Transthoracic echocardiography (M-mode and 2-D scans) was performed in parasternal and apical views. Measurements were averaged for three cardiac cycles in subjects with SR and for 10 cardiac cycles in patients with AF. Left ventricular ejection fraction (LVEF) was estimated using the Simpson’s method. Left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) diameters as well as end-systolic anteroposterior left atrial diameter (LAD) were determined in all subjects.
Blood samples were obtained in EDTA from each subject. Plasma NT-pro-BNP levels were determined using a commercially available enzyme immunoassay run on the Modular Analytics E170 (Elecsys, Roche Diagnostics), according to the manufacturer’s instructions. This assay is reported to have ≤0.001% cross reactivity with bioactive BNP.
Statistical analysis
Continuous variables are presented as mean values ± standard deviations while categorical variables are presented as absolute and relative frequencies (percentages). The Shapiro-Wilk criterion was used for the assessment of normality. In order to assess the difference of distribution of continuous variables among the three groups of our study (controls, paroxysmal AF, permanent AF), one-way analysis of variance (ANOVA) was performed. Student’s t tests were performed in all continuous variables among pairs of study groups. The Bonferroni adjustment for inflation of type I error due to multiple comparisons was used for the purposes of the post-hoc analysis. The mean differences between two groups were evaluated by calculating Student’s t test after controlling for equality of variances with the Levene’s statistic. Due to the non-normal distribution of NT-pro-BNP variable, summary statistics are presented as medians and interquartile ranges, while non-parametric analysis was used (Mann–Whitney U for pair comparisons, Kruskal-Wallis for comparison among the three study groups). Pearson’s chi-square test was used in order to test for any associations between two categorical variables. Forward stepwise linear univariate and multiple regression models were used to examine the independent significant predictors of NT-pro-BNP levels. In order to validate our multiple linear regression models, we tested the normality and heteroscedasticity of the residuals produced. Any potential multicollinearity between the explanatory variables was tested by calculating the variance inflation factor (VIF) and tolerance (1/VIF). No multicollinearity was found since tolerance values were higher than 0.1. Finally the adjusted R 2 was calculated. Our multiple regression model had the highest adjusted R 2 value of all other models, explaining the variability of our dependent variable the best way possible. Using the receiver operating characteristic (ROC) curves, a cut-off point analysis was applied to evaluate NT-pro-BNP levels with the best predictive ability regarding the AF status (paroxysmal or permanent). All reported P values are based on two-sided tests and compared to a significance level of 0.05 (except for multiple comparisons). Data were analyzed using STATA statistical software (Version 9.0, Stata Corporation, College Station, TX 77845, USA).
Results
Patient’s characteristics, echocardiographic and laboratory data are depicted in Table 1. The study included 45 patients with paroxysmal AF (28 males, aged 67.7 ± 8.8 years), 41 patients with permanent AF (26 males, aged 71.9 ± 9.9 years) and 48 controls (27 males, aged 61.3 ± 14.7 years). All subjects were free from symptoms and signs of heart failure and exhibited a preserved LV systolic function (mean LVEF > 50%). Regarding paroxysmal AF group, the number of documented arrhythmic episodes was 4 ± 3.4, whereas the mean duration of AF history was 24 ± 18 months. The mean duration of permanent AF was 7.9 ± 4.2 months. There were statistically significant differences regarding age (P < 0.001), hypertension (P: 0.023), β-blockers (P < 0.001) and calcium channel blockers use (P: 0.036) among the study groups. No statistically significant differences regarding gender, history of diabetes mellitus and hyperlipidemia, and use of inhibitors of the renin-angiotensin system or statins were observed among the three groups (P > 0.05).
The mean LVEF was found reduced in patients with paroxysmal (57.8 ± 5.3%) and permanent AF (55.9 ± 4.1%) in relation to control population (61.2 ± 3.1%) (P < 0.001). The mean LVEDD was not significantly different between the three groups (P > 0.05). LAD was significantly higher in patients with paroxysmal (37 ± 4 mm) and permanent AF (45 ± 5 mm) compared to controls (35 ± 6 mm) (P < 0.001). NT-pro-BNP levels were found significantly elevated in patients with paroxysmal (215 ± 815 pg/ml) and permanent AF (1,086 ± 835 pg/ml) in relation to control population (86.3 ± 77.9 pg/ml) (values in medians ± interquartile ranges, P < 0.001) (Fig. 1). Additionally, NT-pro-BNP levels were significantly higher in patients with permanent AF than those with paroxysmal AF (P < 0.001). The percentage of subjects with normal NT-pro-BNP values (<150 pg/ml as suggested by the manufacturer) among study groups was 66.7% in controls, 31.1% in paroxysmal AF and 0% in permanent AF.
According to univariate linear regression analysis age, hypertension, use of β-blockers, AF (paroxysmal or permanent or both) and LAD exhibited a positive effect on NT-pro-BNP levels, while LVEF displayed a significant inversely linear relationship with NT-pro-BNP levels (P < 0.05) (Table 2). In multiple linear regression analysis, LVEF (B coefficient: −53.030; CI: −95.738 to −10.322; P: 0.015) and LAD (B coefficient: 285.858; CI: 23.731–547.986; P: 0.033) were the only significant and independent determinants of NT-pro-BNP levels.
ROC curve analysis showed that the optimal cut-off point of NT-pro-BNP levels for paroxysmal AF detection was 149 pg/ml [sensitivity = 82.9%, specificity = 80.0%, area under the ROC curve (AUC) = 82.9%] (Fig. 2). Similarly, the optimal cut-off point of NT-pro-BNP levels for permanent AF detection was 244 pg/ml (sensitivity = 94.1%, specificity = 85.0%, AUC = 98.2%) (Fig. 3).
Discussion
The main findings of the present study are the following: (1) NT-pro-BNP levels are significantly elevated in patients with paroxysmal and permanent AF in the setting of preserved LVEF in relation to control population and (2) LVEF and LAD are significant and independent determinants of plasma NT-pro-BNP levels in patients with AF and preserved systolic function.
The increased atrial stretch during AF leads to the activation of the neurohormonal system and is likely to be responsible for the elevated levels of plasma BNP [16]. BNP has been shown to be primarily released from the atria and not from the ventricles in patients with lone AF [8]. Ellinor et al. [6] have additionally reported a discordant pattern of plasma NP concentrations, with a significant rise of NT-pro-BNP in the presence of normal NT-pro-atrial natriuretic peptide (ANP) concentrations in patients with a history of lone AF. On the contrary, Therkelsen et al. [21] showed that plasma ANP and BNP levels are both increased in patients with permanent and permanent AF, including those with lone AF. In the present study, age, hypertension, β-blockers use, LAD, LVEF and AF were correlated with NT-pro-BNP levels. LVEF and LAD were the only independent determinants of plasma NT-pro-BNP levels. A lower LVEF and a higher LAD were associated with increased NT-pro-BNP levels. We also showed that patients with AF, either paroxysmal or permanent, demonstrate an asymptomatic reduction of LVEF regarding symptoms and signs of heart failure compared to control population. The reversibility of LV dysfunction in patients with isolated AF following restoration of SR suggests that this dysfunction can be attributed in part to AF [5]. The left ventricular remodeling occurring in the setting of AF may therefore contribute in part to the elevated NT-pro-BNP concentrations observed in these patients, a fact strongly supported by the inverse relationship between LVEF and NT-pro-BNP levels observed in the present study. Other investigators have demonstrated that LA volume index and LV mass index as well as AF duration were independent predictors of plasma BNP levels in patients with chronic AF and preserved LV systolic function [10, 21].
Natriuretic peptide levels are clearly age- and gender-specific [2]. Therefore, “normal” values vary significantly. Young, healthy adults exhibit NT-pro-BNP levels <70 pg/ml [3]. For acutely dyspneic patients, NT-pro-BNP levels <300 pg/ml are considered optimal for ruling out heart failure, with a negative predictive value of 99% [9]. In the present study, the NT-pro-BNP levels in permanent AF (1,086 pg/ml) in the setting of preserved LVEF were significantly higher compared to the diagnostic thresholds proposed for ruling out heart failure. Similar high NT-pro-BNP concentrations have been reported in previous studies including patients with lone and persistent AF and normal LVEF [4, 18, 22]. Therefore, a higher diagnostic cut-off value should be considered in patients with permanent AF in order to exclude the diagnosis of heart failure. We additionally showed that the optimal cut-off points of NT-pro-BNP levels for detecting paroxysmal and permanent AF in patients with preserved systolic function and without symptoms and signs of heart failure were 149 and 244 pg/ml, respectively. The higher diagnostic threshold in permanent AF is possibly related to the extensive electrical and structural remodeling observed in this condition.
Our study has several potential limitations. Firstly, the number of participants was relative small. Secondly, plasma ANP levels, which might have helped to understand the haemodynamic changes in AF were not measured. Thirdly, echocardiographic parameters of LV diastolic function were not assessed. Finally, we have to acknowledge that patients with paroxysmal AF are a quite heterogeneous group, and thus the relative impact on NT-pro-BNP levels is difficult to be estimated. Most episodes of paroxysmal AF are brief and/or asymptomatic and therefore the exact burden is difficult be assessed, even with repeatedly ambulatory recordings.
In conclusion, plasma NT-pro-BNP levels were higher in subjects with paroxysmal and permanent AF than in those with SR in the setting of preserved LV systolic function. LVEF and LAD were significant and independent determinants of NT-pro-BNP. Plasma NT-pro-BNP levels may therefore reflect both left atrial and ventricular remodeling in patients with AF and preserved systolic function.
References
Cha YM, Redfield MM, Shen WK, Gersh BJ (2004) Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation 109:2839–2843
Daniels LB, Maisel AS (2007) Natriuretic peptides. J Am Coll Cardiol 50:2357–2368.
Daniels LB, Allison MA, Clopton P, Redwine L, Siecke N, Taylor K, Fitzgerald R, Bracker M, Maisel AS (2007) Use of natriuretic peptides in pre-participation screening of college athletes. Int J Cardiol 124:411–414
Efremidis M, Pappas L, Sideris A, Filippatos G (2008) Management of atrial fibrillation in patients with heart failure. J Card Fail 14:232–237
Efremidis M, Sideris A, Xydonas S, Letsas KP, Alexanian IP, Manolatos D, Mihas CC, Filippatos GS, Kardaras F (2008) Ablation of atrial fibrillation in patients with heart failure: reversal of atrial and ventricular remodelling. Hellenic J Cardiol 48:19–25
Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA (2005) Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol 45:82–86
Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG (1995) Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med 155:469–473
Inoue S, Murakami Y, Sano K, Katoh H, Shimada T (2000) Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J Card Failure 6:92–96
Januzzi JL Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB (2005) The N-terminal pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 95:948–954
Kim BJ, Hwang SJ, Sung KC, Kim BS, Kang JH, Lee MH, Park JR (2007) Assessment of factors affecting plasma BNP levels in patients with chronic atrial fibrillation and preserved left ventricular systolic function. Int J Cardiol 118:145–150
Konstam MA (2007) Natriuretic peptides and cardiovascular events: more than a stretch. JAMA 297:212–214
Levin ER, Gardner DG, Samson WK (1998) Natriuretic peptides. N Engl J Med 339:321–328
Lévy S, Camm AJ, Saksena S, Aliot E, Breithardt G, Crijns H, Davies W, Kay N, Prystowsky E, Sutton R, Waldo A, Wyse DG (2003) International consensus on nomenclature and classification of atrial fibrillation; a collaborative project of the Working Group on Arrhythmias and the Working Group on Cardiac Pacing of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Europace 5:119–122
Ndrepepa G, Braun S, Mehilli J, Niemöller K, Schömig A, Kastrati A (2007) A prospective cohort study of prognostic power of N-terminal probrain natriuretic peptide in patients with non-ST segment elevation acute coronary syndromes. Clin Res Cardiol 96:30–37
Ohta Y, Shimada T, Yoshitomi H, Inoue S, Murakami Y, Shimizu H, Nakamura K, Ohta T, Katoh H, Ishibashi Y (2001) Drop in plasma brain natriuretic peptide levels after successful direct current cardioversion in chronic atrial fibrillation. Can J Cardiol 17:415–420
Roy D, Paillard F, Cassidy D, Bourassa MG, Gutkowska J, Genest J, Cantin M (1987) Atrial natriuretic factor during atrial fibrillation and supraventricular tachycardia. J Am Coll Cardiol 9:509–514
Sacher F, Corcuff JB, Schraub P, Le Bouffos V, Georges A, Jones SO, Lafitte S, Bordachar P, Hocini M, Clémenty J, Haissaguerre M, Bordenave L, Roudaut R, Jaïs P (2007) Chronic atrial fibrillation ablation impact on endocrine and mechanical cardiac functions. Eur Heart J 29:1290–1295
Shin DI, Jaekel K, Schley P, Sause A, Müller M, Fueth R, Scheffold T, Guelker H, Horlitz M (2005) Plasma levels of NT-pro-BNP in patients with atrial fibrillation before and after electrical cardioversion. Z Kardiol 94:795–800
Simon T, Becker R, Voss F, Bikou O, Hauck M, Licka M, Katus HA, Bauer A (2008) Elevated B-type natriuretic peptide levels in patients with nonischemic cardiomyopathy predict occurrence of arrhythmic events. Clin Res Cardiol 97:306–309
Thamilarasan M, Grimm RA, Rodriguez LL, Sun JP, Odabashian JA, Agler DA, Morehead A, Chung MK, Klein AL, Thomas JD (2000) Left ventricular diastolic dysfunction in lone atrial fibrillation determined by Doppler tissue imaging of mitral annular motion. Am J Cardiol 86:1026–1029
Therkelsen SK, Groenning BA, Kjaer A, Svendsen JH, Boje Jensen G (2007) ANP and BNP in atrial fibrillation before and after cardioversion—and their relationship to cardiac volume and function. Int J Cardiol 127:396–399
Tveit A, Seljeflot I, Grundvold I, Abdelnoor M, Arnesen H, Smith P (2008) Candesartan, NT-proBNP and recurrence of atrial fibrillation after electrical cardioversion. Int J Cardiol doi:10.1016/j.ijcard.2007.10.028
Wieczorek SJ, Wu AH, Christenson R, Krishnaswamy P, Gottlieb S, Rosano T, Hager D, Gardetto N, Chiu A, Bailly KR, Maisel A (2002) A rapid B-type natriuretic peptide assay accurately diagnoses left ventricular dysfunction and heart failure: a multicenter evaluation. Am Heart J 144:834–839
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Letsas, K.P., Filippatos, G.S., Pappas, L.K. et al. Determinants of plasma NT-pro-BNP levels in patients with atrial fibrillation and preserved left ventricular ejection fraction. Clin Res Cardiol 98, 101–106 (2009). https://doi.org/10.1007/s00392-008-0728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-008-0728-8