Abstract
Background
Chronic heart failure (CHF) is a widespread disease with severe quality of life impairment and a poor prognosis. Beta-blockers are the mainstay of CHF therapy; yet they are under-prescribed and under-dosed in clinical practice. This is particularly evident in elderly patients, which may be due to a fear of side-effects or intolerance. Beta-blockers have further not been adequately tested in patients with diastolic CHF, which is particularly common in elderly patients. Finally, comparative data on the use of different beta-blockers in patients with CHF is scarce.
Aim
To compare the tolerance of bisoprolol and carvedilol in elderly patients with CHF.
Methods
CIBIS-ELD is an investigator-initiated, multi-centre, 1:1 randomised, double-blind, phase III trial comparing bisoprolol and carvedilol in patients ≥65 years with systolic or diastolic CHF. Recruitment started in April 2005 and is anticipated to be completed by April 2008 with at least 800 patients enrolled.
Perspective
This is the first large scale head to head beta-blockers trial in an elderly population with CHF. Besides determining which of two standard beta-blockers is best tolerated in elderly patients with systolic or diastolic CHF, we expect to gain further insight into the treatment of the particular population of patients with diastolic CHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic heart failure (CHF) is a highly prevalent disease with a profound impact on quality of life and has been found to be as malignant as many common types of cancer [25]. In combination with a rising prevalence and incidence in people over 65 years [12], it is a growing epidemic with a not to be underestimated burden on health care systems.
Despite major advances in the treatment of CHF since the pioneering work on the use of beta-blockers 30 years ago [28], prognosis remains poor for patients with systolic CHF [4]. To update, the use of beta-blockers in CHF has been studied extensively in more than 20,000 patients in randomised controlled trials [9, 23] and according to latest ESC guidelines [26], beta-blockers should be used in symptomatic patients with left ventricular systolic dysfunction and in patients with asymptomatic systolic dysfunction due to ischaemic heart disease. Nevertheless, it appears that beta-blockers remain underused and under-dosed particularly in the elderly, both in primary care [7, 20] as well as tertiary care [13] and in patients managed primarily by cardiologists [15]. It is noteworthy that approximately 20% of patients in a large randomised controlled trial received an suboptimal beta-blocker dose or remained completely untreated [8].
More than half of all hospitalised patients with CHF are older than 75 years but recommendations for the use of beta-blockers in the ESC guidelines are regardless of patients’ age despite the fact that elderly have been underrepresented in CHF trials [24] and therefore evidence for the use of beta-blockers in this often multi-morbid population is not as strong as for their younger counterparts.
Beta-blockers are easily available and have proven cost-effective for CHF therapy [27], but comorbidity, polypharmacy as well as fear of intolerance appear to complicate adherence to effective treatment regimens further [2]. The investigators of one non-randomized trial (COLA II) reported good tolerance of beta-blocker therapy in elderly patients with systolic CHF [14]. However, it has been criticised that in this study, 1/4 of the carvedilol target dose was accepted to assess tolerance [6]. Results of the COMET trial suggest that carvedilol improves survival and is better tolerated than metoprolol tartrate [22].
In view of the above described problems, particularly regarding the insufficient evidence in the senior CHF population and tolerance of beta-blockers in this group, investigators initiated the CIBIS-ELD trial. This is the first large scale randomised double blind trial to compare two beta-blockers in an elderly population with CHF. Bisoprolol and carvedilol are compared for tolerance in CHF patients in three months titration, during which the individual maximally tolerated beta-blocker dose is determined.
Design of the CIBIS-ELD trial
The authors confirm that the investigation conforms to the principles outlined in the Declaration of Helsinki. The national and locally appointed ethics committees have approved the research protocol and written informed consent has been obtained from all subjects.
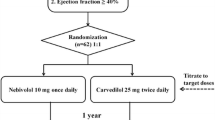
CIBIS-ELD is an investigator-initiated multi-centre randomised parallel, double-dummy, double-blind group phase III trial with ≥65-year-old patients with moderate to severe diastolic or systolic chronic HF. The overall aim of the study is to compare the tolerance of bisoprolol and carvedilol in elderly patients with CHF. The trial is performed in 55 centres in four countries (Germany, Serbia, Montenegro and Slovenia). Fig. 1 shows details of the study flow. Eligible patients are randomised in a 1:1 fashion to receive either bisoprolol or carvedilol; bisoprolol with a 10 mg/d target dose and carvedilol with a target dose of 25 mg b.i.d (patients > 85 kg: 50 mg b.i.d.) (see Fig. 1).
Role of the funding sources and conflict of interest
This project is funded by the German Federal Ministry of Education and Research (BMBF, project number 01GI0205). Some of the authors have received honoraria for speaking on aspects of cardiology by different pharmaceutical companies. No member of the committees is an employee of the pharmaceutical industry nor is paid for attending at meetings or for consulting relating to this trial. Merck KGaA gave an unrestricted grant to support the trial without any rights to influence trial design, data collection, data analysis or publication and will not interfere with the investigators intellectual property rights. The study was initiated and coordinated by investigators of the subproject “multicenter trials” of the Competence Network Heart Failure and is scientifically led by a steering committee consisting of independent researchers. This committee has full responsibility for the study protocol and publications about the study. Sponsor of the trial according to ICH-GCP was Charité - Universitätsmedizin Berlin university hospital. Producer of study medication was the pharmacy of Charité - Universitätsmedizin Berlin. Statistical Analysis is performed by the Coordination Centre for Clinical trials in Leipzig, which is publicly funded as well.
Patient selection
Inclusion and exclusion criteria are listed in Table 1. Patients are identified by their General Practitioners (GPs) or cardiologists, contacted by them and referred to the trial sites for initial assessment. Since April 2005, 670 patients have entered the study. Recruitment is anticipated to be completed by April 2008 with at least 800 patients enrolled (see Table 1).
Up-titration
The internationally recommended target dose for bisoprolol is 10 mg o.d., for carvedilol at 25 and 50 mg b.i.d. for patients >85 kg. To monitor whether these doses achieve a comparable degree of beta-blockade, spiroergometry is performed on a subgroup of 60 patients. The beta-blocker dose is increased to the target dose at fortnightly intervals. Dose titration differs according to the previous medication schedule of each patient. After the initial dose, therapy is commenced with either dose increment 1 or 2 (see Fig. 2). Patients >85 kg receive dose increment five after six or eight weeks. This increment indicates an increase in dosage in the carvedilol group only; in the bisoprolol group the increment is only technically treated like a dose increase for blinding purposes. The highest tolerated dose increment is maintained until the primary endpoint is reached (final visit in week 10 or 12). In case of medication intolerance, patients are instructed not to reduce the dose without medical advice. The trial physician determines whether the intolerance is avoidable by changing the concomitant medication. Is this not the case, the dose is reduced by one increment. The medication is then not further up-titrated to minimise the risk of non-adherence by repeatedly provoking symptoms of intolerance. Is up-titration delayed (e.g. by adjusting concomitant medication) but the dose not reduced, GPs are encouraged to up-titrate to target doses during subsequent treatment, even if it was not attained during the study treatment phase. Due to the exacerbation of sympathetic activity, the beta-blocker is not to be abruptly discontinued after the 12-week therapy phase. GPs are advised to continue prescribing the beta-blocker as standard treatment after study completion.
Endpoints
Primary endpoint
The primary endpoint of CIBIS-ELD is tolerance (yes/no) of the study medication target dose as per cardiology guidelines. Tolerance (=yes) is defined when the target dose was achieved and the study medication dose was not reduced during the titration phase. It is irrelevant whether unscheduled titration-visits took place if the titration target was not affected. Further, the patient is taking the respective target dose at the end of the study and has done so for at least ten consecutive days.
Secondary endpoints
-
Time to treatment failure (TTF)
-
Dose increment attained (% of target dose) for long term treatment
-
Number of adverse events (AE) or serious adverse events (SAE) in total as well as itemised according to their relationship to dose reduction/ discontinuation of treatment
-
NYHA class
-
6-min walk test
-
Echocardiography (left ventricular dimensions, wall thickness, EF according to Simpson or visually determined by an experienced echocardiographer; diastolic function according to ASE including mitral valve Doppler, tissue Doppler, flow propagation time, and pulmonary vein Doppler) before and after beta-blocker titration.
-
Quality of life, depression and physical well-being (measured with the following assessment instruments: SF-36, PHQ-D and FEW-16)
-
Heart rate at rest and under stress
-
Plasma- Brain Natriuretic Peptide (NT-proBNP and BNP) concentration
-
Alteration of total cholesterol, HDL, LDL and triglycerides plasma concentration
-
Investigation of medical treatment parameters at baseline:
-
Frequency and dose of concomitant medication including beta-blockers, diuretics, ACE-inhibitors or angiotensin receptor antagonists
-
Qualitative and quantitative survey of other therapeutic non-pharmacological actions
-
-
Lung function (vital capacity (VC), forced expiratory volume in the first second (FEV1), peak expiratory flow (PEF) and maximal mid-expiratory flow (MEF50))
-
Hospitalisations
-
Days alive and days out of hospital
Laboratory parameters
Blood samples are taken at baseline and during follow up at every visit in order to look for a correlation between both NT-pro BNP and BNP and the influence of beta-blocker therapy. In selected centres these analyses are done at every titration visit to correlate titration performance and BNP/ NT-pro BNP levels. Further blood sample analysis includes haemoglobin, erythrocytes, leucocytes and thrombocytes, sodium, potassium and creatinine serum levels, uric acid, total cholesterol, high and low density lipoprotein and triglycerides as well as CYP P450 2D6 polymorphism testing. In addition, blood samples (1x serology, 2x complete blood count) are taken for the central database from patients who consented to DNA analysis.
Statistical hypothesis and sample size considerations
The major hypothesis of the CIBIS-ELD trial is that the target dose can be reached more often with bisoprolol than with carvedilol. In order to present the difference in the case of opposite direction, the null-hypothesis postulates equality and the alternative hypothesis a difference in any of the two directions. It is estimated that 50% of the patients in the inferior subgroup will tolerate the target dose. Relevant superiority of the other subgroup is ascertained when 60% of patients tolerate the target dose. For the primary endpoint, the Accurate Mann–Whitney U-test will be applied for biometrical analysis. Secondary endpoints: Mann–Whitney contrast with censorship (days alive out of hospital), analysis of variance (metric endpoints), stratified Odds Ratios with Cochrane-Mantel-Haenszel with Breslow-Day-Homogeneity-Test (binary endpoints) and Kaplan Meier (time to event). Exploratory: multiple regression analyses. Guided by power analysis for sample size considerations for a power of 90%, a sample size of 1,040 was determined to be optimal for the purpose of this study. A power of 80% is achieved with 760 patients.
Subgroup analysis
The study will stratify for patients with preserved and impaired LVEF (≤45%), and the findings in these two subgroups will be a secondary endpoint. Further, patients with atrial fibrillation will be analysed as a subgroup to determine potential differences in the effect of beta-blockers in this group.
Discussion
There is an ongoing discussion in clinical practice as to which beta-blocker is advantageous because of their differences in pharmacodynamics and pharmacokinetics. Bisoprolol and metoprolol succinate as selective beta-1-receptor-blockers, and carvedilol as an alpha-1-, beta-1- and beta-2-receptor-blocker have shown to be effective in reducing the risk of all cause mortality in patients with CHF by approximately 35% with a supporting ACE-inhibitors therapy, whereas results for nebivolol were partly disappointing (non-significant 12% fall in all cause mortality) [5, 10, 17, 19]. Bisoprolol shows the strongest beta-1-selectivity of all beta-blockers approved for CHF therapy. The strong selectivity might be a possible benefit for CHF patients with accompanying chronic obstructive pulmonary disease [16]. On the other hand it has been discussed whether the anti-adrenergic effects of carvedilol in addition to beta-1-blockade prolongs survival [18].
The effectiveness of beta-blockers specifically in elderly CHF patients has been investigated prospectively in two major trials: In CIBIS III bisoprolol, when compared with enalapril, reduced overall mortality by 28% (secondary endpoint) in patients ≥65 years after 6 months mono-therapy [29]. In SENIORS, nebivolol reduced the risk for the combined endpoint of all cause mortality or cardiovascular hospital admission in heart failure (HF) patients (HR 0.86, 95% CI 0.74–0.99, P = 0.039) [10]. Although the benefit of nebivolol appeared to be less in patients >75 years, there was no significant interaction between age as continuous or categorical variable and the effect of nebivolol.
Beta-blocker therapy is approved for patients with HF due to severe systolic dysfunction only [3]. Patients with an LVEF > 35% have been excluded from almost every beta-blocker trial. Nevertheless, CHF with normal or mildly impaired systolic function has received much attention over the last years. A recent trial showed that isolated diastolic dysfunction is present in more than 40% of patients with CHF [4]. More than half of CHF patients have a preserved EF–their mortality rate is as high as in systolic CHF. So far there is no proven treatment for these patients. There is some evidence for AT1-Blockers from the CHARM-Preserved trial, which showed a non-significant reduction in the composite endpoint of cardiovascular death or HF hospitalisation with candesartan [21]. The benefit of digoxin could not be shown in a subgroup analysis of the DIG trial [1]. An objective measurement of diastolic function was not performed in these trials, though. Therefore results do not permit any conclusion about the treatment of diastolic heart failure. From a pathophysiologic point of view it is worth asking for the role of beta-blockers in the treatment of diastolic HF as they prolong the diastole and energy is preserved for the relaxation process. In the SENIORS trial, nebivolol reduced the composite of all-cause mortality and cardiovascular hospital admission, regardless of EF, in patients ≥70-years-old [10]. More than 700 Patients had an EF ≥ 35%. Although an improvement in EF and a reduction of ventricular size could be shown in patients with systolic HF, the mechanism of benefit in the patients with preserved HF remained unclear. No changes of diastolic function assessed by pulsed mitral valve Doppler-echocardiography could be found. Tissue Doppler was not performed.
The authors argue that elderly patients and those with preserved diastolic function should not be excluded from beta-blocker trials any longer, as a high percentage of HF patients have a preserved EF and especially the elderly are affected. It appears mandatory to evaluate the possible mechanisms leading to clinical improvement by thorough assessment of diastolic function. This can be achieved by tissue Doppler, which is one of the examinations performed in the CIBIS-ELD trial.
Further trials are needed to evaluate the effect of beta-blockers on mortality concentrating on patients with CHF and preserved EF or diastolic heart failure. The J-DHF trial, which assesses the effects of beta-blockers in diastolic heart failure, is expected to finish in 2009 [11]. The results of CIBIS-ELD will be helpful in identifying the beta-blocker with the best tolerance in elderly patients with CHF.
Perspective
Beta-blockers are standard therapy in heart failure, though they are still under-prescribed. Good tolerance of beta-blockers is a key factor in successfully initiating therapy, reaching the target dose and establishing it for long term therapy. Elderly patients were under-represented in almost every CHF-trial, even though they present the largest group of CHF patients. They are thought to be particularly affected by intolerance to beta-blocker therapy. CIBIS-ELD is the first trial comparing the tolerance of two commonly used beta-blockers in heart failure patients as the primary outcome measure. The beta-blocker identified as being better tolerated should be used as standard for the therapy of elderly CHF patients. In addition, this study will provide important insights into the therapy of patients with diastolic heart failure, and results will need to be considered in the further development of guidelines.
Committees
Steering committee
Members are Prof. Dr. Hans-Jürgen Becker (Frankfurt/M.), Dr. Hans-Dirk Düngen (Berlin), Prof. Dr.Thomas Eschenhagen (Hamburg), PD Dr. Roland Hardt (Trier), Prof. Dr. Friedrich Luft (Berlin), Prof. Dr. Bernhard Rauch (Ludwigshafen), Prof. Dr. Elisabeth Steinhagen-Thiessen (Berlin), Prof. Dr. Ruth Strasser (Dresden), Prof. Dr. Finn Waagstein (Göteborg).
The Committee advises on the scientific direction of the trial, protocol design, scientific policies, monitoring of trial progress, sub-studies, co-operation with the Data and Safety Monitoring Committee, as well as on the reporting and publication of trial results. The Committee is scheduled to meet once per year during the trial, and is informed regarding trial progress, protocol adherence and other aspects as necessary. A decision on premature termination of the trial will be made by the steering committee after consultation of the funding organisation, the external advisory board, the DMSC, and the biometrician.
Independent data and safety monitoring committee (IDSMC)
Members are Dr. Ulrike Bauer (Berlin), Dr. Stephan Beckmann (Berlin) and Dr. Jürgen Waigand (Berlin). The principal responsibility of the IDSMC is protecting the safety of patients in the trial.
External advisory board
Members are Univ.-Prof. Dr. Georg Ertl (Würzburg), Herr Prof. Dr. Ferenc Follath (Zürich) and Prof. Dr. Thomas Meinertz (Hamburg).
List of Principal Investigators by country
Germany: Dr. S. Baumbach, Dr. St. Beckmann, Dr. St. Czischke, Dr. W. Dausch, Dr. H.-Ch. Deyda, Dr. A. Dietze-Richter, Dr. H.D. Düngen, Prof. Dr. R. Erbel, Prof. Dr. E. Fleck, Dr. E. Frohburg, Dr. Ch. Gerischer, Dr. O. Hagen, Dr. F. Hartmannn, Dr. J. Heckmann, Dr. J. Honneth, PD H.-U. Kreider-Stempfle, Dr. I. Kruck, Dr. H. Leinberger, Prof. Dr. A. Mügge, Dr. E. Müller, Prof. Dr. M. Oeff, Prof. Dr. B. Pieske, Dr. N. Prokynitopoulos, Dr. H.-E. Sarninghausen, Dr. Ch. Schmitt, Dr. K.-H. Schöll, Dr. R.-J. Schulz, PD Dr. H.-Y. Sohn, Prof. Dr. R. Strasser, Dr. H. Streich, Dr. J. Taggeselle, Prof. Dr. P. Weismüller, Dr. S. Zimmermann, Prof. Dr. R. Zotz.
Serbia: Ass. Prof. Dr. S. Apostolović, Dr. S. Ćatović, Dr. V. Čelić, Prof. Dr. S. Dimković, Dr. D. Košević, Prof. Dr. M. Krotin, Prof. Dr. M. Miloradović, Dr. J. Milosavljević, Dr. Z. Naumović, Prof. Dr. M. Pavlović, Dr. V. Petrović, Ass. Prof. Dr. B. Putniković, Prof. Dr. D. Sakač, Dr. N. Trifunović, Prof. Dr. Z. Vasiljević, Dr. S. Živković.
Slovenia: Ass. Prof. Dr. M. Lainščak, Dr. D. Kovač, Dr. A. Marolt, Dr. N. Škrabl-Močnik,
Montenegro: Ass. Prof. Dr. A. Bosković, Dr. B. Knezević.
References
Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Gheorghiade M (2006) Effects of digoxin on morbidity and mortality in diastolic heart failure. Circulation 114:397–403
Baxter AJ, Spensley A, Hildreth A, Karimova G, O’Connell JE, Gray CS (2002) Beta-blockers in older persons with heart failure: tolerance and impact on quality of life. Heart 88:611–614
Böhm M, Werner N, Kindermann M (2006) Drug treatment of chronic heart failure. Clin Res Cardiol 95:36–54
Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL (2006) Systolic and diastolic heart failure in the community. JAMA 296:2209–2216
CIBIS-II Investigators and Committees (1999) The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet 353:9–13
Cleland JG, Charlesworth A, Lubsen J, Swedberg K, Remme WJ, Erhardt L, Di Lenarda A, Komajda M, Metra M, Torp-Pedersen C, Poole-Wilson PA, COMET Investigators (2006) A comparison of the effects of carvedilol and metoprolol on well-being, morbidity, and mortality (the “patient journey”) in patients with heart failure: a report from the carvedilol or metoprolol european trial (COMET). J Am Coll Cardiol 47:1603–1611
Cleland JG, Cohen-Solal A, Aguilar CJ, Dietz R, Eastaugh J, Follath F, Freemantle N, Gavazzi A, van Gilst WH, Hobbs FDR, Korewicki J, Madeira HC, Preda I, Swedberg K, Widimsky J (2002) Management of heart failure in primary care (the IMPROVEMENT of heart failure programme): an international survey. Lancet 360:1631–1639
Dobre D, van Veldhuisen DJ, Mordenti G, Vintila M, Haaijer-Ruskamp FM, Coats AJ, Poole-Wilson PA, Flather MD, SENIORS Investigators (2007) Tolerance and dose-related effects of nebivolol in elderly patients with heart failure: data from the study of the effects of nebivolol intervention on outcomes and rehospitalisation in seniors with heart failure (SENIORS) trial. Am Heart J 154:109–115
Dulin BR, Haas SJ, Abraham WT, Krum H (2005) Do elderly systolic heart failure patients benefit from Beta-blockers to the same extent as the non-elderly? Meta-analysis of >12,000 patients in large-scale clinical trials. Am J Cardiol 95:896–898
Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Böhm M, Anker SD, Thompson SG, Poole-Wilson PA, SENIORS Investigators (2005) Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26:215–225
Hori M, Kitabatake A, Tsutsui H, Okamoto H, Shirato K, Nagai R, Izumi T, Yokoyama H, Yasumura Y, Ishida Y, Matsuzaki M, Oki T, Sekiya M; The J-DHF, Program Committee (2005) Rationale and design of a randomized trial to assess the effects of beta-blocker in diastolic heart failure; Japanese diastolic heart failure study (J-DHF). J Card Fail 11:542–547
Kannel WB (1987) Epidemiology and prevention of cardiac failure: Framingham study insights. Eur Heart J 8(Suppl F):23–26
Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen-Solal A, Dietz R, Gavazzi A, Van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J, Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology (2003) The Euroheart failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J 24:464–474
Krum H, Hill J, Fruhwald F, Sharpe C, Abraham G, Zhu JR, Poy C, Kragten JA (2006) Tolerance of beta-blockers in elderly patients with chronic heart failure: the COLA II study. Eur J Heart Fail 8:302–307
Lainščak M, Moullet C, Schoen N, Tendera M (2007) Treatment of chronic heart failure with carvedilol in daily practice: the SATELLITE survey experience. Int J Cardiol 122:149–155
Maack C, Tyroller S, Schnabel P, Cremers B, Dabew E, Südkamp M, Böhm M (2001) Characterization of beta(1)-selectivity, adrenoceptor-G(s)-protein interaction and inverse agonism of nebivolol in human myocardium. Br J Pharmacol 132:1817–1826
MERIT-HF Study Group (1999) Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 353:2001–2007
Packer M (2003) Do beta-blockers prolong survival in heart failure only by inhibiting the beta1-receptor? A perspective on the results of the COMET trial. J Card Fail 9:429–443
Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106:2194–2199
Peters-Klimm F, Müller-Tasch T, Schellberg D, Remmpis A, Barth A, Holzapfel N, Jünger J, Herzog W, Szecsenyi J (2008) Guideline adherence for pharmacotherapy of chronic systolic heart failure in general practice: a close look on evidence-based therapy. Clin Res Cardiol 97:244–252
Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S; CHARM Investigators, Committees (2003) Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. Lancet 362:759–766
Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A (2003) Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol european trial (COMET): randomised controlled trial. Lancet 362:7–13
Shibata MC, Flather MD, Wang D (2001) Systematic review of the impact of beta-blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail 3:351–357
Shibata MC, Soneff CM, Tsuyuki RT (2005) Utilization of evidence-based therapies for heart failure in the institutionalized elderly. Eur J Heart Fail 7:1122–1125
Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV (2001) More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 3:315–322
Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Lévy S, Linde C, Lopez-Sendon JL, Nieminen MS, Piérard L, Remme WJ (2005) Task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005). Eur Heart J 26:1115–1140
Varney S (2001) A cost-effectiveness analysis of bisoprolol for heart failure. Eur J Heart Fail 3:365–371
Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I (1975) Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 37:1022–1036
Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, Krum H, Ponikowski P, Skene A, van de Ven L, Verkenne P, Lechat P; CIBIS III Investigators (2005) Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized cardiac insufficiency bisoprolol study (CIBIS) III. Circulation 112:2426–2435
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
This trial was supported by the Competence Network of Heart Failure funded by the Federal Ministry of Education and Research (BMBF, project number 01GI0205) and is registered with number ISRCTN34827306 at http://www.controlled-trials.com.
Rights and permissions
About this article
Cite this article
Düngen, HD., Apostolović, S., Inkrot, S. et al. Bisoprolol vs. carvedilol in elderly patients with heart failure: rationale and design of the CIBIS-ELD trial. Clin Res Cardiol 97, 578–586 (2008). https://doi.org/10.1007/s00392-008-0681-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-008-0681-6