Abstract
Purpose
The primary aim of this study was to characterise complications, identify predictors of postoperative morbidity and mortality and to evaluate existing risk prediction models in elderly rectal cancer patients.
Methods
An observational single-centre study of 330 consecutive patients >75 years treated in 1994–2006. Analyses were performed by age group: 75–79 years, 80–85 years and >85 years.
Results
Total observed in-hospital morbidity was 48.7 %. In multivariate analysis, age (OR 1.04, 95 % CI 1.01–1.08, p = 0.04), ASA grade ≥ 3 (p = 0.01), acute presentation (OR 1.67, 95 % CI 1.2–13.2, p = 0.02) and major surgery (APR OR 3.72, 95 % CI 1.37–10.15, p = 0.01, LAR OR 2.98, 95 % CI 1.14–7.79, p = 0.03, Hartmann OR 5.46, 95 % CI 1.60–19.28, p = 0.02) were independent risk factors for postoperative morbidity.
The 30-day mortality was 6.3, 6.4 and 14.3 % (p = 0.146) in the three age groups, and the 100-day mortality was 8.7, 10.1 and 22.2 % (p = 0.03), respectively. ASA group 3 (OR 6.21, 95 % CI 4.39–27.69, p = 0.017), ASA group 4 (OR 32.6, 95 % CI 5.12–207.75, p < 0.001) and acute presentation (OR 6.48, 95 % CI 1.62–25.99, p = 0.008) increased the risk of 100-day mortality.
The Physiological and Operative Severity Score for enUmeration of Mortality and Morbidity (POSSUM) observed/estimated (O/E) ratio for morbidity was 1.05. For 30-day mortality, the colorectal POSSUM (Cr-POSSUM) O/E ratio was 0.74, Surgical Risk Scale 0.61 and the Association of Coloproctology of Great Britain and Ireland (ACPGBI) mortality model 0.63, and for 100-day mortality, ratios were 1.12, 0.91 and 0.95, respectively.
Conclusion
In this series, age increased the risk of in-hospital morbidity and 100-day mortality. Cr-POSSUM, SRS and ACPGBI overestimated 30-day mortality but predicted 100-day mortality with a high degree of accuracy. POSSUM correctly predicted in-hospital morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The median age of rectal cancer patients in Norway is 70 years, and age-specific incidence peaks at 80 years (135/100.000) [1]. Due to an increased life expectancy, the number of elderly patients with rectal cancer is increasing. Although both surgical and oncological treatment have become more differentiated, major surgery is still the cornerstone of rectal cancer treatment. There is evidence that elderly people should not be denied surgical treatment on the basis of their chronological age alone [2–4], but the consequences of complications are more often severe [5]. In clinical practice, elderly patients receive less curative surgery, less radiochemotherapy and often modified surgical treatment when operated [6], and no consensus on the treatment of elderly patients exists [7].
Increasing accuracy of preoperative staging, multidisciplinary team conferences [8] and new treatment modalities facilitate the principles of tailored treatment [9], but still, it may be questioned if clinical judgments alone are sufficient to make safe decisions for fragile patients. Before deciding on treatment, objective individual evaluation through risk prediction scoring systems and comprehensive geriatric assessment (CGA) [10] may be equally important.
There are several risk prediction models, including the Physiological and Operative Severity Score for enUmeration of Mortality and Morbidity (POSSUM), colorectal POSSUM (Cr-POSSUM), Surgical Risk Scale (SRS) and the Association of Coloproctology of Great Britain and Ireland mortality model (ACPGBI mortality model), estimating the risk of postoperative morbidity and mortality after surgery [11, 12]. In Norway, the use of these tools has been limited, and no validation of the different models exists.
The primary aim of this study was to characterise the complications after surgery for rectal cancer and to identify potential factors increasing the risk of postoperative morbidity and mortality in a cohort of rectal cancer patients >75 years.
Secondly, the aim was to evaluate the ability of the POSSUM, Cr-POSSUM, SRS and ACPGBI scoring systems to predict mortality and morbidity in the same cohort of patients.
Material and methods
The Norwegian Colorectal Cancer Registry (NCCR) is part of the Cancer Registry of Norway and has prospectively registered data on all patients diagnosed with rectal cancer in Norway since 1993 [13]. From this registry, we identified 837 patients diagnosed between January 1994 and December 2006, with invasive adenocarcinoma located within 16 cm from the anal verge, at St. Olavs Hospital, Trondheim, Norway. The present series includes all 330 patients (39.4 %) aged >75 years at diagnosis. St. Olavs Hospital is a third-line referral hospital for the health region Mid-Norway with 700,000 inhabitants and a second-line hospital for a population of 200,000 inhabitants. The end of follow-up was 31 December 2011, 5 years after inclusion of the last patient, and the follow-up was complete.
Data on patient and tumour characteristics, local recurrences, metastases and survival from the NCCR were combined with data from a local prospective register of complications after surgery at the Department of Gastrointestinal Surgery, St. Olavs Hospital. The local register of complications was established in 1993, and complications during admission on all patients undergoing surgical treatment are registered. The complications have been defined according to the Clavien-Dindo classification [14] and sub-divided into medical (cardiovascular, pulmonary, urogenital and others) and surgical complications (re-operation, bleeding, wound dehiscence, anastomosis leakage and ileus). Additional information, specifically regarding comorbidity and readmissions, was assigned by a retrospective assessment of each patient’s medical record. Comorbidity was recorded according to the adapted version of the Charlson index [15].
Morbidity and mortality scores were calculated for each patient by using the POSSUM, Cr-POSSUM, SRS and ACPGBI systems. If one parameter in the calculation was missing, a normal value was assigned and the patient was included in the analyses, but if more than one value was missing, the patient was excluded from the analyses [16]. The risk scores were calculated by use of an online calculator [17], with the exception of SRS which was calculated manually. The following equations were used:
where R is the predicted risk of death. The estimated risk of mortality or morbidity for the cohort was obtained by using the mean score of the calculated values. These estimates were compared to the observed operative mortality after 30 days and 100 days as well as the in-hospital morbidity. Mortality was defined as death, whatever the cause. The ability of the different scoring systems to predict mortality and morbidity was assessed by observed/estimated ratio (O/E ratio).
The SPSS version 21 for Windows was used for statistical analyses. Categorical data were analysed by use of Pearson chi-square test or Fisher’s exact test. For analysis of factors predicting frequency of mortality and in-hospital morbidity, univariate and multivariate logistic regression analyses were performed. The characteristics and outcomes of the three age groups 75–79 years, 80–85 years and >85 years were studied by comparative analyses. Two-sided p values <0.05 were considered significant.
The study was approved by the regional ethics committee.
Results
The characteristics of the 330 patients aged >75 years are given in Table 1. The mean age was 80.8 years (SD 4.6 years, range 75–99 years). Thirty-two patients (9.7 %) did not receive any surgical treatment. The reason was advanced cancer in 13 patients, comorbidity in 11 patients (Charlson index >2) and 8 patients did not want any surgical treatment. The mean age of the non-operated group was 83.4 years (SD 6.3 years).
The proportion of patients with comorbidity increased with age from 68.4 % for the patients 75–79 years to 82.7 % for patients >85 years (p = 0.066). Being independent in day-to-day care decreased from 89.8 % among patients 75–79 years compared to 66.7 % for patients >85 years (p < 0.001). There was no difference in pathological tumour node metastasis (pTNM) stage between the age groups (p = 0.188).
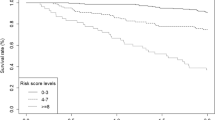
The 5-year overall survival was 42.8 % for the patients aged 75–79 years, 35.1 % for the patients aged 80–85 years and 19.5 % for the patients >85 years (p < 0.001). The 5-year relative survival was 57.2 % (46.0–68.1), 55.5 % (41.4–69.9) and 49.9 % (29.0–74.7) for the same three groups, respectively (p = n.s).
Mortality
The overall 30-day mortality was 6.3 % for patients 75–79 years, 6.4 % for patients 80–85 years and 14.3 % for patients >85 years (p = 0.166) (Table 2). In a multivariate logistic regression analysis, ASA group 3 (OR 6.21, 95 % CI (4.39–27.69), p = 0.017), ASA group 4 (OR 32.6, 95 % CI (5.12–207.75), p < 0.001) and acute presentation (OR 6.48, 95 % CI (1.62–25.99), p = 0.008) increased the risk of 30-day mortality when adjusted for gender, pTNM stage, type of surgery and comorbidity, and no significant effect was observed for age (OR 1.06, 95 % CI (0.95–1.18), p = 0.289) in this analysis.
The overall 100-day mortality was 12.1 % for the group of patients receiving an operation and increased significantly with age by 8.7, 10.1 and 22.2 % for the age groups 75–79, 80–85 and >85 years, respectively (p = 0.03). In a multivariate regression analysis evaluating 100-day mortality, acute presentation (OR 4.87, 95 % CI (1.49–15.90), p = 0.009) and ASA 3 (OR 3.89, 95 % CI (1.29–11.74), p = 0.016) and ASA 4 (OR 14.30, 95 % CI (3.66–55.92), p < 0.001) increased the risk of 100-day mortality when adjusted for pTNM stage, comorbidity, type of surgery and radiochemotherapy. There was no certain effect of age when analysed as a continuous variable (HR 1.08, 95 % CI (0.99–1.18), p = 0.073).
Morbidity
In-hospital morbidity was observed in 48.7 % of the patients and increased with age from 41.3 % for the patients 75–79 years to 58.6 % for the patients over 85 years (p = 0.062) (Table 2). The medical morbidity was 33.3 and 49.2 % (p = 0.091) for the same two groups, respectively. The overall frequency of surgical morbidity was 27.0, 25.7 and 36.5 % (p = 0.291) in the three age groups, respectively, and the frequency of reoperation was 9.5, 7.3 and 15.9 % in the same groups, respectively (p = 0.196). Anastomotic leakage was observed in 4.8 and 3.9 % in the youngest groups, compared to 15.9 % for patients >85 years (p = 0.156). No certain effect of age on the frequency of surgical morbidity was observed.
In a univariate logistic regression analysis, age (OR 1.07, 95 % CI 1.02–1.13, p = 0.009), ASA score (p = 0.013), pTNM stage (p = 0.009), acute presentation (OR 4.46, 95 % CI 1.6–12.3, p = 0.004) and type of surgery (OR 8.93, 95 % CI 3.4–23.5, p < 0.001) affected the risk of postoperative morbidity. No effect was observed for gender, comorbidity and preoperative chemoradiation. In the multivariate logistic regression analysis, age (OR 1.04, 95 % CI 1.01–1.08, p = 0.04), ASA grade = 3 (OR 1.90, 95 % CI 1.31–2.77, p = 0.01), ASA grade = 4 (OR 3.01, 95 % CI 1.65–5.52, p = 0.01), acute presentation (OR 1.67, 95 % CI 1.2–13.2, p = 0.02) and major surgery (APR OR 3,72, 95 % CI 1.37–10.15, p = 0.01, LAR OR 2.98, 95 % CI 1.14–7.79, p = 0.03, Hartmann OR 5.46, 95 % CI 1.60–19.28, p = 0.02) were independent risk factors for postoperative morbidity when adjusted for gender, comorbidity and stage of disease (Table 3).
Risk prediction
The comparison of observed and estimated mortality using the Cr-POSSUM, SRS and ACPGBI, and observed and estimated morbidity using POSSUM are given in Table 4. Univariate logistic regression analysis revealed that all risk prediction models analysed as continuous variables were significantly predictive of death (Cr-POSSUM OR 1.05, 95 % CI (1.03, 1.08), p < 0.001; SRS OR 1.06, 95 % CI (1.02, 1.10), p = 0.003; ACPGBI OR 1.14, 95 % CI (1.08, 1.20), p < 0.001) and that the POSSUM score was a significant predictor of postoperative morbidity (OR 1.04, 95 % CI (1.02, 1.06), p < 0.001). The observed morbidity was 48.7 %, the POSSUM estimated morbidity was 46.5 % and the observed/estimated (O/E) ratio was 1.05. For Cr-POSSUM, the O/E ratio was 0.74, for SRS 0.61 and for ACPGBI 0.63 when comparing observed and estimated 30-day mortality. The O/E ratios for observed 100-day and estimated mortality for Cr-POSSUM, SRS and ACPGBI were 1.12, 0.91 and 0.95, respectively.
Discussion
In this series, almost half of the patients over 75 years developed complications after rectal cancer surgery, and the in-hospital morbidity increased with age. Both the 30-day mortality and the 100-day mortality increased by age, and the rates were 2.5 times higher for patients >85 years compared to patients 75–79 years.
Validated risk score models predict the risk of postoperative morbidity and mortality with a high degree of accuracy and are useful during patient information and when deciding upon a specific type of treatment.
An individual treatment strategy should be based on accurate clinical staging, objective preoperative risk prediction and patient preference. The most important outcome in this process is the mortality related to treatment. In this study, acute presentation and ASA group ≥ 3 increased the risk of mortality. Deaths after operation frequently occur later than 30 days, and some have argued that mortality after 100 days may be a more natural time horizon [18]. In the present study of elderly patients selected to different treatment strategies, one of five patients over 85 years receiving an operation for rectal cancer died within 100 days, and this is an important aspect in the process of decision-making.
The effect of age on the risk of postoperative mortality and morbidity differs between studies. A French multicentre study from 2005 [19] and a Dutch study from 2006 [20] claim that age is an independent risk factor, but others have argued that comorbidity, differences in mode of presentation and tumour stage in a better way explain why the elderly have increased morbidity after rectal cancer surgery [21, 22]. The conflicting results may be a result of selection bias as there are no randomised controlled trials addressing the question. There is evidence that sub-groups of the elderly tolerate surgery as well as their younger counterparts [2, 7], and several variables are highly relevant when deciding upon treatment.
The frequency of comorbid conditions at diagnosis increased with age, but there was no impact of this comorbidity on the risk of postoperative morbidity and mortality. This is also disputed in the literature, whilst some have shown that specific comorbid conditions increase the risk of complications [23], others have argued this effect to be negligible [24]. The lack of a clear correlation between comorbidity and complications means that the number of comorbid conditions is not a good measure in decision-making. Some have suggested the use of a CGA during preoperative evaluation [10, 25]. CGA has proven to be a significant predictor of postoperative morbidity in colorectal cancer patients. During a thorough preoperative multidimensional assessment of the patient, both the pathology and reduced functional capacity of multiple organ systems are identified. These findings can be used in selection to different treatment strategies and/or to tailor an individual intervention plan in the concept of preoperative habilitation.
Validation of the predictive models
The POSSUM, SRS and ACPGBI scoring systems were originally developed to adjust for differences in population between different hospitals in order to have systems for surgical audit. In addition, ACPGBI was also designed for preoperative counselling [26]. The use of validated predictive scoring systems can potentially predict surgical outcome and can also be a part of the surgeons’ decision-making and the patients’ informed consent to treatment.
Studies have shown SRS, ACPGBI and Cr-POSSUM to be effective tools for predicting death [27, 28], but all systems seem to overestimate the risk of mortality, especially in low-risk groups and in the elderly [29]. Also, in the present study, Cr-POSSUM, ACPGBI and SRS overestimated the risk of postoperative mortality; however, when compared with the observed 30-day mortality, Cr-POSSUM was marginally more accurate. SRS and ACPGBI, on the other hand, have the advantages of not including any perioperative parameters and of being quicker and easier to assess. When comparing the estimated values with the observed 100-day mortality, the accuracy improved, possibly due to improvements in intensive care medicine during the last 20 years.
The POSSUM score predicted the risk of postoperative morbidity very accurately in the present cohort, and it seems like such scoring systems may add valid information when considering major surgery on old, fragile subjects with considerable comorbidity.
Strength and limitations
There are several limitations to this study. The most important may be the limited sample size and, hence, the risk of not detecting differences that may exist in a larger cohort. As few patients died within 30 days, and also within 100 days, the small numbers made any multivariable model unfit to analyse mortality.
The patients were included in the study in a prospective manner, but St. Olavs Hospital is a third-line referral hospital for the health region of Mid-Norway, and this may have introduced a selection bias, and the present results may not be valid for other hospital series. In particular, two patient groups are overrepresented, as all patients receiving TEM surgery and patients with the most advanced cancers are treated at St. Olavs Hospital.
In the study period, there were few laparoscopic procedures, which may have influenced the frequency of complications.
Conclusion
In-hospital morbidity increased with age, ASA ≥ 3, acute mode of presentation and after major surgery, and the risk of postoperative complications can be predicted accurately with the POSSUM scoring system.
Thirty-day and 100-day mortality increased with ASA grade ≥ 3 and acute presentation. Cr-POSSUM, SRS and ACPGBI overestimated the risk of 30-day mortality, but they predicted the 100-day mortality with a high degree of accuracy. Thus, they seem valuable in surgical audit and also in daily practice for decision-making on surgery for rectal cancer in elderly patients.
References
Kreftregisteret: Cancer in Norway 2011 (2011) Available at: http://www.kreftregisteret.no/no/Generelt/Publikasjoner/Cancer-in-Norway/Cancer-in-Norway-2011/
Arenal JJ, Tinoco C, Labarga F et al (2012) Colorectal cancer in nonagenarians. Color Dis 14:44–47
Tan K, Kawamura Y, Mizokami K et al (2009) Colorectal surgery in octogenarian patients—outcomes and predictors of morbidity. Int J Color Dis 24:185–189
Alley PG (2000) Surgery for colorectal cancer in elderly patients. Lancet 356:956
Rutten HJ, den Dulk M, Lemmens VE et al (2008) Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol 9:494–501
Audisio RA, Bozzetti F, Gennari R et al (2004) The surgical management of elderly cancer patients; recommendations of the SIOG surgical task force. Eur J Cancer 40:926–938
Manceau G, Karoui M, Werner A et al (2012) Comparative outcomes of rectal cancer surgery between elderly and non-elderly patients: a systematic review. Lancet Oncol 13:E525–E536
Tudyka V, Blomqvist L, Beets-Tan RG et al (2014) EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. EJSO 40:469–475
Wibe A, Law WL, Fazio V, Delaney CP (2013) Tailored rectal cancer treatment: a time for implementing contemporary prognostic factors? Color Dis 15:1333–1342
Kristjansson SR, Nesbakken A, Jordhoy MS et al (2010) Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol 76:208–217
Copeland GP, Jones D, Walters M (1991) POSSUM: a scoring system for surgical audit. Br J Surg 78:355–360
Tekkis PP, Prytherch DR, Kocher HM et al (2004) Development of a dedicated risk-adjustment scoring system for colorectal surgery (colorectal POSSUM). Br J Surg 91:1174–1182
Wibe A, Moller B, Norstein J et al (2002) A national strategic change in treatment policy for rectal cancer: implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 45:857–866
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Tran Ba Loc P, du Montcel ST, Duron JJ et al (2010) Elderly POSSUM, a dedicated score for prediction of mortality and morbidity after major colorectal surgery in older patients. Br J Surg 97:396–403
Smith JJ, Tekkis PP. Risk prediction in surgery. Available at: www.riskprediction.org.uk
Strasberg SM, Linehan DC, Hawkins WG (2009) The accordion severity grading system of surgical complications. Ann Surg 250:177–186
Alves A, Panis Y, Mathieu P et al (2005) Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 140:278–283, discussion 284
Shahir M, Lemmens V, Poll-Franse L et al (2006) Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 42(17):3015–3021
Takeuchi K, Tsuzuki Y, Ando T et al (2004) Should patients over 85 years old be operated on for colorectal cancer? J Clin Gastroenterol 38:408–413
Lemmens V, Janssen-Heijnen M, Verheij C et al (2005) Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg 92:615–623
Pedrazzani C, Cerullo G, De Marco G et al (2009) Impact of age-related comorbidity on results of colorectal cancer surgery. World J Gastroenterol 15:5706–5711
Janssen-Heijnen ML, Lemmens VE, van den Borne BE et al (2007) Negligible influence of comorbidity on prognosis of patients with small cell lung cancer: a population-based study in the Netherlands. Crit Rev Oncol Hematol 62:172–178
Audisio RA, Pope D, Ramesh HS et al (2008) Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 65:156–163
Richards CH, Leitch EF, Anderson JH et al (2011) The revised ACPGBI model is a simple and accurate predictor of operative mortality after potentially curative resection of colorectal cancer. Ann Surg Oncol 18:3680–3685
Bromage SJ, Cunliffe WJ (2007) Validation of the CR-POSSUM risk-adjusted scoring system for major colorectal cancer surgery in a single center. Dis Colon Rectum 50:192–196
Sutton R, Bann S, Brooks M, Sarin S (2002) The Surgical Risk Scale as an improved tool for risk-adjusted analysis in comparative surgical audit. Br J Surg 89:763–768
Ferjani AM, Griffin D, Stallard N, Wong LS (2007) A newly devised scoring system for prediction of mortality in patients with colorectal cancer: a prospective study. Lancet Oncol 8:317–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stornes, T., Wibe, A. & Endreseth, B.H. Complications and risk prediction in treatment of elderly patients with rectal cancer. Int J Colorectal Dis 31, 87–93 (2016). https://doi.org/10.1007/s00384-015-2372-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2372-x